Abstract
BACKGROUND AND PURPOSE: Early detection of arterial occlusion and perfusion abnormality is necessary for effective therapy of hyperacute cerebral ischemia. We attempted to assess the utility of the fast fluid-attenuated inversion recovery (fast-FLAIR) sequence in detecting occluded arteries as high signal (referred to as intraarterial signal) and to establish the role of fast-FLAIR in detecting ischemic penumbra of hyperacute stroke within 24 hours after ictus.
METHODS: We studied 60 patients with hyperacute cerebral ischemia caused by occlusion of intracranial major arteries. We compared intraarterial signal on FLAIR images with time of flight (TOF) on MR angiograms, flow voids on T2-weighted images, hyperintense lesions on diffusion-weighted images, and results of follow-up CT or MR scans.
RESULTS: In 58 (96.7%) patients, FLAIR detected intraarterial signals as early as 35 minutes after stroke onset. In 48 (80.0%) patients, intraarterial signal on FLAIR images coincided with lack of TOF on MR angiograms. In 41 (74.5%) of 55 patients, the intraarterial signals of fast T2-weighted imaging depicted occlusion better than did deficient flow void on T2-weighted images. In 25 (41.7%) of 60 patients, the area of intraarterial signal distribution was larger than the hyperintense lesion measured on diffusion-weighted images. Areas of final infarction had sizes between those of intraarterial signal distribution on FLAIR images and lesions measured on diffusion-weighted images. In 35 (87.5%) of 40 patients, areas of intraarterial signal distribution were equal to regions of abnormal perfusion.
CONCLUSION: Intraarterial signal on FLAIR images is an early sign of occlusion of major arteries. FLAIR combined with diffusion-weighted imaging can be helpful to predict an area at risk for infarction (ischemic penumbra). FLAIR plays an important role for determining whether a patient should undergo perfusion study.
Diffusion-weighted MR imaging has become an essential study for patients with symptoms of ischemic stroke (1, 2). Diffusion imaging has made it possible to delineate early ischemic changes caused by Na+-, K+-pump failure that T2-weighted imaging misses. However, by the time most lesions appear hyperintense on diffusion-weighted images, they are irreversible and develop into final infarct. Lesions visible on diffusion-weighted images nearly correspond to the center of ischemia where cell death has already occurred. Lesions detected by diffusion-weighted imaging during the first 24 hours of onset are generally smaller than the final infarct (3, 4). Prediction of the presence and extent of tissue at risk is difficult with diffusion-weighted imaging alone (2). The therapeutic window of cerebral ischemia requires MR imaging techniques that are not only sensitive to the presence of infarction, but also capable of delineating areas at risk for infarction. Early detection of arterial occlusion and perfusion abnormality before lesion detection by diffusion imaging is necessary for effective thrombolytic therapy and improvement of clinical outcome.
Fluid-attenuated inversion recovery (FLAIR), which is based on the fast spin-echo sequence, is widely available on standard clinical MR systems and is used to improve the visibility of small lesions close to CSF. Recently, FLAIR has been reported to show occluded arteries as high signal in patients with hyperacute ischemic stroke (5–7). Noguchi et al (5) compared the utility of FLAIR for detecting occluded arteries in acute cerebral infarction with that of fast T2-weighted imaging. In a case report comparing CT with MR imaging, Maeda et al (6) described high signal of an occluded artery. Cosnard et al (7) reported the accuracy of FLAIR compared with MR angiography for identifying occluded arteries in 53 patients with hyperacute stroke. We sought to assess the utility of fast-FLAIR for detecting occluded arteries as high signal (intraarterial signal). On the basis of our findings, we recommend the role of fast-FLAIR in detecting ischemic penumbra of hyperacute stroke within 24 hours after ictus in conjunction with MR angiography, diffusion-weighted imaging, and perfusion study.
