Abstract
BACKGROUND AND PURPOSE: The lens of the eye is sensitive to radiation. Children undergoing CT of the head and patients undergoing repeated CT scanning of the head are vulnerable to this complication. The purpose of this study was to test the ability of a heavy metal, bismuth, in reducing radiation to the lens of the eye during routine cranial CT.
METHODS: Both phantom and human studies were done. Using a standard head-attenuating phantom, scanning was performed with detectors placed over the eye, first without the protectors, and then with shielding by one (1T), two (2T), or three thickness (3T) of bismuth-coated latex. The patient study included 30 patients randomized into one of three groups with eye protection provided by 1T, 2T, or 3T of the bismuth-coated latex. Control measurements were done using thermoluminescent dosimeters over the forehead above each eye. Image artifact from the bismuth shields was assessed.
RESULTS: The phantom study demonstrated that the use of bismuth-coated shielding over the eyes decreased radiation dosage by 48.5%, 59.8%, and 65.4% using 1T, 2T, and 3T, respectively. The effect of eye shielding in decreasing radiation dosage to the eye was highly significant for all three thicknesses (P = 2.9 × 10−81 to 1.9 × 10−89). In the patient study, the use of 1T, 2T, and 3T of bismuth-coated latex saved an average radiation dose of 39.6%, 43.5%, and 52.8%, respectively. While the use of shielding was statistically significant in saving radiation for all thicknesses (P = 2.2 × 10−10 to 1.4 × 10−21), there was no statistical difference between 1T, 2T, and 3T of bismuth-coated latex shielding found in patients. However, the trend was for increased radiation savings to the eye with increased thickness of shielding used. A review of all 30 studies showed no significant artifact caused by the eye shielding, regardless of thickness.
CONCLUSION: Bismuth-coated latex shielding of the eye during cranial CT is simple to apply, inexpensive, and causes up to a 50% reduction in radiation to the lens of the eye.
During CT of the brain, the eye receives approximately 50 milliGray (mGy), or 5 rads, of radiation (1–11). The lens of the eye is particularly radiosensitive; as little as 0.5–2 Gray (50–200 rads) causes detectable opacities, and exposures of over 4 Gray (400 rads) causes visual impairment secondary to cataracts (12, 13). The eyes of children are especially radiosensitive, with less than half this exposure causing cataracts (14). Controlling radiation exposure to the eye is important, especially in patients with visual impairment, cataracts, young or sensitive eyes, and in patients who require multiple scans.
Other than positioning the eyes outside the scan, no other radiation protection has been used for the eye during cranial CT. Using bismuth-impregnated latex, we evaluated the ability of this heavy metal to attenuate radiation dosages to the lens of the eye during cranial CT.
Methods
A single batch of thermoluminescent dosimeters (TLDs) were acquired and used for this study to measure radiation dose. For both the phantom and clinical studies, the dosage to each protector set was manually measured, the TLDs were annealed to remove any residual dose, and the TLDs were then reused. The dosage of each of the three detectors in each set was determined and averaged. Because the TLD detector does not significantly attenuate X-ray, it is ideal for in-plane radiation dose measurement.
Phantom Study
A standard head phantom (Phantom Laboratory, Cambridge, NY) was scanned 75 times by using a standard head scanning protocol (17-cm field of view [FOV], 512 × 512 matrix, 120 kV, 350 mAs, conventional CT, filter setting 0) on the same CT scanner (PQ5000, Marconi Medical Systems, Highland Heights, OH). For each scan, three TLDs were initially placed next to each other over the center of the eye, without any radioprotection in place. Following this, the eye was covered with a randomized thickness of radioprotective material and three TLDs were placed over the center of both eyes on top of the material, and the eye was rescanned. The eye shield was carefully cut to protect the lens from both the front and the sides and to conform to the nose so that no air gaps or wrinkles occurred that could produce an artifact. Twenty-five scans were performed with a single thickness (1T) of bismuth-coated latex, 25 with a double thickness (2T), and 25 with a triple thickness (3T). Exposure data (measured in mGy) from the TLDs, as well as the mAs, were recorded for each phantom case.
Patient Study
Thirty patients were recruited into the study under the auspices of an institutional review board. All patients were undergoing medically indicated head CT. The 30 patients were randomized into one of three groups: 1) eyes protected with 1T of bismuth-coated latex, 2) patients with eyes protected by 2T of bismuth-coated latex, or 3) patients with eyes protected by 3T of bismuth-coated latex.
