Abstract
BACKGROUND AND PURPOSE: Liquid n-butyl cyanoacrylate (n-BCA) use for the treatment of arteriovenous malformations (AVM) in the brain has become part of medical practice. However, no study has led to the Food and Drug Administration’s approval of n-BCA for intravascular use. The purpose of this study was to verify the effectiveness and safety of an n-BCA/Tantalum Powder/Ethiodized Oil mixture, compared with conventional treatment (Trufill polyvinyl alcohol [PVA]) for preoperative embolization of cerebral AVM.
METHODS: Between October 15, 1996, and March 24, 1999, 104 patients at 13 centers were prospectively randomized to undergo embolization using an n-BCA/Tantalum Powder/Ethiodol mixture or Trufill PVA. The pre-embolization therapy goals were determined in terms of the number of pedicles to be embolized and the percent of nidus reduction expected. Embolization results were evaluated by a central laboratory. Subsequent surgical resection data were recorded. Safety evaluation data included recording device complications, procedure complications, and intracranial events/overall neurologic outcomes, which could be either device-related, procedure-related, or both.
RESULTS: The reduction of AVM dimensions (79.4% in the n-BCA group and 86.9% in the PVA group) and the mean number of vessels embolized (2.2 in the n-BCA group and 2.1 in the PVA group) was similar in the two groups. Coils were used more commonly with PVA embolization (P<.0001). No differences were detected in surgical resection time, number of patients who required tranfusion, volume and number of transfusion units, or type and volume of fluid replacement. Glasgow Outcome Scale scores were not significantly different between the two groups before treatment, after embolization, or after resection. Two of 42 patients who underwent resection and had been treated with n-BCA experienced post-resection hematoma, compared with eight of 45 patients who underwent resection and had been treated with PVA (P<.05).
CONCLUSION: This prospective, randomized trial showed that n-BCA is equivalent to PVA as a preoperative embolic agent for treatment of cerebral AVM as determined by percent of nidus reduction and number of feeding pedicles embolized.
Embolization of cerebral arteriovenous malformations (AVM) is a well-accepted treatment adjunct to aid in surgical resection (1–6). Polyvinyl alcohol (PVA) sponge particles and platinum coils have been shown to be useful in that regard and are approved by the Food and Drug Administration for intravascular use (7). Liquid n-butyl cyanoacrylate (n-BCA) has also become a part of medical practice based on theories presented as early as the 1960s regarding the advantages of depositing liquid embolic agents into the AVM nidus (8). However, until now, no study has led to approval of n-BCA for intravascular use by the Food and Drug Administration. A number of large case series of preoperative cyanoacrylate embolization have been published (4–6, 9–14), but no prospective direct comparison of the safety and effectiveness of particulate agents versus n-BCA has been performed. A previous retrospective analysis of PVA versus n-BCA use has suggested that n-BCA is at least equivalent to PVA in aiding resectability and that it is perhaps superior in decreasing clinical complications (15).
We have recently completed, and herein report, the first prospective, multi-center, single-blind randomized study to verify that n-BCA opacified with Ethiodized Oil and Tantalum Powder is as safe and effective as (and, perhaps in some patients, superior to) PVA or coil treatment for preoperative devascularization of cerebral AVM.
Methods
One hundred four patients with cerebral AVM were randomized to undergo embolization with either PVA or n-BCA opacified with Ethiodized Oil and Tantalum Powder (Cordis Neurovascular, Inc., Miami Lakes, FL) as preoperative treatment to aid in surgical resection. The study was conducted at 13 centers. The number of patients included from each center was 17, 13, 13, 11, 11, 10, nine, five, five, four, three, two, and one, respectively. The treating physicians were experienced in AVM embolization and participated in laboratory instruction in the use of the liquid embolic system. The mean patient age was 39.1 years (62 male and 42 female patients), mean Spetzler-Martin grade was 2.9 (range, 1–5), and mean lesion volume was 21.7 cc for the PVA group and 22.1 cc for the n-BCA group. Inclusion criteria were as follows: 1) patient had Spetzler-Martin grade 3, 4, or 5 cerebral AVM (16); or 2) patient had Spetzler-Martin grade 1 or 2 AVM and AVM feeding pedicle was in area difficult to surgically access or anticipated benefit of embolization was greater than risk (eg, embolization of a known pedicle/intranidal aneurysm while the patient’s condition is stabilizing before resection, which is a circumstance that might occur in the setting of acute intracerebral hemorrhage) (17, 18).
After randomization, the operators assessed the angioarchitectural characteristics of each AVM individually. Based on medical judgment, the operators chose the microcatheter, microguidewire, and, depending on the randomization assignment, either the n-BCA/Ethiodized Oil/Tantalum Powder ratio or the PVA particle sizes to treat the AVM. If indicated, the operators also selected the configuration, length, and width of the coils appropriate for a particular AVM. Although coils were to be used preliminarily to close shunts and reduce PVA passage through the AVM, they were not allowed as a final occlusive agent to block the pedicle embolized with PVA.
