Abstract
BACKGROUND AND PURPOSE: Sensorineural hearing loss (SNHL) is a rare complication of stapes surgery that may arise for many reasons. Usually, the pathogenesis of SNHL can be established by clinical and CT examinations. The purpose of this study was to evaluate the utility of MR imaging when CT findings are normal or not contributive.
METHODS: Eleven patients with SNHL (in some instances, associated with vertigo) after stapedectomy, in whom CT showed no well-defined cause, were examined by MR imaging.
RESULTS: MR studies established the additional findings of reparative intravestibular granuloma (n = 2), intralabyrinthine hemorrhage (n = 1), and bacterial labyrinthitis (n = 1). In five cases, MR findings were similar to CT findings. In two cases, CT and MR results were normal. Revision surgery was performed in five patients and confirmed the MR findings in each case.
CONCLUSION: If CT is not contributive as to the origin of SNHL and vertigo occurring after stapes surgery, then MR imaging may be helpful in these patients.
Sensorineural hearing loss (SNHL), often associated with vertigo, is a rare complication after stapes surgery (1). The pathogeneses are varied and depend mainly on the duration of the symptoms (1). Direct trauma to the membranous labyrinth, bulging of the prosthesis into the vestibule, postoperative reparative granuloma, oval window fistula, suppurative or nonsuppurative labyrinthitis, and endolymphatic hydrops are the primary known etiopathogenic factors (2).
Until recently, systematic surgical revision was the method of choice for identifying some of these underlying causes of SNHL. Currently, high-resolution CT (HRCT) is considered the first-line imaging technique for delineating the extent of disease, and it may improve the results of surgical intervention (3). Postoperatively, HRCT can depict the status of the reconstructed prosthetic ossicular chain, identify the position of the tip of the prosthesis, and may identify those cases that require repeat surgery. Moreover, HRCT can depict a malpositioned prosthesis, postoperative middle ear granuloma, perilymphatic fistula, and rare inner ear malformations at the origin of perioperative “gusher” syndrome.
The purpose of this study was to evaluate the contribution of MR imaging to the diagnosis of membranous labyrinthine complications occurring after stapes surgery when clinical and CT exploration fail to identify the cause of SNHL and vertigo.
Methods
This prospective study, conducted between 1994 and 1999, included 11 patients aged 30 to 76 years (mean age, 50 years). Inclusion criteria were 1) documentation of SNHL after stapes surgery and 2) persistent unknown origin of SNHL after clinical and CT examinations.
Surgical procedures included stapedectomy in eight patients and small fenestral stapedotomy in three patients. After surgery, all 11 patients experienced severe SNHL, and six patients also had profound hearing loss (in the operated ear). Vertigo was encountered in nine patients (90%) and tinnitus in two (20%). No facial palsy was encountered.
All patients underwent HRCT of the temporal bone in both axial and coronal planes with 1-mm contiguous slices and a 512 × 512 matrix. CT scans were examined to determine the position of the prosthesis; a tip protruding more than 2 mm into the vestibule defined an intravestibular bulging of the prosthesis. Other signs evaluated included mineralization of the long process of the incus and bony labyrinth as compared with the contralateral healthy ear, aeration of the oval window and the sinus tympani, ossification of the membranous labyrinth, and associated inner ear malformation (eg, vestibular aqueduct dilatation, abnormal cochlear segmentation). MR imaging was performed in all patients 1 to 7 days after CT exploration on a 1.0-T superconducting active shielded magnet. Three-millimeter-thick contiguous 2D spin-echo T1-weighted images (500/15/4 [TR/TE/excitations]) without and with administration of intravenous gadopentetate dimeglumine and 4-mm-thick axial 2D spin-echo T2-weighted sequences (2500/90/1) with a 0.8-mm gap were obtained in axial and coronal planes. Finally, 1-mm-thick contiguous axial 3D Fourier transformed constructive interference in steady state (20/8/1) sequences centered on the inner ear were obtained.