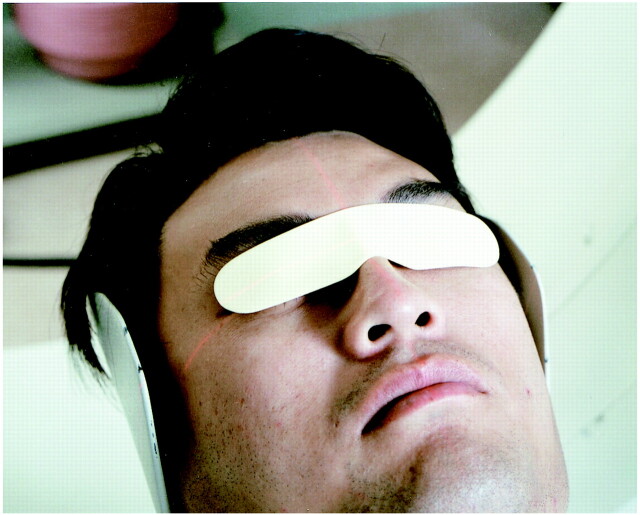
The same special shape of the shield used for the phantom study was used for the clinical studies (Fig 1). Both eyes of each patient were evaluated. Three TLDs were carefully taped over the closed eyelids of both eyes. The radioprotective material was then applied and a second set of three TLDs was placed just above the eyebrow, sagittally aligned with the original set. This second set of detectors was placed above the shield to place them out of the image plane of the first TLD set and the shield.
fig 1.
The bismuth eye shield is simple to place and covers only the eye
Patients were scanned by a standard head CT protocol (17-cm FOV; 512 × 512 matrix; 120 kV; 375 mAs; slice thickness = 4-mm foramen magnum to top clivus, 8-mm rest of the brain; conventional CT; standard algorithm). The same CT scanner was used for all studies (PQ5000, Marconi Medical Systems, Highland Heights, OH). Each scan was carefully evaluated to detect any artifact caused by the shield.
Statistical Methodology
A mixed effects linear model was used to analyze significant differences between shielded and unshielded TLDs within a given shield thickness type and between shield thickness types in this repeated measures design. For this model, the fixed effects were shield type (1T, 2T, 3T), shielded (yes or no), and the interaction of these two factors. A heterogeneous covariance structure was imposed on the model, allowing the variance to depend on the shield type by shield status combination to account for both within-case and between-case correlations (15). Bonferroni adjustments were made to P values and confidence intervals (CI) for the six contrasts of interest to adjust for multiple comparisons, such that the overall probability of a Type I error (α) was 0.05 (16). Assessment of model fit was evaluated using residual diagnostics.
Results
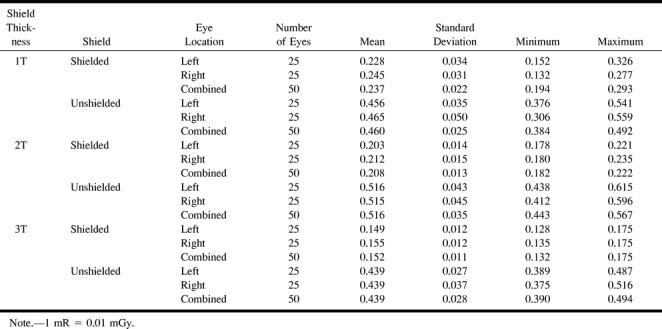
For the phantom study, shielding with 1T of radioprotective shielding decreased the average radiation dose 48.5% (95% CI: −51.6 to −45.4; P = 2.9 × 10−81). A progressive decrease in eye radiation was achieved by the addition of 2T and 3T shields (additional 11.3% and 16.9% decrease, respectively). The increased shielding effects of 2T and 3T were significant when compared with 1T alone (P = 1.7 × 10−17 and P = 2.6 × 10−12, respectively). For example, 1T radioprotection provided a mean reduction from 0.460 to 0.237 mrad/mAs. With 2T and 3T, the means decreased from 0.516 to 0.208 mrad/mAs and 0.439 to 0.152 mrad/mAs, respectively. Data of the phantom study are summarized in Tables 1 and 2.