This prospective study was designed to show equivalence between experimental n-BCA opacified with Ethiodized Oil and Tantalum Powder and conventional treatment (PVA and coils) for the presurgical embolization of cerebral AVM. The primary efficacy hypothesis was that the degree of vascular occlusion achieved (as measured by percent of nidus reduction and number of feeding vessels treated) by using n-BCA is not inferior to that achieved by using PVA. The secondary efficacy hypotheses were as follows: 1) the length of time to resect the AVM is equivalent between the two treatment groups; and 2) the number of transfusions required as a reflection of total blood loss during surgery is equivalent between the two treatment groups.
Pre-embolization evaluations included medical/surgical history, physical examination, and neurologic evaluation, including National Institutes of Health Stroke Scale (NIHSS) and Glasgow Outcome Scale (GOS), conducted by a nurse or physician examiner not involved in the embolization procedure. The pre-embolization angiography was performed with 10-mm metal markers on four sides of the head to measure AVM dimensions. Pre-embolization therapy goals were determined by the investigator and recorded as the number of pedicles to be embolized and the percent of nidus reduction expected.
Intraprocedural data collection included indication for embolization, randomization assignment, embolic agent used, duration of procedure, fluoroscopy time, use of coils, size of PVA particles or n-BCA/Ethiodized Oil/Tantalum Powder mixture ratio, and types of catheters and guidewires used.
Post-embolization evaluations included angiography with measurement markers in place and neurologic evaluation, including GOS and NIHSS, performed by an unbiased practitioner. Post-embolization efficacy results were determined by comparing post-embolization percent of nidus reduction and number of pedicles occluded, as measured by a central core laboratory at the University of South Florida, Tampa, FL, to the pre-procedural goal. AVM dimensions were measured: maximun length (L), maximum width (W), and maximum height (H) on biplane films with 10-mm metal markers on both sides of the head. Volume was determined according to the formula: (H × L × W)/2 = Volume.
Surgical resection data included length of time to resect the AVM, volume and number of blood units transfused, and type and volume of fluid replacement. Safety evaluation data included the following: 1) device complications, defined as product malfunctions, unintended occurrences, or user error that caused a neurologic or clinical adverse event (including catheter occlusion, catheter glued in place, early or late n-BCA polymerization, and embolic agent pulmonary embolization); 2) procedure complications, defined as adverse events that resulted from performing the embolization procedure (including pulmonary embolism, vessel perforation, vessel dissection, incorrect vessel occluded, AVM rupture, vasospasm, and hematoma); and 3) intracranial events/overall neurologic outcomes, which could be either device-related, procedure-related, or both (including ischemia, subarachnoid or parenchymal hemorrhage, seizure, and postoperative hematomas).
The safety end point determination was made by the investigator at the site. No post-occurrence adjudication was performed. Other safety measures, although not end points (clinical neurologic examination, GOS, NIHSS), were summarized at each of the follow-up time periods (after embolization, after surgical resection, and before hospital discharge). Intent-to-treat analyses of all safety and efficacy measures were conducted. Additionally, per-protocol analyses of the secondary efficacy measures were conducted.
Results
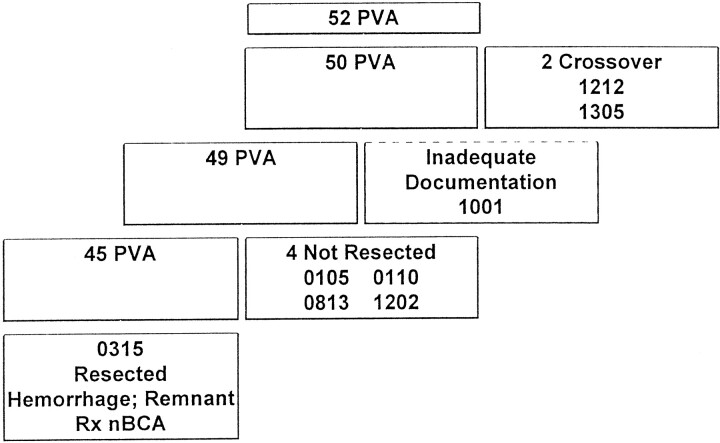
One hundred four patients were randomized between October 15, 1996, and March 24, 1999, 52 to undergo PVA treatment and 52 to undergo n-BCA/Ethiodized Oil/Tantalum Powder treatment. Of the 104 patients, 88 (84.6%) completed the treatment plan, including AVM resection. Two patients in the PVA group crossed over to treatment with n-BCA after receiving partial therapy with PVA/coils and were not included in the efficacy analysis. Forty-five (86.5%) of the 52 patients who underwent PVA treatment finished the treatment course per protocol (Fig 1). An additional patient was eliminated from analysis because of inadequate source documentation. Four patients underwent embolization with PVA but subsequently did not undergo resection: one died after experiencing post-embolization intracerebral hemorrhage; one failed provocative testing with amytal injection during stage 2 and did not undergo further embolization or resection; one underwent embolization, withdrew consent for resection, and was lost to follow-up; and one did not undergo surgery because of increased intracranial pressure but exhibited normal function at the 1-year follow-up examination.
Fig 1.
PVA group treatment flow chart.