Methods
Subjects
We studied 60 patients (26 men and 34 women aged 27–93 years; mean age, 70.3 years) who had suffered hyperacute cerebral ischemia caused by occlusion of intracranial major arteries (internal carotid artery, vertebral artery, basilar artery, anterior cerebral artery, middle cerebral artery, and posterior cerebral artery). All patients fulfilled at least one of the criteria for major arterial occlusion: 1) deficient time of flight (TOF) on MR angiogram (58 patients), 2) perfusion abnormality in the territory fed by hemispheric branches (40 patients), or 3) final infarct involving the hemispheric branch territory identified on follow-up CT or MR scans (50 patients). In 10 patients, major arterial occlusion was confirmed by MR angiography or perfusion study, but final infarction was localized only in the territory fed by the perforating artery. All patients underwent emergency MR imaging within 24 hours after onset of neurologic symptoms: 25 patients within the first 3 hours, 14 patients from 3 to 6 hours, nine patients from 6 to l2 hours, nine patients from 12 to 18 hours, and three patients from 18 to 24 hours. We clinically diagnosed ischemic stroke by onset, course, and neurologic status. Reliable time of clinical ictus was determined from the medical records, by checking with the referring neurologists or neurosurgeons, or by direct patient interview.
Imaging Studies
MR examinations were performed with a clinical whole-body imager operating at 1.5 T (MAGNETOM Vision; Siemens AG, Erlangen, Germany). Fast-FLAIR parameters were as follows: 8000/10/1 (TR/TE/excitation); TI, 2000 ms; echo train length, 7; field of view, 21 cm; matrix size, 238 × 256; axial sections, 5 mm thick; and scan time, 3 minutes 36 seconds.
We performed fast T2-weighted imaging (4500/96/1, 5-mm section thickness, 21-cm field of view, and 266 × 512 matrix) and single-shot echo-planar (EPI) diffusion-weighted imaging (500/123/1, 24-cm field of view, 5-mm-thick axial sections, 128 × 128 matrix, diffusion gradient with b value of 1200 sec/mm2 applied along slice axis). We also completed 3D-TOF MR angiography around the circle of Willis (6.4/31 or 6.8/32 [TR/TE], 15-degree flip angle, 72-mm slab thickness, 266 × 512 matrix) as well as a dynamic-enhanced perfusion study with a single-shot, gradient-echo, EPI sequence (1000/54, 128 × 128 matrix) during the first pass of a 0.2 mmol/kg bolus of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany). Dynamic data were acquired for 60 seconds. Postcontrast MR angiography followed perfusion study with the same parameters as above.
T2-weighted imaging was performed using the fast spin-echo sequence in 55 patients and spin-echo-type EPI in the remaining five. Dynamic-enhanced perfusion study was done in 40 patients, and postcontrast 3D-TOF MR angiography was carried out in 11 patients.
We determined final infarction by follow-up CT or MR imaging performed 2 to 30 days after symptom onset. An abnormally hyperintense lesion measured on T2-weighted images or a hypodense lesion on a CT scan was confirmed to be the final infarction. In one patient with lack of TOF and perfusion abnormality in left middle cerebral artery, neurologic deficits were completely improved and no infarction was detected by follow-up CT or MR imaging.
Informed consent was obtained from all patients or their family before MR examinations.
Data Analysis
Two experienced neuroradiologists (K.T., M.I.), who were informed of the clinical status and blinded to the areas of final infarction, assessed all MR images retrospectively. The presence of intraarterial signal on FLAIR images was noted, and the signal quality of intraarterial signal in relation to the cerebral parenchyma was classified as hyperintense or isointense. A visual comparison was made for each patient to determine which study (intraarterial signal on FLAIR images versus flow void on T2-weighted images and lack of TOF on MR angiograms) better showed arterial occlusion. Areas of intraarterial signal on FLAIR images were compared with areas of perfusion abnormality, the area disclosing a hyperintense lesion on diffusion-weighted images, and the area of final infarction.