TABLE 1:
Phantom study—mean exposure in mR/mAs (average TLDs within each subject)
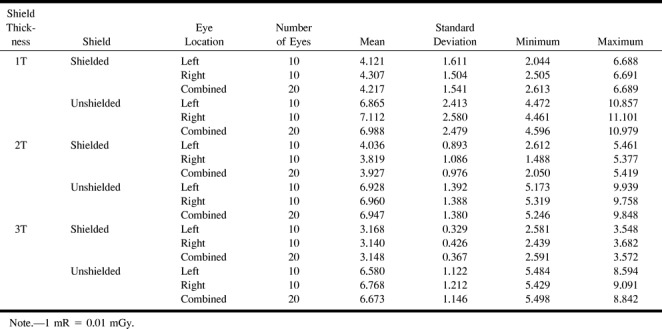
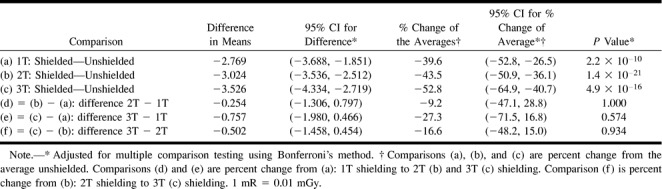
Similar results were achieved in the patient study. Mean exposure with 1T of radioprotective shielding resulted in a mean decrease in radiation to the eye from 6.988 to 4.217 mrad/mAs, a 39.6% reduction (95% CI: −52.8 to −26.5; P = 1.1 × 10−10). Some increased protection was found with 2T shielding, which reduced the mean dose from 6.947 to 3.927 mrad/mAs. This additional 0.254 mrad/mAs savings in radiation, however, was not statistically different from 1T shielding (P = 1.0). 3T shielding saved 52.8% (mean unshielded 6.673; shielded 3.148 mrad/mAs) of radiation dose to the eye versus 39.6% for 1T shielding only. There was, however, no statistical difference between 3T and 1T shielding (P = .574). The results of the patient study are included in Tables 3 and 4. The disparity between the clinical and phantom studies is likely a reflection of the homogeneity of the phantom and lack of motion, and because the phantom studies were all performed on the same head versus different heads, as in the clinical study. In addition, the control detectors for the patient studies were placed over the forehead rather than over the eye, as with the phantom study.
TABLE 3:
Patient study—mean exposure in mR/mAs (average TLDs within each subject)
The patient studies were reviewed for artifacts projected into the brain. No artifacts were observed for any of the shielding thicknesses. Specifically, no beam-hardening artifact into the deeper orbit, or especially into the cerebrum, was identified on any case. There was, however, significant artifact projected into the superficial orbit and the lens.
Discussion
While constituting only a small percentage of X-ray studies performed in the general population each year, by the early 1990s, CT, of both the brain and body, contributed about 20% of the collective dose of radiation to that population (17–18). The current widespread use of helical, and now multi-slice, CT, plus the development of new applications, has increased this collective dose significantly. The dose from cranial CT absorbed by the lens varies from 22.4 to 100 mGy (2.23 to 10 rad), depending upon beam collimation, reconstruction interval, kV, mAs, angulation, and scanner brand (1–11, 19). While the radiation exposure to the eye from a single scan is only a small fraction of the 4 Gy (400 rad) needed to cause cataracts (11, 12), the frequency of cranial CT examination (especially repeated scans performed on the same patient), and the effect of the radiation on the more sensitive pediatric eye should be considered. Merriam and Focht (14) reported that cataracts may occur with doses as low as 2 Gy (200 rad). The radiation effects on patients with smaller head sizes also lead to increased absorbed doses to the lens (20). In addition, the threshold for accelerating cataract formation in patients with preexisting lens disease cannot be underestimated (20). While there is no report that cataracts are directly attributable to diagnostic X-ray, the long latency period and the age-related natural occurrence of senile cataracts make such evaluations difficult (2).
Despite these uncertainties, some authors (8, 21) have recommended that the lens be excluded on routine brain CT. Maclennan and Hadley (8) found that the average orbital dose can be decreased to 18.5 mGy (1.85 rad) by aligning brain CT scans along the supraorbital meatal baseline. Yeoman et al (21), however, found that only 32% of sites routinely avoided the eye during brain CT. Most users prefer to begin the scan at or below the level of the foramen magnum and include a portion of the skull base within the study. As a result, head scans usually include all or the upper half of both orbits, making lens irradiation much higher.