In the n-BCA group, 43 (82.7%) patients completed the study per protocol (Fig 2). Of the nine patients not completing the study, one patient was not treated because of an unsafe feeding vessel and location characteristics. In two patients, inability to select the perforating artery feeders led to withdrawal from the study; both underwent surgical resection and achieved good outcomes. One patient refused to proceed with the study after stage 1 embolization with 96 coils and withdrew from further treatment. Two patients had successful n-BCA injections and then PVA injections in later stages when the microcatheter could not be advanced close enough for safe n-BCA deposition. These patients were not included in the efficacy analysis. Two patients who underwent embolization did not undergo resection, one after an adverse event with cerebral ischemia and another who was operated on for hematoma evacuation but not AVM removal before re-hemorrhage and death occurred. The ninth patient was lost to complete data analysis because of a prolonged, ongoing hospital course despite good post-embolization status.
Fig 2.
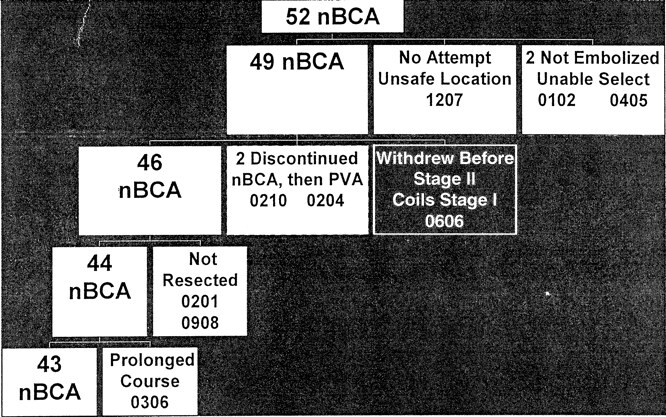
n-BCA group treatment flow chart.
AVM and pretreatment patient characteristics are outline in Table 1. No significant differences in patient groups were found. AVM volumes and deep venous drainage were equally distributed.
TABLE 1:
Arteriovenous malformations and patient characteristics
| n-BCA | PVA | |
|---|---|---|
| Number of patients | 52 | 50 |
| Spetzler grade, mean (number in each grade 1–5) | 2.9(5,13,20,11,3) | 2.9(5,14,17,10,3) |
| Volume(cm3) | 22.2 | 21.7 |
| >6 cm | 4 | 4 |
| Deep venous drainage | 26 | 23 |
| Age (mean years) | 40.5 | 37.6 |
| Sex | 33 male: 19 female | 27 male: 23 female |
| History | ||
| Intracerebral hemorrhage | 14 | 12 |
| Subarachnoid hemorrhage | 7 | 3 |
| Seizures | 24 | 21 |
| Altered mental status | 6 | 6 |
| Pounding/pulsatile headache | 14 | 15 |
| Physical examination deficits | ||
| Vision | 7 | 7 |
| Motor/Coordination | 10 | 11 |
| Sensory | 6 | 6 |
| Speech | 4 | 6 |
| Mentation | 6 | 3 |
| % Pretreatment Glasgow Outcome Scale score = 5 | 93.5% | 87.5% |
Note.—n-BCA indicates n-butyl cyanoacrylate; PVA, polyvinyl alcohol.
Treatment parameters are outlined in Table 2. Coils were used in 70.9% of PVA procedures but in only 19% of n-BCA procedures (P < .0001). Although 39 patients in the n-BCA group underwent embolization without coil use, only nine patients in the PVA group required no coil use.
TABLE 2:
Treatment parameters
| n-BCA | PVA | |
|---|---|---|
| Number of patients | 52 | 49 |
| Number of stages | 77 | 86 |
| Duration of embolization (min) | 178 | 168 |
| Duration of fluoroscopy (total min) | 93.8 | 109.6 |
| Coils used (stages) | 15 | 61* |
| Coils used (mean) | 204(14.4)** | 559(10.0)% |
| Number of patients, no coils used | 39 | 9 |
| Particle size used among procedures | ||
| <500 μm | 18(36.7%) | |
| <500 μm only, no coils | 5(10.6%) | |
| 500–710 μm | 42(29.6%) | |
| 710–1000 μm | 22(15.5%) | |
| 1000–1400 μm | 5(3.5%) | |
| Infusion catheters | ||
| 3-French infusion | 31(31.3%) | 53(52%) |
| Flow-directed catheter | 62(62.6%) | 33(32.4%) |
| Other | 6(6.1%) | 16(15.7%) |
| Guidewires | ||
| 0.018 | 0 | 5(3.8%) |
| 0.016 | 10(11.5%) | 28(21.5%) |
| 0.014 | 11(12.6%) | 34(26.2%) |
| 0.010 | 51(58.6%) | 41(31.5%) |
Note.—n-BCA indicates n-bytyl cyanoacrylate; PVA, polyvinyl alcohol.
P < .0001.
95 coils used in one patient.
PVA particles >500 μm were used in 48.6% of PVA uses. Particles <500 μm were used in only 36.7% of patients, 24.5% with and 12.2% without adjunctive coil use.
Three-French infusion catheters and larger guide wires (0.014–0.018 in) were typically used in PVA embolizations. Embolizations with n-BCA usually involved the use of flow-guided catheters and smaller 0.010-in guidewires.
By-volume percentages of n-BCA varied. The median n-BCA concentration in Ethiodized Oil was 25% (range, 10–70%).
