Abstract
BACKGROUND AND PURPOSE: Several studies have shown that procedural outcomes are better at high-volume institutions, possibly due to greater physician experience (learning) or practice (repetition). Our purpose was to determine whether outcomes for coil embolization improved with the experience of the practitioner, after adjusting for the perceived risk of treatment.
METHODS: We identified all unruptured aneurysms treated with coil embolization at our institution from 1990 through 1997. A clinical nurse specialist abstracted the characteristics from cases that met the entry criteria. Two neurologists independently determined the complications by using definitions established a priori. The influence of experience of the treating-physician on complications was evaluated with univariate and multivariable logistic regression analyses.
RESULTS: Sixteen complications occurred in 94 patients (17%) treated with coil embolization. Complications occurred in 53% of the first five cases that each of three physicians treated, and in 10% of later cases (P < .001). After an adjustment for all other predictors, including physician assessment of the risk of the procedure, the odds of a complication decreased with increasing physician experience (odds ratio, 0.69 for every five cases treated; 95% confidence interval: 0.50, 0.96; P=.03).
CONCLUSION: The risk of complications with coil embolization of unruptured aneurysms appears to decrease dramatically with physician experience. Because the physicians in this study were highly experienced in other endovascular techniques at study onset, the rate of learning may not be generalizable to other centers.
Outcomes of complex procedures improve with the experience of the practitioners (1–3). This association, termed the learning curve, has been demonstrated in several complex procedures, including laparoscopic cholecystectomy, laparoscopic fundoplication, coronary angioplasty, and laser-assisted in situ keratomileusis (LASIK) surgery of the eye (4–8).
Endovascular therapy with platinum coils (Guglielmi detachable coils [GDCs]) was introduced in 1990 as an alternative to surgical clip placement for the treatment of cerebral aneurysms (9, 10). Endovascular therapy with coils is a complex procedure, and extensive training has been recommended (11, 12). With its appeal as a minimally invasive technique, an increasing number of centers are treating unruptured and ruptured aneurysms with endovascular coil embolization.
We sought to determine whether outcomes for endovascular coil embolization improved with the experience of the practitioner after adjusting for the perceived risk of treatment.
Methods
Cohort Definition
We searched a computerized database of hospital admissions at the University of California San Francisco (UCSF) Medical Center for all unruptured aneurysms treated by endovascular means from 1990 through 1997, as previously described (13). Medical records were reviewed to confirm that patients met the following inclusion criteria: endovascular coil embolization of an unruptured aneurysm, age 18 years or older at follow-up, no associated arteriovenous malformation, no subarachnoid hemorrhage from a different aneurysm within 6 months before treatment, and no aneurysm treated on a second occasion within 2 months of treatment. This cohort was developed as a part of larger study to compare endovascular and surgical treatment (13).
To obtain an overall estimate of procedural risk, the following information was presented to three neurointerventional radiologists who were unaware of the actual treatment modality and outcome: 1) patient age, past medical history, symptoms, and signs on presentation based on medical records abstraction and 2) relevant preprocedural radiographic images. Physicians familiar with a case were excluded from its review. Anticipated procedural risk was rated on a four-point Likert scale. Neurointerventional radiologists (R.T.H., C.F.D., V.V.H.) were asked to estimate the aneurysm size and neck-to-dome ratio.
Outcomes
The occurrence of a procedure-related complication was the primary outcome for this analysis. A procedure-related complication was defined as an adverse event that was clearly a result of the procedure and that led to prolonged hospitalization or a change in the Rankin Scale score of 1 or more points at discharge. This outcome was believed to be important because it reflected morbidity and resource consumption, although it was likely that previously applied definitions increased the detection of complications.
Two neurologists (S.C.J., D.R.G.) independently determined the procedure-related complications for the entire course of treatment (13). Agreement on whether a procedure-related complication occurred was substantial ( 54=0.86) (14). Disagreements were resolved through discussion. The effectiveness of treatment was estimated by assessing the presence of residual neck or coil compaction during initial follow-up angiography (generally performed within 6 months) that required additional coil embolization. The length of stay was recorded as the number of days that the patient spent in the hospital; this included acute as well as all follow-up hospitalizations. Hospital charges were obtained from institutional administrative databases and included those from initial and all follow-up care at UCSF.