MR imaging is considered a safe procedure for patients who have undergone stapedectomy; previous studies have shown that the piston of the prosthesis is not ferromagnetic, with the exception of the McGee stapedectomy piston prosthesis (4, 5). All CT and MR examinations were evaluated by two radiologists. The MR images were studied for T1 and T2 signal intensity of the anterior and posterior labyrinth, especially in the region of the oval and round windows, and for membranous labyrinthine enhancement after injection of contrast material.
Results
The study population included nine women and two men (age range, 30–76 years; mean age, 50 years). The left ear was the most frequently involved (in nine cases, versus two in the right ear). Symptom onset varied from 2 days to 20 years after stapedectomy: in three cases, onset was within 2 days; in two cases, within 2 months; in four cases, within 1 to 5 years; and in two cases, onset was more than 5 years after stapedectomy. In all patients, symptoms were represented by SNHL, which was either total (n = 7) or partial (n = 6). Vertigo was present in eight patients, tinnitus in two (Table 1). No patient had facial palsy. Three patients received medical treatment with antibiotics and steroids, resulting in partial amelioration.
TABLE 1:
Clinical and imaging findings
CT Findings
CT findings were normal in two cases and abnormal in nine. CT showed hypodensity in the oval niche in one patient (case 1), periprosthetic hypodensity in four patients (cases 2, 5, 8, and 9), hypodensity in the vestibular aqueduct associated with enlargement of the internal auditory canal and modiolus in one patient (case 3), and periprosthetic granuloma associated with a neuroma of the belly of the stapedius muscle in one patient (case 5). In five patients, CT showed intravestibular bulging of the prosthesis, which was sufficient to explain the SNHL (cases 3, 5, 7, 9, and 11). In the other cases, CT abnormalities were insufficient to explain the SNHL.
MR Findings
MR studies showed postoperative hemorrhagic labyrinthitis, as determined by T1 and T2 hyperintensity of the membranous labyrinth in one patient (case 2) (Fig 1). In two patients, a reparative intralabyrinthine granuloma (or intravestibular granuloma), as determined by intermediate signal intensity on T1-weighted images and hypointensity on T2-weighted images of the membranous labyrinth and enhancement after contrast medium administration, was present (cases 3 and 8) (Fig 2). Suppurative labyrinthitis, depicted by an intense enhancement of the posterior membranous labyrinth associated with a decrease of the normal high signal intensity on T2-weighted images, was present in one patient (case 6) (Fig 3).
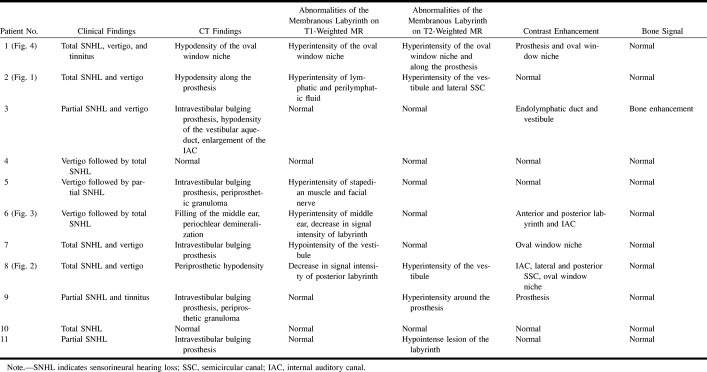
fig 1.
58 year-old woman with postoperative hemorrhagic labyrinthitis 2 months after left-sided stapedectomy.
A, Coronal CT scan of left temporal bone shows a hypodensity along a well-located prosthesis (arrow). The lateral semicircular canal is normal.
B, Axial T1-weighted MR image shows abnormal signal intensity of the left lateral semicircular canal, as compared with right ear.
C, Axial T2-weighted MR image (2500/90) shows normal hyperintensity of the lateral semicircular canal (arrow).
D, Contrast-enhanced axial T1-weighted MR image (500/15) shows no change in the signal intensity of the labyrinth.