Primary Efficacy Results
AVM reduction was similar in the two groups. The percent of AVM reduction achieved by stage was 81.1% in the n-BCA group and 79.9% in the PVA group. The percent of AVM reduction achieved per patient was 79.4% in the n-BCA group and 86.9% in the PVA group. The mean number of vessels embolized per stage (1.5 in the n-BCA group and 1.3 in the PVA group) and per patient (2.2 in the n-BCA group and 2.1 in the PVA group) was also similar in the two groups.
Secondary Efficacy Results
The number of stages was greater in the PVA group. Intent-to-treat analysis of fluoroscopy time showed no significant difference between groups. Recorded duration of fluoroscopy was greater in the PVA group (n = 43) than in the n-BCA group (n = 41) per protocol (109.6 versus 93.8 min), with a 15.8-min (16.8%) difference.
The mean surgical time required to resect the AVM was equivalent in both groups (n-BCA, 381 min; PVA, 413 min). A nonsignificant trend toward longer surgery time was also suggested in the fourth quartile for patients in the PVA group (691 versus 561 min). Six patients in the PVA group required more time than the longest time required in the n-BCA group (>680 min).
Per-protocol transfusion and fluid replacement data are included in Table 3. Patients in the PVA group required a larger number of transfused units, but the data were skewed by two patients requiring 33 and 61 units, respectively. No statistical differences were detected in the number of patients who required transfusions, the volume and number of transfusion units, or the type and volume of fluid replacement.
TABLE 3:
Transfusions/fluid replacement, surgery time
| n-BCA | PVA | |
|---|---|---|
| Number of patients transfused | 13 | 13 |
| Number of units transfused (mean, median, range) | 51 (3.4, 2, 1–16) | 135 (10.4, 3,1–61) |
| Fluid, colloid replacement (cc) | 3918 | 4053 |
| Surgery min (mean) | 381 (n = 41) | 413 (n = 42) |
Note.—n-BCA indicates n-butyl cyanoacrylate; PVA, polyvinyl alcohol.
Safety Results
A total of 104 patients were enrolled for safety evaluation in the clinical trial. The embolization complications of two patients who were randomized to undergo PVA treatment but who also underwent n-BCA treatment after failed attempts to effectively embolize with PVA were ascribed to the agent used when they occurred (ie, during n-BCA or PVA embolization). Four of the five complications these patients experienced occurred during the PVA embolization stage and were therefore listed as PVA complications. One complication (other: considerable bleeding) occurred during resection after n-BCA embolization and was therefore listed as an n-BCA complication. Therefore, the number of patients used for calculation of the incidence of adverse events in the n-BCA group is 54. Fifty percent (27 patients) of the n-BCA group and 53.8% (28 patients) of the PVA group experienced at least one complication each.
Complications were classified by the investigator as either device-related or procedure-related. In some instances, investigators classified individual complications under more than one category (eg, AVM rupture and vessel perforation). Some of the complications might easily have been deleted by an adjudication committee focusing on clinically significant events. All reported complications, whether they were categorized as device-related or procedure-related, are listed in Table 4. The complications are listed in descending order according to frequency as observed in the n-BCA treatment group.
TABLE 4:
Incidence of complications
| Complications | n-BCA (n = 54) | PVA (n = 52) |
|---|---|---|
| Seizure | 5(9.3%) | 5(9.6%) |
| Catheter glued inside vessel | 4(7.4%) | 0(0.0%) |
| Late polymerization | 3(5.6%) | 0(0.0%) |
| Occluded catheter | 3(5.6%) | 5(9.6%) |
| Parenchymal hemorrhage | 3(5.6%) | 6(11.5%) |
| Vasospasm | 3(5.6%) | 7(13.5%) |
| AVM rupture | 2(3.7%) | 1(1.9%) |
| Early polymerization | 2(3.7%) | 0(0.0%) |
| Inability to subselect vessel | 2(3.7%) | 4(7.7%) |
| CVA (stroke) | 2(3.7%) | 3(5.8%) |
| Death | 1(1.9%) | 3(5.8%) |
| Hematoma | 1(1.9%) | 1(1.9%) |
| Incorrect vessel(s) occluded | 1(1.9%) | 0(0.0%) |
| Infection/inflammation | 1(1.9%) | 0(0.0%) |
| Over-the-wire system could not be advanced | 1(1.9%) | 1(1.9%) |
| Thromboembolism | 1(1.9%) | 1(1.9%) |
| Vessel dissection | 1(1.9%) | 1(1.9%) |
| Vessel perforation | 1(1.9%) | 3(5.8%) |
| Cranial ischemia (TIA) | 0(0.0%) | 2(3.8%) |
| Catheter rupture | 0(0.0%) | 1(1.9%) |
| Failure to access vessel | 0(0.0%) | 2(3.8%) |
| Flow too high for safe infusion of embolic agent | 0(0.0%) | 2(3.8%) |
| Headache | 0(0.0%) | 2(3.8%) |
| Pulmonary embolism | 0(0.0%) | 1(1.9%) |
| Subarachnoid hemorrhage | 0(0.0%) | 2(3.8%) |
| Provocative test failed | 0(0.0%) | 1(1.9%) |
| Uncooperative patient | 0(0.0%) | 2(3.8%) |
| Other | 9(16.7%) | 9(17.3%) |
Note.—n-BCA indicates n-butyl cyanoacrylate; PVA, polyvinyl alcohol; AVM, arteriovenous malformation; CVA, cerebrovascular accident; TIA, transient ischemic attack.