Statistical Analysis
Physician-anticipated risk ratings were transformed to z scores so that the mean response for each reviewer was 0 with a standard deviation of 1. For each patient, z scores were averaged so that each reviewer contributed equally to the risk assessment. The case number in a rank series for a given practitioner was used as a surrogate for that practitioner’s experience. For univariate analyses, we compared cases from the first half of the collective experience with later cases. To illustrate changes in outcomes with experience, we plotted the proportion with complications by the case number.
Univariate comparisons of continuous variables that were not normally distributed were performed with the Wilcoxon rank-sum test. The chi-square test was used for comparing the categorical variables unless any cell in a 2 × 2 table was less than five, in which case we used the Fisher exact test. We used logistic regression to determine the independent association of experience on the risk of complications after we adjusted for risk assessments, age, female sex, Rankin score at admission, presenting symptoms, number of aneurysms, aneurysmal location, aneurysmal size, aneurysmal neck-to-dome ratio.
Results
A total of 98 patients were treated by means of coil embolization for unruptured aneurysms; of these, 94 met the inclusion criteria. Compared with the other patients, those treated in the later period were slightly younger and less likely to have presented with compressive symptoms, such as cranial neuropathies (Table 1). In the initial treatment group, more aneurysms were in the posterior circulation, whereas in the later group, aneurysms were more common in the anterior circulation. The neck-to-dome ratio was greater in the aneurysms that were treated later. Anticipated procedural risks estimated by the neurointerventional radiologists were similar in the two groups.
TABLE 1:
Patient characteristics at admission
| Characteristic | Initial Cases(n=45) | Later Cases(n=49) |
|---|---|---|
| Patient demographics | ||
| Female sex | 35 (78) | 41 (84) |
| Male sex | 10 (22) | 8 (16) |
| Age (y)* | 60 ± 12 | 58 ± 15 |
| Presenting Symptoms | ||
| Incidental | 24 (53) | 31 (63) |
| Compressive | 21 (47) | 18 (37) |
| Rankin score at | ||
| 1, no handicap | 7 (16) | 6 (12) |
| 2, minor handicap | 27 (60) | 29 (59) |
| 3, moderate handicap | 5 (11) | 8 (16) |
| 4, moderate-severe handicap | 4 (8) | 6 (12) |
| 5, severe handicap | 2 (4) | 0 (0) |
| Aneurysmal location | ||
| Anterior circulation | 31 (69) | 38 (78) |
| Posterior circulation | 14 (31) | 11 (22) |
| Multiple aneurysms | 5 (11) | 8 (16) |
| Aneurysmal size (mm) | ||
| <5 | 3 (7) | 7 (14) |
| 5–9 | 13 (29) | 19 (39) |
| 10–25 | 21 (46) | 20 (41) |
| >25 | 7 (15) | 3 (6) |
| Aneurysmal neck-to-dome ratio* | 0.54 ± 0.17 | 0.59 ± 0.15 |
| Anticipated procedure risk† | 0.0004 | −0.0004 |
Note.—Unless otherwise specified, data are means or numbers of patients; data in parentheses are percentages.
Data are the means ± the standard deviations.
Data the z scores.
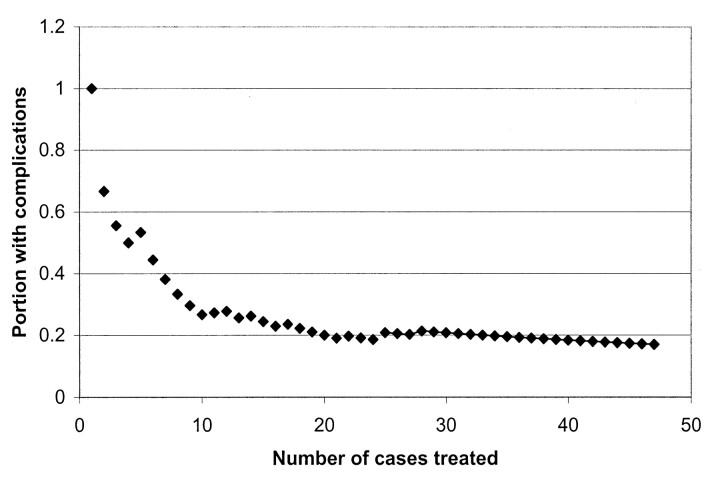
When cases were dichotomized at the midpoint of practitioner experience, 24% of the initial cases and 10% of the later cases had a complication P=.07 (Table 2). There were no significant differences in the types of adverse events (Table 3). An additional analysis was performed, in which we considered only those adverse events that were neurologically or directly related to the procedure. The proportion of these adverse events was 15 33% in the initial group and nine (18%) in the later group. The total length of stay, intensive care days, and hospital charges, including all follow-up care at UCSF, were similar in both groups. Further embolization was required less frequently in the later cases (Table 2). The cumulative proportion with complications dramatically decreased with the number of cases previously treated by each neurointerventional radiologist (Fig 1).