E, Contrast-enhanced coronal T1-weighted MR image shows an enhancing mass engulfing the prosthesis (arrow). Note the normal enhancement of the tympanic portion of the facial nerve (arrowhead) under the abnormal lateral semicircular canal.
F, Follow-up axial T1-weighted MR image at 1 year shows disappearance of the high signal intensity of the lateral semicircular canal.
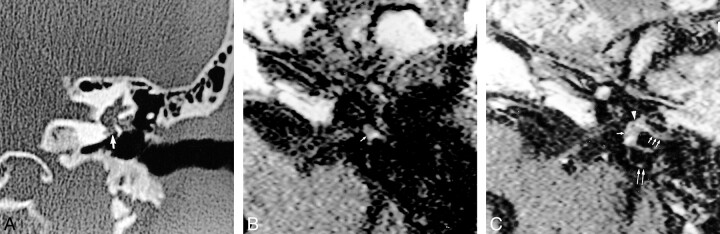
fig 2.
58 year-old man referred for SNHL and vertigo 2 days after stapedectomy with a reparative intravestibular granuloma, confirmed by surgery.
A, Coronal CT scan of the left temporal bone shows a soft tissue mass filling the oval window niche (arrow) and surrounding the tympanic portion of the facial nerve and the prosthesis.
B, Noncontrast T1-weighted MR image (500/15) reveals slight high signal intensity of the vestibule (arrow).
C, Axial contrast-enhanced T1-weighted MR image shows an enhancing mass of the oval window niche (arrowhead) spreading within the vestibule (single arrow) and the posterior (double arrows) and lateral (triple arrows) semicircular canals.
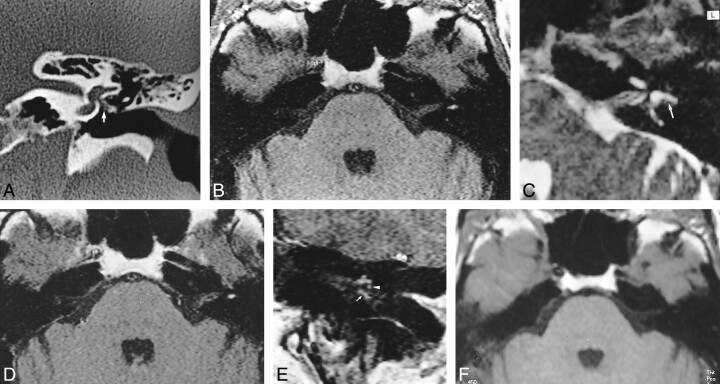
fig 3.
39 year-old woman referred for SNHL and vertigo 2 months after stapedectomy complicated by suppurative labyrinthitis.
A, Coronal CT scan shows complete filling of the middle ear and oval window niche, enlargement of the oval window (double arrows), osseous erosion of the promontory and the lateral semicircular canal (single arrow), and enlargement of the vestibule and superior semicircular canal (arrowhead).
B, Contrast-enhanced coronal T1-weighted MR image shows enhancement of an inflammatory mass of the epitympanum in communication with the lateral semicircular canal (arrow). Note associated abnormal enhancement of the vestibule, superior semicircular canal (arrowhead), and internal auditory canal.
In two patients, MR and CT examinations were normal (cases 4 and 10), but there was a long delay (5 months and 4 years, respectively) between clinical symptoms and imaging examination. MR findings were similar to CT findings in five cases, showing a periprosthetic granuloma associated with a neuroma of the belly of the stapedius muscle (n = 1, case 5), intravestibular bulging of the prosthesis (n = 2, cases 7 and 11), intravestibular bulging of the prosthesis associated with periprosthetic granuloma (n = 1, case 9), and a granuloma filling the oval window niche and periprosthetic region (n = 1, case 1) (Fig 4).