On only one occasion did an n-BCA device-related complication result in an adverse neurologic or clinical event. Reflux of the n-BCA mixture into the middle cerebral main trunk from a temporal branch occurred, leading to middle cerebral artery occlusion and cerebral infarction.
Hemorrhagic complications are summarized in Table 5. Four vessel perforations occurred during the embolization procedures (in one of these cases, subarachnoid hemorrhage was also noted on the post-procedural CT scan). Three-French over-the-wire microcatheters were used during three of these procedures, whereas only flow-guided catheters were used in the fourth case. Subarachnoid hemorrhage was discovered after embolization on one additional occasion after difficult catheterizations using 3- and 1.9-French over-the-wire catheters.
TABLE 5:
Hemorrhagic complications
| n-BCA (n = 54) | PVA (n = 52) | |
|---|---|---|
| Total hemorrhagic complications | 7(13.0%) | 15(28.9%) |
| Vessel perforation, SAH* using 3- or 1.9-French OTW catheter | 1(1.9%) | 3(5.8%) |
| Vessel perforation, SAH* using flow-guided catheter only | 0 | 1(1.9%) |
| Postembolization, presurgical intracerebral hemorrhage | 4(7.4%) | 3(5.8%) |
| Postoperative hemorrhage | 2(3.7%)** | 8(15.4%)** |
| AVM volume cm3 mean (range) | 21.8(10.4–32.4) | 20.2(6.3–105.3) |
| Deep venous drainage (n) | 2 | 6 |
| Coils, mean, range | 12, 0–24 | 17.4, 0–50 |
Note.—n-BCA indicates n-butyl cyanoacrylate; PVA, polyvinyl alcohol; SAH, subarachnoid hemorrhage; AVM, arteriovenous malformation.
Subarachnoid hemorrhage identified on early postembolization CT scan; not identified at fluoroscopy.
P < .05 in intent-to-treat analysis.
Post-embolization hemorrhage occurred in four patients in the n-BCA group and three in the PVA group. One of the n-BCA patients presented with a cerebellar hematoma. The primary anterior inferior cerebellar artery feeder was embolized before intentional hematoma evacuation without AVM removal. The AVM rebled within 24 hr, and the patient died. A second patient in the n-BCA group, who required thrombolysis of an occluded PCA branch, received administration of heparin and experienced hemorrhage within 24 hr.
NIHSS and GOS scores were not significantly different between the two groups before treatment, after embolization, or after resection. A GOS score of 5 (good recovery with minor neurologic/psychologic deficit) after resection but before discharge was documented for 73.7% of the patients in the n-BCA group and 66.7% of the patients in the PVA group. Longer term outcomes were not measured.
Four deaths occurred in the study. One death occurred in each group as a result of hemorrhage after embolization but before resection, and two occurred in the PVA group after embolization and after resection.
Discussion
This study was designed to evaluate the use of n-BCA/Ethiodized Oil/Tantalum Powder compared with PVA treatment for patients with planned AVM surgical resection. The goal of embolization was typically to reduce flow or occlude the deep feeders to make surgery safer. Eighty-five percent of the patients ultimately underwent surgical resection of their AVM. This comparison study confirms that n-BCA and PVA are similar in safety and effectiveness for preoperative embolization based on percent of nidus reduction, number of pedicles embolized, surgical resection time, surgical blood loss, fluid replacement, and Glasgow Outcome Scale score. This study confirms the opinion presented by Wallace et al (15) that n-BCA is at least comparable in reducing nidus size and in aiding the ability to surgically resect cerebral AVM.
A variety of theoretical advantages of n-BCA use were not addressed in this study, including the potential advantage of increased permanence and durability of n-BCA embolization as compared with PVA embolization. In addition, smaller lesions that might be totally obliterated by embolization or embolization plus stereotactic radiosurgery were not considered (19). Only one AVM in the study was totally obliterated (after partial, inadequate embolization with PVA and then complete embolization using n-BCA). Although case reports of n-BCA recanalization exist (20, 21), 15% to 20% of patients treated with PVA before undergoing stereotactic radiosurgery experienced recanalization, as shown by follow-up arteriograms obtained 2 to 3 years later (22, 23).
Our study design allowed a subjective assessment of the treatment goal (estimate of desired volume reduction and number of pedicles to be occluded) by the operator and an objective assessment of the results by central core laboratory analysis. The results were essentially equivalent. Because this study was designed before the use of liquid coils and hybrid flow-guided/over-the-wire catheters to aid distal pedicle particulate embolization, it was anticipated that embolization failures and fluoroscopy time might be different between the two groups. However, this was not shown. Failure to access the lesion or inability to pass 3-French over-the-wire catheters was reported four times in the study. Failures due to the inability to subselect the vessel occurred a total of six times, including twice in the n-BCA group. The first involved a posterior choroidal artery-supplied AVM that could not be safely accessed. The second involved a frontal callosal AVM with hemorrhage. In this case, a nidal component with short en passage feeders could not be safely catheterized for embolization. Both lesions were resected, and good outcomes were achieved. It is thought that these two cases represent catheterization failures, not n-BCA failures, that would not have been successfully embolized if they had been randomized to the PVA group. Two other patients with successful n-BCA stages then received PVA treatment when the microcatheter could not be positioned close to the nidus for additional n-BCA delivery.