TABLE 2:
Patient characteristics at discharge
| Characteristic | Initial Cases*(n=45) | Later Cases*(n=49) | P Value |
|---|---|---|---|
| Complications† | 11 (24) | 5 (10) | 0.07‡ |
| Multiple embolization sessions§ | 20 (59) | 8 (30) | 0.03‡ |
| Length of stay (d) | |||
| Total | 5 | 3 | 0.23 |
| Intensive care | 2 | 1.5 | 0.14 |
| Hospital charges ($) | |||
| Total | 32,895 | 32,335 | 0.32 |
| Minus initial hospitalization | 26,401 | 26,874 | 0.3 |
| Minus follow-up care | 5,790 | 5,348 | 0.27 |
Data are means or numbers of patients; data in parentheses are percentages.
A complications was defined as an adverse event that was clearly a result of the procedure and that led to prolonged hospitalization or a change in the Rankin scale score of 1 or more points at discharge.
This P value was determined with the Pearson χ2 test. All other P values were determined by using Wilcoxon rank-sum tests.
Additional coil embolization was needed for the treatment of an aneurysm during follow-up.
TABLE 3:
Adverse events during initial follow-up hospitalization
| Adverse Event | Initial Cases(n=45) | Later Cases(n=49) |
|---|---|---|
| Neurologic | ||
| Cranial neuropathy | 6 (13) | 3 (6) |
| Cortical deficit (ischemic and/or compressive) | 3 (7) | 2 (4) |
| Intra- or postoperative aneurysm rupture | 2 (4) | 3 (6) |
| Pain | 3 (7) | 1 (2) |
| Vessel dissection | 1 (2) | 0 (0) |
| Non-neurologic | ||
| Arrhythmia | 0 (0) | 1 (2) |
| Pneumonia | 2 (4) | 0 (0) |
| Urinary tract infection | 0 (0) | 1 (2) |
| Groin and/or wound complication | 2 (4) | 1 (2) |
| Respiratory decompensation (intubation) | 1 (2) | 0 (0) |
| Intravenous line infection | 1 (2) | 0 (0) |
| Sodium disturbance | 1 (2) | 0 (0) |
| Bowel ischemia | 0 (0) | 1 (2) |
Note.—Data are means or numbers of patients; data in parentheses are percentages. None of the differences were statistically significant on the basis of results of the Fisher exact test.
Fig 1.
Plot shows the cumulative proportion of cases with complications by practitioner experience. The proportion with complications decreases with increasing physician experience, which is represented by the number of cases that each practitioner treated previously.
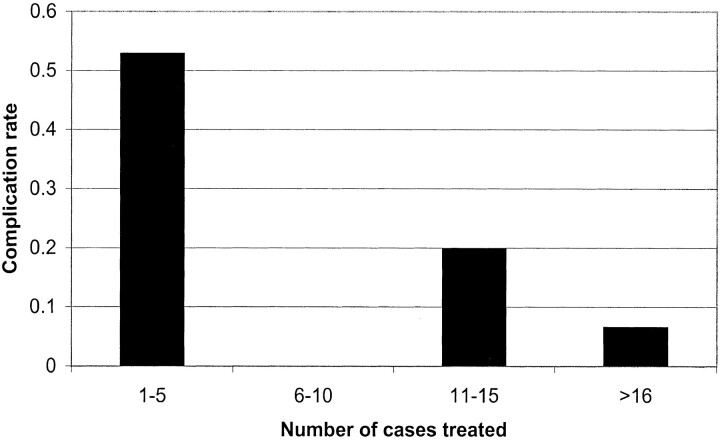
We compared complications for the first five cases for each of these neuroradiologists with those of subsequent cases (Fig 2). Complications occurred in 53% of the first five cases that each of the three physicians treated and in 10% of later cases (P < .001). After we adjusted for all other predictors, the odds of complication were lower with increasing physician experience (odds ratio, 0.69 for every five cases treated; 95% confidence interval: 0.50, 0.96; P= .03); the result correspond to a 30% odds reduction for complication for every five cases treated.
Fig 2.