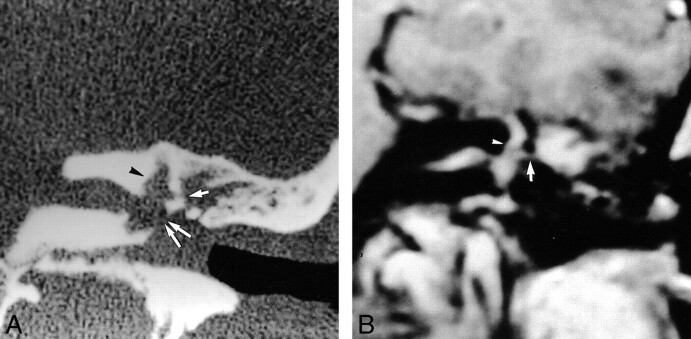
fig 4.
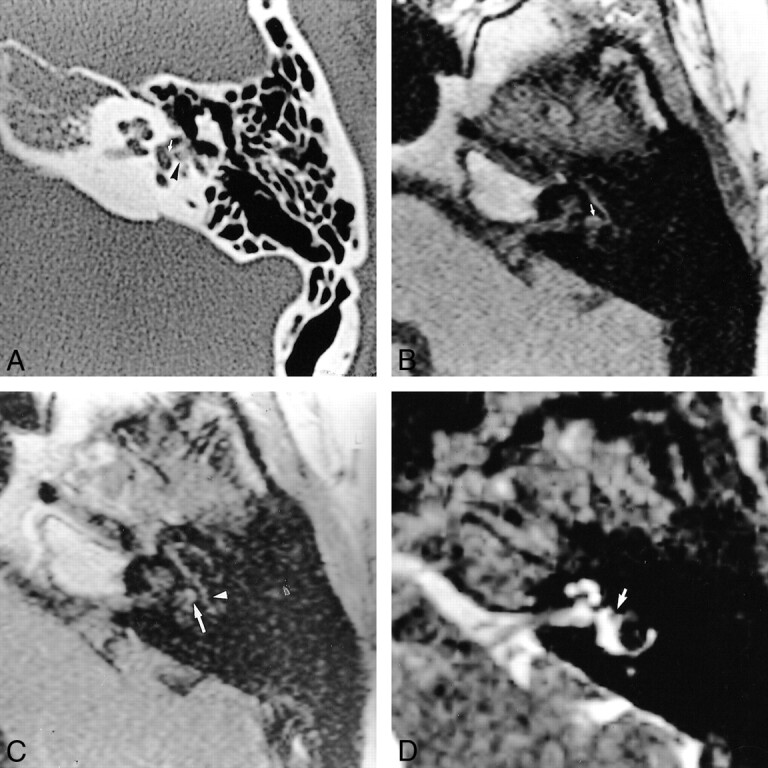
44-year-old woman referred for SNHL and vertigo 6 weeks after stapedectomy with a granuloma located around the prosthesis and in the oval window niche.
A, Axial CT scan of the left temporal bone shows well-located prosthesis (arrow) and partial filling of the posterior part of the oval window niche (arrowhead).
B, Noncontrast axial T1-weighted MR image (500/15) shows normal signal intensity of the labyrinthine fluid, except for slightly high intensity around the oval window (arrow).
C, Contrast-enhanced axial T1-weighted MR image (500/15) shows mild enhancement of the oval window (arrow). Tympanic portion of the facial nerve is seen laterally (arrowhead).
D, Axial T2-weighted MR image shows normal high signal intensity of the labyrinthine fluid. A discrete intravestibular bulging of the prosthesis is clearly depicted (arrow).