Radiation protection for patients' eyes during neuroradiologic procedures is not a new subject. Isherwood et al (22) showed that leaded glasses worn by patients during angiography significantly decreased the amount of radiation to the lens. However, such a technique in CT is unwise, as the sharp edges and air gap between the shield and the eyelid would cause serious artifacts in the image. Superficial radioprotective clothing has been proposed for CT. Hopper et al (23), using the same bismuth latex material as in this study, found a significant reduction in absorbed dose to breasts during thoracic CT.
Other metals, especially lead, could be used for radioprotective shielding. However, lead-impregnanted latex is thicker than bismuth latex, making it much more difficult to conform to the surface of the eyes and nose. Any air spaces or wrinkles in the shielding substance will result in significant beam hardening artifact being projected into the brain.
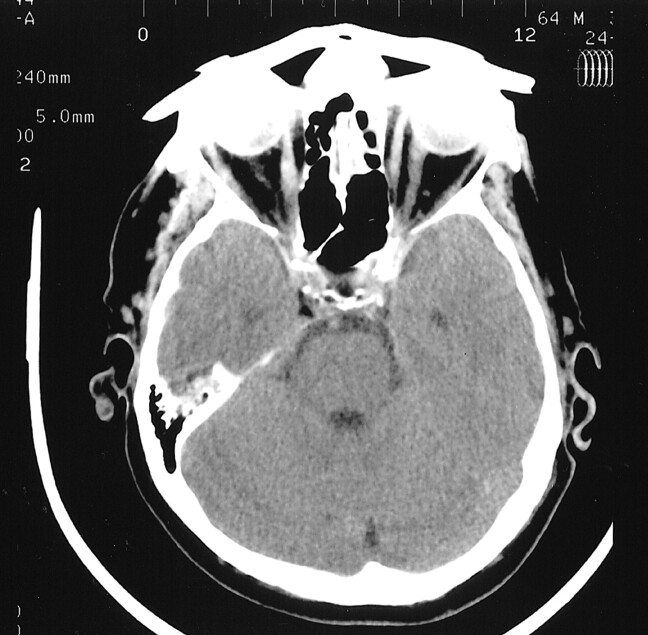
The results of this study show that it is a simple process to shield the eyes during CT. As much as half of the radiation to the lens can be avoided by using elastic, form-fitting, inert, bismuth-coated latex shields that are easy to place. These eye shields are now commercially available ($100 per package of 20, F&L Medical, One Parks Bend, Box 3, Vandergrift, PA 15690). As shown by the data in this study, no deleterious artifact is projected into the diagnostic portion of the image from such shielding (Fig 2). Indeed, perhaps an even greater thickness of shielding could be used without artifact. Such shielding is especially useful in patients with preexisting eye disease, individuals undergoing multiple cranial X-ray studies, and in children.
fig 2.
This adult patient has a 3T eye shield in place. While artifact is seen into the globe, no artifact is transmitted into the brain
TABLE 2:
Phantom study—comparisons from mixed effects linear model for mean exposure (mR/mAs)
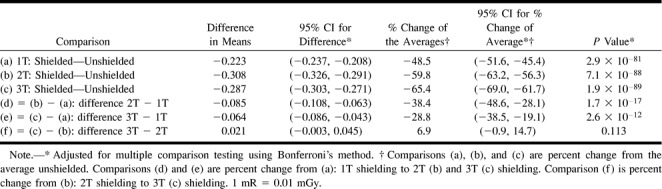
TABLE 4:
Patient study—comparisons from mixed effects linear model for mean exposure (mR/mAs)
Footnotes
Address reprint requests to Kenneth D. Hopper, MD, Department of Radiology, Penn State University, P.O. Box 850, Hershey, PA 17033.