No statistically significant difference in fluoroscopy time was documented. The absolute 15.8-min difference (mean) in fluoroscopy time overall does translate into a reduced radiation dose in patients in the n-BCA group, which is difficult to ignore considering that every radiation exposure is associated with potential long-term sequelae. The number of digital images obtained during the procedures was not recorded, and no other dose estimates were performed.
Complications associated with the use of n-BCA included four catheters glued in place, which is a long-known risk unique to adhesive cyanoacryates (24, 25). Two of the catheters were glued in place during prolonged intranidal injections. No adverse clinical sequelae related to the glued-in catheters were documented, although one patient with a seizure history suffered a seizure the day after the procedure and another patient suffered a post-resection hematoma of uncertain cause. Three of the catheters were fixed to the femoral puncture site and were completely removed at the time of AVM resection 1 to 3 days later. One catheter was broken with gentle traction at the time of fixation, leaving approximately 10 cm of the catheter in the middle cerebral artery after resection.
Injected n-BCA may polymerize prematurely and block the microcatheter or may incompletely penetrate the nidus. Early polymerization with microcatheter blockage occurred in two patients, again with no untoward clinical sequelae. Premature n-BCA polymerization in the feeding pedicle with little nidus penetration occurred twice in the study; additional embolization and uneventful surgical resection were then performed. Polymerization problems are unique to n-BCA use, but blockage of catheters also occurred in the PVA group with no untoward sequelae. One catheter rupture occurred during PVA use, also without clinical sequelae.
Late polymerization of n-BCA may occur, leading to venous passage and venous occlusion. Although venous occlusion may be a goal when attempting intranidal embolization to achieve complete occlusion, it can lead to venous/nidal hypertension if inflow persists and outflow is obstructed (26). Polymerization in draining veins was documented three times but caused no adverse neurologic events or hemorrhage. Surgery was expedited in two of the cases, and good outcomes were achieved.
It is possible to release small amounts of n-BCA into non-target vessels during catheter removal, either as an adherent droplet dislodged from the catheter tip or perhaps milked from the microcatheter as it is retracted through bends of the catheterized vessel and through the guide catheter tip. Non-target vessel embolization of n-BCA occurred twice in the study, but the agent embolized to vessels also supplying the AVM, leading to no adverse clinical events.
The advantage of n-BCA solidifying within the nidus while uncommonly passing through to major veins and the lungs may have limited significance (27). However, two episodes of PVA passage into the pulmonary bed were noted during the study. PVA was discovered in the pulmonary microvasculature at autopsy in a patient suffering post-embolization hemorrhage and death (without premortem adverse respiratory effect documented). The second episode was documented intraprocedurally in an otherwise healthy, 45-kg, 34-year-old woman with decreasing oxygen saturation after PVA injection through a flow-guided microcatheter.
The embolic agent cannot be viewed independently from the delivery system when considering procedure complications. The ability to deliver n-BCA using smaller, flow-guided catheters to more distal locations would seem a potential safety advantage. There may not be an advantage to n-BCA in smaller, compact-nidus, proximal AVM, where PVA ≤500 μm in size can be delivered successfully through smaller flow-guided microcatheters. However, PVA particles >500 μm, which cannot easily be injected through small, currently available flow-guided microcatheters were used in 63% of the procedures. When larger microcatheters and guidewires to accommodate larger particles or coils must be used, vessel perforations and subarachnoid hemorrhage can be anticipated (28, 29). In this study, three vessel perforations were documented fluoroscopically during cases involving 3-French over-the-wire catheters. One other was discovered on post-embolization CT scans in a case in which both 3- and 1.9-French over-the-wire catheters were used. The only case of perforation with a flow-guided catheter was documented after injection of n-BCA in a cross-over patient who had initially received PVA and liquid coils.
This study showed that coils were used more commonly with PVA embolization (P < .0001). Where larger arteriovenous communications exist, coils must be used to prevent excessive PVA embolization to the lungs, and these coils may create relatively proximal obstructions that may not obliterate the nidus. Two of three patients who experienced hemorrhage after embolization but before surgery in the PVA group required more than the average number of coils placed during the procedure.
Proximal occlusion may allow collateral supply, sometimes deeper and less surgically controllable, to vascularize the nidus, making surgery more difficult and blood loss greater. This may have been a factor affecting surgical blood loss in this study; blood loss, as manifested by units of blood transfused, was greater in the PVA group. The occurrence of post-resection hemorrhage (n-BCA: two [4.7%] of 43 patients, one of whom required repeat craniotomy; PVA: eight [17.8%] of 45 patients, five of whom required repeat craniotomy; P < .05) may be a reflection of residual nidus, less control of perfusion pressure alterations, or recruitment or persistence of collateral flow sources that served as delayed bleeding sources.