Surgical Outcome
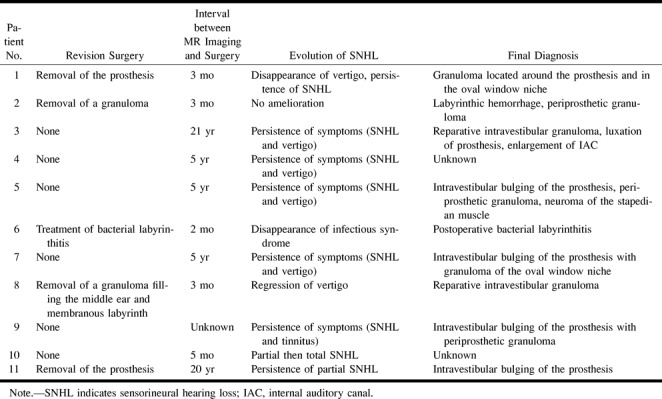
Revision surgery was performed in five cases (Table 2). Auditory function never improved after revision surgery. In four patients, indications for revision surgery were identified after MR examination, as follows: suppurative labyrinthitis (n = 1), in which surgery effected resolution of an infectious syndrome and regression of vertigo; reparative intravestibular granuloma (n = 2), in which, in one case only, removal of the prosthesis with oval fossa revision was followed by resolution of vertigo; and a hemorrhagic labyrinth associated with an intravestibular granuloma (n = 1), which had complicated a prior stapedectomy revision performed for a granuloma involving the oval window niche (only part of the posterior labyrinth was involved, the vestibule and the lateral semicircular canal). Because of the long delay (20 years) between surgery and onset of symptoms, MR examination was indicated to look for an associated disease at the origin of the SNHL. Treatment in this patient (case 11) consisted of removal of the piston of the prosthesis with interposition of a graft on the oval window niche. Revision surgery failed to resolve the vertigo attacks and hearing loss, and follow-up studies at 1 year showed complete regression of the abnormal T1 and T2 signal intensity of the posterior membranous labyrinth without any labyrinthine calcification detectable on CT scans.
TABLE 2:
Diagnosis and evolution of sensorineural hearing loss
Discussion
Otospongiosis is a common disease affecting young persons, especially Caucasian women. It may be inherited as an autosomal dominant trait, but frequently occurs as an isolated event. The disease is bilateral in 85% of patients and often symmetrical (3). The well-established treatment of otospongiosis is stapedectomy. Stapedectomy was first introduced in 1956 by Shea, who used a polyethylene strut placed on a vein graft in the oval window (6, 7). This procedure entails placement of a stapes prosthesis, extending from the oval window to the incus, and resection of the stapes superstructure.
Over the years, surgical techniques have continued to improve. Stapedectomy has increasingly been replaced by stapedotomy (the so-called small fenestra method), which results in better hearing in the low and middle frequencies, and prostheses have undergone improvements in shape, size, and material. Today, the shaft diameter of a prosthesis ranges from 0.3 to 0.8 mm (6), and pistons made of Teflon are the most widely used for reconstruction of the ossicular chain.
Complications of stapedectomy are infrequent (around 0.2% to 1%) (1). SNHL may appear suddenly after surgery or after a long delay (more than 5 years); vertigo is often the chief complaint in patients with profound SNHL, and may lead to revision stapedectomy (8). Many reports emphasize the need for rapid surgical revision in patients with deterioration of cochlear function after stapes surgery in order to prevent deafness (8). Complications of stapes surgery are always difficult to manage. Surgical revision may cause total hearing loss or disabling vertigo (1, 8, 9). Some authors have recommended the use of steroids, antibiotics, and vasodilators after surgical failure; for example, in a study by Mann et al (1), recovery of some hearing was found in six of 12 patients who followed this protocol and who did not undergo revision surgery.
Postoperative SNHL may manifest at different time intervals after surgery, depending on the underlying cause. SNHL that arises in the immediate postoperative period is most often caused by the insertion of a prosthesis that is too long, an intralabyrinthine hemorrhage, a perilymphatic fistula, or perioperative acoustic trauma. SNHL that occurs within a 2- to 5-month period after surgery is often the result of a middle ear granuloma, which may involve the vestibule (1). After several months, intravestibular granuloma, perilymphatic fistula, or prosthesis migration may be the cause of SNHL and vertigo.