References
- 1.Moström U, Ytterbergh C, Bergström K. Eye lens dose in cranial computed tomography with reference to the technical development of CT scanners. Acta Radiol Diagn (Stockh) 1986;27:599-606 [DOI] [PubMed] [Google Scholar]
- 2.Tweed JJ, Davies ML, Faulkner K, Rawlings DJ, Forster E. Patient dose and associated risk due to radiological investigation of the internal auditory meatus. Br J Radiol 1991;64:447-451 [DOI] [PubMed] [Google Scholar]
- 3.Lund E, Halaburt H. Irradiation dose to the lens of the eye during CT of the head. Neuroradiology 1982;22:181-184 [DOI] [PubMed] [Google Scholar]
- 4.Sim LH, Case CC. A comparison of the radiation dose to the lens of the eye from four modern CT scanners. Aust Phys Eng Sci Med 1988;11:76-80 [PubMed] [Google Scholar]
- 5.Siddle KJ, Sim LH, Case CC. Radiation doses to the lens of the eye during computerised tomography of the orbit; a comparison of four modern computerised tomography units. Australas Radiol 1990;34:323-325 [DOI] [PubMed] [Google Scholar]
- 6.Geleijns J, Van Unnik JG, Zoetelief J, Zweers D, Broerse JJ. Comparison of two methods for assessing patient dose from computer tomography. Br J Radiol 1994;67:360-365 [DOI] [PubMed] [Google Scholar]
- 7.Poletti JL. Patient doses from CT in New Zealand and a simple method for estimating effective dose. Br J Radiol 1996;69:432-436 [DOI] [PubMed] [Google Scholar]
- 8.Maclennan AC, Hadley DM. Radiation dose to the lens from computed tomography scanning in a neuroradiology department. Br J Radiol 1995;68:19-22 [DOI] [PubMed] [Google Scholar]
- 9.Sim L, Siddle K. Radiation doses to the lens of the eye during procedures on a 9800 Quick CT scanner. Radiation Protection in Australia 1987;5:122-126 [Google Scholar]
- 10.Henson PW. Acceptance tests and patient dose measurements on a Siemens Somatom 2 CT scanner. AJPESM 1982;5:102-112 [Google Scholar]
- 11.Nishizawa K, Maruyama T, Takayama M, Okada M, Hachiya J, Furuya Y. Determinations of organ doses and effective dose equivalents from computed tomographic examination. Br J Radiol 1991;64:20-28 [DOI] [PubMed] [Google Scholar]
- 12.Dalrymple GV, Goulden ME, Kollmorgen GM, Vogel H. Medical Radiation Biology.. Philadelphia: WB Saunders; 1973:235
- 13.Martin A, Harbison S. An Introduction to Radiation Protection.. London: Science Paperbacks; 1972:41
- 14.Merriam GR, Focht EF. A clinical study of radiation cataracts and the relationship to dose. AJR Am J Roentgenol 1957;77:759-785 [PubMed] [Google Scholar]
- 15.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System For Mixed Models.. Cary, NC: SAS Institute, Inc. 1996;
- 16.Woolson RF. Statistical Methods for the Analysis of Biomedical Data.. New York: John Wiley & Sons; 1987:260–267, 337
- 17.Shrimpton PC, Hart D, Hillier MC, et al. Survey of CT Practice in the UK. Part 1: Aspects of Examination Frequency and Quality Assurance (NRPB-R248).. Chilton: National Radiological Protection Board 1991;
- 18.Shrimpton PC, Jones DG, Hillier MC, et al. Survey of CT Practice in the UK. Part 2: Dosimetric Aspects (NRPB-R249).. Chilton: National Radiological Protection Board 1991;
- 19.Rothenberg LN, Pentlow KS. Radiation dose in CT. Radiographics 1992;12:1225-1243 [DOI] [PubMed] [Google Scholar]
- 20.Brasch RC, Boyd DP, Gooding CA. Computed tomographic scanning in children: comparison of radiation dose and resolving power of commercial CT scanners. AJR Am J Roentgenol 1978;131:95-101 [DOI] [PubMed] [Google Scholar]
- 21.Yeoman LJ, Howarth L, Britten A, Cotterill A, Adam EJ. Gantry angulation in brain CT: dosage implications, effect on posterior fossa artifacts, and current international practice. Radiology 1992;184:113-116 [DOI] [PubMed] [Google Scholar]
- 22.Isherwood I, Young IM, Bowker KW, Bramall GK. Radiation dose to the eyes of the patient during neuroradiological investigation. Neuroradiology 1975;10:137-141 [DOI] [PubMed] [Google Scholar]
- 23.Hopper KD, King SH, Lobell ME, TenHave TR, Weaver JS. The breast: in-plane x-ray protection during diagnostic thoracic CT—shielding with bismuth radioprotective garments. Radiology 1997;205:853-858 [DOI] [PubMed] [Google Scholar]