Six of eight patients with post-resection hemorrhage in the PVA group had deep venous drainage, and six of eight received the average or greater than the average number of coils placed during embolization. Only one patient with post-resection hemorrhage had a residual nidus, and none had confirmed venous occlusion. Three of the 10 post-resection hemorrhages occurred in AVM that were larger than the average AVM size during the study, two in the PVA group and one in the n-BCA group. Patients who had intra- or post-procedural hemorrhage detected were more likely to have received heparin during embolization. Wallace et al (15)also found hemorrhagic complications to be greater in the PVA group, with numbers too small to be significant.
Seizures occurring after embolization might occur for a variety of reasons. Many patients with AVM have experienced seizures and may have already been receiving anti-epileptic treatment before embolization. No new seizures occurred in the patients in the n-BCA group, whereas three patients in the PVA group experienced new seizures. Insofar as surgical therapy usually includes seizure prophylaxis, the 6% post-embolization incidence of new seizures in the PVA group suggests that seizure prophylaxis should be considered before performing presurgical embolization.
No specific PVA particle size and no specific coil size or configuration has been or can be prescribed for AVM treatment; similarly, no specific n-BCA concentration can be prescribed for AVM treatment. The unique variability and range of n-BCA concentrations possible after mixing with radiopaque Ethiodized Oil and Tantalum Powder provides a potentially distinct advantage to n-BCA use.
Presurgical embolization with n-BCA mixed with Ethiodized Oil and Tantalum Powder can be considered equivalent in effectiveness to embolization using PVA and coils. Fewer vessel perforations and subarachnoid hemorrhage with flow-guided microcatheters, which are typically used for liquid n-BCA embolization, represents an additional safety factor. Analysis of clinical parameters that are particularly pertinent to procedure performance and patient safety seem to confirm and reinforce the opinion presented by Wallace et al (15) that distinct advantages to n-BCA use may exist in certain settings.
Conclusion
This prospective, randomized trial showed that n-BCA combined with Ethiodized Oil and Tantalum Powder is equivalent to PVA as a preoperative embolic agent for cerebral AVM treatment as determined by percent of nidus reduction and number of feeding pedicles embolized. PVA was usually combined with the use of coils and larger over-the-wire micrcocatheters. The liquid n-BCA mixture allowed the placement of fewer coils and the use of smaller flow-guided microcatheters with smaller guidewires. Additionally, patients undergoing n-BCA treatment experienced fewer adverse clinical sequelae, especially post-resection hemorrhage. Although certain unique device risks are inherent to n-BCA use, the clinical safety advantages of using smaller, safer microcatheters to deliver nidal n-BCA occlusion may outweigh those risks. The availability of n-BCA in the neurointerventional armamentarium is an important development in the management of cerebral AVM.
Appendix
Principal Investigator: Thomas A. Tomsick, MD, FACR
The n-BCA Trial Investigators
Phillip Purdy, MD, Michael Horowitz, MD, Thomas Kopitnik, MD, Duke Samson, MD, The University of Texas Southwestern Medical Center, Zale Lipshy Hospital, Dallas, TX; Jacques Dion, MD, Gregory Joseph, MD, Robert Dawson, MD, David Owens, MD, Danial Barrow, MD, Emory University Hospital, Atlanta, GA; John Barr, MD, Stephen Powers, MD, Kevin Cockroft, MD, Brian Holmes, MD, Maria Sumas, MD, Penn State Geisinger Health System, Hershey Medical Center, Hershey, PA; Robert Wallace, MD, Thomas Masaryk, MD, John Perl, MD, Douglas Chyatte, MD, The Cleveland Clinic Foundation, Cleveland, OH; Thomas Tomsick, MD, John M. Tew, Jr., MD, Harry van Loveren, MD, Mario Zuccarello, MD, Departments of Radiology and Neurosurgery, University of Cincinnati, Cincinnati, OH; Michael Marks, MD, Alexander Norbash, MD, Gary Steinberg, MD, Department of Radiology, Stanford Hospital, Stanford Stroke Center, Stanford, CA; Van Halbach, MD, Randall Higashida, MD, Christopher Dowd, MD, Michael Lawton, MD, Charles Wilson, MD, University of California, San Francisco Medical Center, San Francisco, CA; Cameron McDougall, MD, Robert Spetzler, MD, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, AZ; Gary Nesbit, MD, Stanley Barnwell, MD, Oregon Health Sciences University, Dotter Institute, Neurointerventional Program, Portland, OR; Douglas Nichols, MD, Kent Thielen, MD, John Atkinson, MD, Fredric Meyer, MD, David Piepgras, MD, Mayo Clinic, Rochester, MN; In Sup Choi, MD, John D. Day, MD, Lahey Clinic, Burlington, MA; Alejandro Berenstein, MD, Avi Setton, MD, Johnny Pryor, MD, Yasunari, Niimi, MD, Eugene Flamm, MD, Beth Israel Medical Center, North Division, New York, NY; Andrew DeNardo, MD, John Scott, MD, Methodist Hospital of Indiana, Indianapolis, IN; F. Reed Murtagh, MD, University Diagnostic Institute, University of South Florida, Tampa, FL.
Footnotes
Performed with research grant funding from Cordis Neurovascular Inc., Miami Lakes, FL.