Many complications of stapes surgery can be identified by CT examination, especially prosthesis dislocation and graft retraction (3). Damage to the tympanic membrane and postoperative otitis are frequent (3). Cholesteatoma formation has been reported as a poststapedectomy complication (3). Abnormal protrusion of the prosthesis into the vestibule, postoperative granuloma, suppurative labyrinthitis, and perilymphatic fistula responsible for SNHL may be identified or suggested on CT scans (1, 3). Intravestibular bulging of the prosthesis is easy to identify on CT studies; it is defined by the tip of the prosthesis protruding more than2 mm into the vestibule (3). Postoperative granuloma may be suggested by the persistence of soft tissue in the vicinity of the oval window 1 month after surgery (3). Suppurative labyrinthitis (10) may be suspected at a late stage if CT scans show labyrinthine bone demineralization with effacement of the intracartilaginous tissue and pseudoenlargement of the labyrinth. Perilymphatic fistula may be suspected when a fluid-filled middle ear is associated with the presence of air bubbles along the tip of the prosthesis (11).
MR imaging may be helpful for understanding the mechanism of SNHL and may reveal complementary findings to those on CT scans. Serous or serofibrinoid labyrinthitis is relatively common in the immediate postoperative period, and may cause transient SNHL, as a result of intralabyrinthine bleeding (2). CT is not contributive to this diagnosis. MR examination may be helpful by illustrating high signal intensity of the labyrinth on noncontrast T1-weighted images, related to a posttraumatic hemorrhagic lesion. Postsurgical labyrinthine bleeding is a local phenomenon involving only the vestibule and one or more semicircular canals. This occurrence underscores the value of a dedicated MR examination in these patients. Hearing loss may occur when bleeding involves the posterior membranous labyrinth, probably because of a transient biochemical modification of the endo- and perilymphatic fluid (12). When such complication occurs, the surgeon may have to remove the prosthesis, allowing decompression of the membranous labyrinth. This diagnosis has to be made within the first months, as spontaneous T1 hyperintensity signal disappears rapidly. Calcifications can be found at a late stage of a hemorrhagic process, allowing a retrospective diagnosis of hemorrhage (12). In our experience with serofibrinoid labyrinthitis, based on follow-up findings in only a few patients, no calcifications were evident. Moreover, abnormalities of the labyrinthine fluid are transient, which is why if diagnosis of a postoperative serofibrinoid labyrinthitis is suspected, MR imaging should be performed in the weeks immediately after the surgical procedure (12). The pathologic value of T1 hyperintensity in the immediate postsurgical phase has to be established by a larger cohort of patients.
Suppurative labyrinthitis is a rare but severe complication of stapes surgery. It may occur within days after surgery or even after a long delay (13, 14). The routes of infection from the middle ear to the labyrinth are probably through the space between the stapes footplate and the rim of the oval window, a subluxated footplate facilitating the spread of a middle ear infection in the membranous labyrinth. At a late stage of the disease, CT may show demineralization of the labyrinthine bone and effacement of the enchondral bone surrounding the footplate, with pseudoenlargement of the membranous labyrinth (10). Although labyrinthine bone demineralization is infrequent in bacterial labyrinthitis, it is often observed in otosyphilis. In iatrogenic tympanogenic labyrinthitis, the inflammatory process seems to be more aggressive than in other cases of bacterial labyrinthitis, perhaps because the bacteria have been introduced directly into the ear during surgery. MR imaging allows an early diagnosis, as it can show abnormalities of the membranous labyrinth, manifesting primarily as areas of T2 hypointensity associated with T1 enhancement after contrast administration. MR images may depict extension of the suppurative process into the internal auditory meatus along the acousticofacial bundles, which can promote intracranial complications, such as meningitis, sigmoid sinus thrombosis, and temporal lobe abscess. Consequently, in patients referred for meningitis and who have a history of stapes surgery, MR imaging should be performed to assess the inner ear as the possible origin of the infectious process.