Graph shows the proportion with complications by practitioner experience. Cases were rank-ordered for each practitioner and grouped accordingly.
Discussion
In our study of three practitioners at a single center, the risk of complications with coil embolization of unruptured aneurysms dramatically decreased with physician experience, even after adjustment for case complexity. As endovascular coil embolization is introduced in an increasing number of centers, the recognition that the practitioner’s lack of initial experience with the technique is likely to be associated with rates of complications that are higher than those reported in the literature (13, 15). Similar to other complex procedures, coil embolization is associated with a steep learning curve.
One prior group (16) evaluated the learning curve for coil embolization, comparing the first 100 cases with the second 100 treated at a single institution. The rate of procedure-related complications decreased from 14% to 7%. This study included patients with ruptured aneurysms, and the definition of complications in that study was more conservative than ours. Results from unruptured aneurysm treatment provide more direct information about the procedural risk because patients with this condition have minimal aneurysm-related impairment prior to treatment (17). Although our study was smaller, we only included cases in which new deficits could be attributed to treatment, and we were able to control for the procedural risk to isolate the influence of experience on outcome.
Two main limitations of this study should be acknowledged. First, advances in coil technology, catheters, wires, and imaging modalities could simplify the procedures and result in more rapid training. These advances may account for some of the improvement seen in the later part of the study. Second, this study included experienced neurointerventional radiologists who were trained in other endovascular techniques prior to the study period; therefore, the results may not be reproducible in a community-based practice. Those with less experience who are performing endovascular techniques may be expected to improve more slowly with coil embolization.
Conclusion
Practitioners should be aware of the influence of the learning curve on the procedure-related complication rate for coil embolization. Extensive formal training could reduce early complications. Recognition of the steep learning curve may also be important in considering referrals for coil embolization.
References
- 1.Barrat C, Cueto-Rozon R, Catheline JM, Rizk N, Champault G. Impact of learning and experience on the laparoscopic treatment of gastroesophageal reflux. Chirurgie 1999;124:675–80 [DOI] [PubMed] [Google Scholar]
- 2.Hawasli A, Lloyd LR. Laparoscopic cholecystectomy: the learning curve—report of 50 patients. Am Surg 1991;57:542–4 [PubMed] [Google Scholar]
- 3.Kopacz DJ, Neal JM, Pollock JE. The regional anesthesia “ learning curve”. What is the minimum number of epidural and spinal blocks to reach consistency? Reg Anesth 1996;21:182–90 [PubMed] [Google Scholar]
- 4.Buonanno C. Transluminal coronary angioplasty: is a learning curve necessary? G Ital Cardiol 1986;16:457–62 [PubMed] [Google Scholar]
- 5.Blanckaert J, Sallet G. Lasik learning curve: clinical study of 300 myopic eyes. Bull Soc Belge Ophtalmol 1998;268:7–12 [PubMed] [Google Scholar]
- 6.Cagir B, Rangraj M, Maffuci L, Herz BL. The learning curve for laparoscopic cholecystectomy. J Laparoendosc Surg 1994;4:419–27 [DOI] [PubMed] [Google Scholar]
- 7.Lekawa M, Shapiro SJ, Gordon LA, Rothbart J, Hiatt JR. The laparoscopic learning curve. Surg Laparosc Endosc 1995;5:455–8 [PubMed] [Google Scholar]
- 8.Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication: definable, avoidable, or a waste of time? Ann Surg 1996;224:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach, 1: electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1–7 [DOI] [PubMed] [Google Scholar]
- 10.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach, II: preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 11.Higashida RT. Evolution of a new multidisciplinary subspecialty: interventional neuroradiology/neuroendovascular surgery. AJNR Am J Neuroradiol 2000;21:1151–2. [PMC free article] [PubMed] [Google Scholar]
- 12.Higashida RT, Hopkins LN, Berenstein A, Halbach VV, Kerber C. Program requirements for residency/fellowship education in neuroendovascular surgery/interventional neuroradiology: a special report on graduate medical education. AJNR Am J Neuroradiol 2000;21:1153–9 [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston SC, Wilson CB, Halbach VV, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol 2000;48:11–9 [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–74 [PubMed] [Google Scholar]
- 15.Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 1999;30:470–6 [DOI] [PubMed] [Google Scholar]
- 16.Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176–83 [DOI] [PubMed] [Google Scholar]
- 17.Solomon RA, Fink ME, Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg 1994;80:440–6 [DOI] [PubMed] [Google Scholar]