Address reprint requests to Thomas A. Tomsick, MD, FACR, Department of Radiology, The University Hospital, P.O. Box 670762, 234 Goodman Street, Cincinnati, OH 45267
References
- 1.Luessenhop AJ, Presper JH. Sugical embolization of cerebral arteriovenous malformations through internal carotid and vertebral arteries. J Neurosurg 1975;42:443–451 [DOI] [PubMed] [Google Scholar]
- 2.Stein BM, Wolpert SM. Arteriovenous malformations of the brain: current concepts and treatment. Arch Neurol 1980;37:69–75 [DOI] [PubMed] [Google Scholar]
- 3.Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg 1987;67:17–28 [DOI] [PubMed] [Google Scholar]
- 4.Samson D. Surgical treatment of intracranial arteriovenous malformations. Tex Med 1983;79:52–59 [PubMed] [Google Scholar]
- 5.Debrun G, ViñAtnuela F, Fox A, Drake CG. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg 1982;56:615–627 [DOI] [PubMed] [Google Scholar]
- 6.Frizzel RT, Fisher WS. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery 1995;37:1031–1040 [DOI] [PubMed] [Google Scholar]
- 7.Purdy PD, Samson D, Batjer HH, Risser RC. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol 1990;11:501–510 [PMC free article] [PubMed] [Google Scholar]
- 8.Sano K, Jimbo M, Saito I, Terao H, Hirakawa K. Artificial embolization with liquid plastic. Neurol Med Chir 1966;8:198–201 [Google Scholar]
- 9.Bank WO, Kerber CW, Cromwell LD. Treatment of intracerebral arteriovenous malformations with isobutyl 2-cyanoacrylate: initial clinical experience. Radiology 1981;139:609–616 [DOI] [PubMed] [Google Scholar]
- 10.Viñuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 c J Neurosurg 1991;75:856–864 [DOI] [PubMed] [Google Scholar]
- 11.Berthelson B, Lofgren J, Svendsen P. Embolization of cerebral arteriovenous malformations with bucrylate: experience in a first series of 29 patients. Acta Radiol 1990;31:13–21 [PubMed] [Google Scholar]
- 12.DeMeritt JS, Pile-Spellman J, Mast H, et al. Outcome analysis of preoperative embolization with N-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol 1995;16:1801–1807 [PMC free article] [PubMed] [Google Scholar]
- 13.Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg 1993;78:60–69 [DOI] [PubMed] [Google Scholar]
- 14.Wikholm G, Lundqvist C, Svendsen P. Transarterial embolization of cerebral arteriovenous malformations: improvements of results with experience. AJNR Am J Neuroradiol 1995;16 ;1811–1817 [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace RC, Flom RA, Khayata MH, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experiences of a single institution. Neurosurgery 1995;37:606–618 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery 1994;34:2–7 [PubMed] [Google Scholar]
- 17.Perata H, Tomsick TA, Tew JM. Feeding artery pedicle aneurysms: association with parenchymal hemorrhage and arteriovenous malformation in the brain. J Neurosurg 1994;80 ;631–634 [DOI] [PubMed] [Google Scholar]
- 18.Marks M, Lane B, Steinberg GK, Chang PJ. Hemorrhage in intracereral arteriovenous malformations: angiographic determinants. Radiology 1990;176 ;807–813 [DOI] [PubMed] [Google Scholar]
- 19.Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 1996;85 ;19–28 [DOI] [PubMed] [Google Scholar]
- 20.Fournier D, Terbrugge K, Rodesch G, Lasjaunias P. Revascularization of brain arteriovenous malformations after embolization with brucrylate. Neuroradiology 1990;32 ;497–501 [DOI] [PubMed] [Google Scholar]
- 21.Gruber A, Mazal PR, Bavinzski G, Killer M, Budka H, Richling B. Repermeation of partially embolized cerebral arteriovenous malformations: a clinical, radiologic, and histologic study. AJNR Am J Neuroradiol 1996;17:1323–1331 [PMC free article] [PubMed] [Google Scholar]
- 22.Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol 1995;16:299–306 [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 1998;42 ;1239–1247 [DOI] [PubMed] [Google Scholar]
- 24.Bank WO, Kerber CW, Cromwell LD. Treatment of arteriovenous malformations with isobutyl 2-cyanocacrylate: initial clinical experience. Radiology 1981;139 ;609–616 [DOI] [PubMed] [Google Scholar]
- 25.Debrun GM, Aletich VA, Shownkeen H, Ausman J. Glued catheters during embolisation of brain AVMs with acrylic glue. Intervent Neuroradiol 1997;3:13–19 [DOI] [PubMed] [Google Scholar]
- 26.Debrun GM, Aletich V, Ausman JI, Charbel F, Dujovny M. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery 1997;40 ;112–120 [PubMed] [Google Scholar]
- 27.Pelz D, Lownie S, Fox A, Hutton L. Symptomatic pulmonary complications from liquid acrylate embolization of brain arteriovenous malformations. AJNR Am J Neuroradiol 1995;16:19–26 [PMC free article] [PubMed] [Google Scholar]
- 28.Purdy PD, Batjer HH, Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg 1991;74:205–211 [DOI] [PubMed] [Google Scholar]
- 29.Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Hieshima GB. Management of vascular perforations that occur during neurointerventional procedures. AJNR Am J Neuroradiol 1991;12:319–327 [PMC free article] [PubMed] [Google Scholar]