Reparative granuloma is one of the most frequent complications of stapes surgery, and becomes manifest between the sixth and 15th day after surgery (12). By 1 month after surgery, no postoperative soft tissue debris should persist in the vicinity of the oval window (3). When such abnormality is discerned on CT scans, granuloma formation should be suspected (1). Intravestibular spread of reparative granuloma occurs in 2% of cases. Surgical removal of the granuloma and the prosthesis, and replacement with a different graft and prosthesis, is often successful in preventing permanent SNHL. MR examination may reveal a focal decrease of the normal T2 labyrinthine fluid signal and enhancement after contrast medium administration, especially when a lesion of the same signal fills the oval window niche. Therefore, MR imaging should be performed when CT shows a periprosthetic granuloma in a patient with SNHL.
Inner ear malformations may on occasion simulate otospongiosis clinically. As no imaging studies are usually required before stapes surgery, diagnosis of such inner ear malformations (endolymphatic sac dilatation, semicircular canal dysplasia, or incomplete segmentation of the cochlea) may manifest with a CSF gusher in the perioperative period, or if postoperative SNHL or disabling vertigo occurs. A CSF gusher is defined by fluid outflow through the oval window related to an abnormal congenital communication between the subarachnoid and perilymphatic spaces (12). Identification of congenital abnormalities of the inner ear that can give rise to a gusher is easily accomplished on CT studies. However, MR imaging may also be contributive by showing an endolymphatic sac dilatation or an abnormal segmentation of the cochlea.
In some cases, MR imaging does not allow precise determination of the cause of postoperative complications, although it may contribute suggestive findings. For instance, perilymphatic fistula, defined by the abnormal presence of endolabyrinthic fluid within the middle ear, occurs as early as the first week after stapes surgery and as late as 6 years after surgery (12, 15) and persists until an endosteal membrane forms at the oval window. Perilymphatic fistula occurs more frequently after stapedectomy than stapedotomy (15), manifesting as fluctuating or permanent SNHL, vertigo, and tinnitus. It can be confused with postoperative endolymphatic hydrops (15). The diagnosis of perilymphatic fistula is difficult and CT findings may be subtle. CT studies may show fluid within the middle ear, depending on the pressure within the vestibule. This finding may suggest perilymphatic fistula if associated with a pneumolabyrinth: air bubbles at the tip of the prosthesis are an indirect sign of perilymphatic fistula (3, 2). CT is better than MR imaging for the depiction of intravestibular bubbles. One recent study has shown that the presence of a pneumolabyrinth can sometimes explain postoperative vertigo and is a good prognostic sign, as, most often, a pneumolabyrinth regresses with the vertigo within a few days (unpublished data; Veillon F, Riehm G, Roedicht N, Lévépue C, Tongio J, Merli D; Congress of Radiological French Society, Paris France, 1999). In some cases of perilymphatic fistula arising early in the postoperative period, gusher syndrome has to be considered in the differential diagnosis. Indeed, a CSF gusher may simulate otospongiosis clinically and may manifest as a persistent perilymphatic fistula after surgery. In the first 2 months after surgery, perilymphatic fistula and granuloma may have the same appearance on CT studies. MR imaging can distinguish perilymphatic fistula from postoperative debris, as the CSF is hyperintense on T2-weighted images of the labyrinth.
Intravestibular bulging of the prosthesis may occur within days after surgery. It is defined on CT scans by the tip of the prosthesis protruding more than 2 mm into the vestibule (3). Sometimes, the tip of the prosthesis may be evident as a hypointensity on gradient-echo MR sequences; however, a magnetic susceptibility artifact may cause inaccurate localization of the tip and create a false-positive MR finding of intravestibular bulging. Thus, CT remains the standard of reference for diagnosis of this complication. In our study, five patients had intravestibular bulging of the prosthesis on both MR and CT studies.
Conclusion
CT is currently the standard of reference for depicting pathologic processes necessitating surgical revision in patients with SNHL after surgery for otospongiosis. CT scans can show a prosthesis bulging into the vestibule, a postoperative granuloma, findings suggestive of perilymphatic fistula, or occult pericochlear abnormalities. When CT findings are normal or fail to explain the clinical signs, MR imaging may be contributory. Indeed, MR examination may reveal an intralabyrinthine hemorrhage, spread of infections or inflammatory labyrinthitis, or intravestibular spread of a reparative granuloma.
Acknowledgments
We thank Daniel Rocher for skillful technical assistance in preparing the illustrations, Patrice Tran Ba Huy for supplying one of the cases (patient 6), and Walter Grauer for help in clarifying the manuscript.
Footnotes
Presented at the International Congress of Head and Neck Radiology, Strasbourg, October 1997.
Address reprint requests to K. Marsot-Dupuch, MD, Department of Neuroradiology, Centre Hospitalo-Universitaire de Bicêtre, 78, rue du Général Leclerc, 94275 Le Kremlin Bicêtre, France.
References
- 1.Mann WJ, Amedee RG, Fuerst G, Tabb HG. Hearing loss as a complication of stapes surgery. Otolaryngol Head Neck Surg 1996;115:324-328 [DOI] [PubMed] [Google Scholar]
- 2.Schuknecht HF, Mendoza AM. Cochlear pathology after stapedectomy. Am J Otolaryngol 1981;2:173-187 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz JD, Harnsberger HR. Imaging of the Temporal Bone.. 3rd ed. New York: Thieme; 1998:294–310
- 4.Applebaum EL, Valvassori GE. Effects of magnetic resonance imaging fields on stapedectomy prostheses. Arch Otolaryngol 1985;11:820-821 [DOI] [PubMed] [Google Scholar]
- 5.Applebaum EL, Valvassori GE. Further studies on the effects of magnetic resonance fields on middle ear implants. Ann Otol Rhinol Laryngol 1990;99:801-804 [DOI] [PubMed] [Google Scholar]
- 6.Grolman W, Tange RA, de Brugin AJG, Hart AAM, Shouwenburg PF. A retrospective study of the hearing results obtained after stapedotomy by the implantation of two Teflon pistons with a different diameter. Eur Arch Otorhinolaryngol 1997;254:422-424 [DOI] [PubMed] [Google Scholar]
- 7.Persson P, Harder H, Magnuson B. Hearing results in otosclerosis surgery after partial stapedectomy, total stapedectomy and stapedotomy. Acta Otolaryngol 1997;117:94-99 [DOI] [PubMed] [Google Scholar]
- 8.Mawson SR. Management of complications of stapedectomy. J Laryngol Otol 1975;89:145-149 [DOI] [PubMed] [Google Scholar]
- 9.Wiet RJ, Harvey SA, Bauer GP. Complications in stapes surgery: options for prevention and management. Otolaryngol Clin North Am 1993;26:471-490 [PubMed] [Google Scholar]
- 10.Matz GJ, Lockhart HB, Lindsay JR. Meningitis following stapedectomy. Laryngoscope 1968;78:56-63 [DOI] [PubMed] [Google Scholar]
- 11.Bordure P, Legent F, Calais C, Loheac D, Beauvillain C. Pneumolabyrinth and perilymphatic fistula after stapedectomy. Ann Otolaryngol Chir Cervicofac 1990;107:359-362 [PubMed] [Google Scholar]
- 12.Schuknecht HF. Pathology of the Ear.. 2nd ed. Philadelphia: Lea & Febiger; 1993
- 13.Jablokow VR, Kathuria S. Fatal meningitis due to Serratia marcescens after stapedectomy. Arch Otolaryngol 1982;108:34-35 [DOI] [PubMed] [Google Scholar]
- 14.Brown JS. Meningitis following stapes surgery: the pathway of spread to the intracranial cavity. Laryngoscope 1967;77:1295-1303 [DOI] [PubMed] [Google Scholar]
- 15.Harrison WH, Shambaugh GE Jr, Derlacki EL, Clemis JD. Perilymph fistula in stapes surgery. Laryngoscope 1967;77:836-849 [DOI] [PubMed] [Google Scholar]