For the analysis of perfusion study, ΔR2* was calculated from the dynamic-enhanced data on the MR scanner. ΔR2*was equal to −ln (St/S0)/TE, where ΔR2* was Δ(1/T2*), S0 was the precontrast signal intensity, and St was the signal intensity at time t. Time-ΔR2* curves of regions of interest (ROI) were used for side-by-side comparison. Symmetrical mirror ROI were placed in the bilateral hemispheres to avoid large vessels and partial volume effect. The size of ROI was defined as 1.0 cm2. We did not deconvolute arterial input or venous output function. Time-to-peak, transit time, peak height, and washout pattern during the first passage on time-ΔR2* curves were visually compared among hemispheres. Perfusion abnormality was defined as areas of increased time-to-peak, elongated transit time, reduction of peak height, and delayed washout.
Raters discussed discordant findings, and determined results by consensus.
Results
In 58 (96.7%) of 60 patients, intraarterial signals were detected on FLAIR images (Table 1). Intraarterial signal was visible as early as 35 minutes after stroke symptom onset (Fig 1). In 25 patients studied within 3 hours after onset, the sensitivity of intraarterial signal was 100%. Intraarterial signals were hyperintense in 52 of the 58 patients and isointense in the remaining six. FLAIR imaging yielded no false-positive findings of arterial occlusion, which would have appeared as hyperintense or isointense intraarterial signal in the healthy contralateral hemisphere, in any patient.
TABLE 1:
Detection of intraarterial signal on FLAIR images

fig 1.
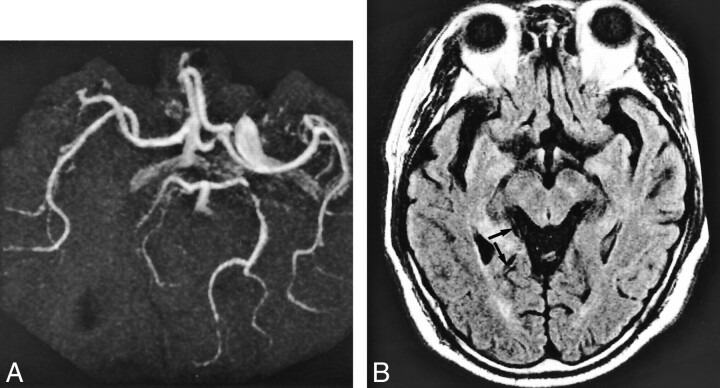
A 78-year-old woman who underwent imaging 35 minutes after onset of right hemiparesis and aphasia.
A and B, FLAIR images (8000/10/1 [TR/TE/excitation]), TI = 2000) show contiguous intraarterial signal, which is hyperintense to cerebral parenchyma in M1, M2, and M3 segments of the left middle cerebral artery (arrows).
C, MR angiogram (32/6.8, flip angle = 15 degrees) shows corresponding lack of TOF effect in M1, M2, and M3 segments of the left middle cerebral artery.
D, Diffusion-weighted image (500/123/1, b = 1200) shows faintly hyperintense lesion localized in the area fed by left lateral lenticulostriate artery (arrow); however, there is no change in the hemispheric territory of the left middle cerebral artery.
E, Postcontrast MR angiogram (32/6.8, flip angle = 15 degrees) shows intraluminal enhancement in the left middle cerebral artery except for in the distal potion of the M1 segment. Arrow indicates complete obstruction in distal portion of left M1 segment.
F, T2-weighted image (4500/96/1) obtained 7 days after onset confirmed final infarction is in the entire territory of the left middle cerebral artery. Note the diffusion-weighted lesion is still smaller than the area of intraarterial signal distribution. Intraarterial signal consists of not only complete obstruction but also slow flow. The area of final infarct corresponds to the area of intraarterial signal distribution.
Two patients with localized cortical infarction (10 and 22.5 hours after onset) had negative FLAIR results. Neither FLAIR nor MR angiography could detect occluded arteries in these patients.
In 48 (80.0%) of 60 patients, intraarterial signal on FLAIR images coincided with lack of TOF on MR angiograms (Fig 1A-C, Fig 2A-C). MR angiography showed no T1-shortening effect corresponding to intraarterial signal. In nine of the 60 patients, intraarterial signal on FLAIR images was inferior to lack of TOF on angiograms in detecting occluded arteries. Three of those nine patients had posterior cerebral artery occlusion (Fig 3). In five of those nine patients (13 hours, 15 hours, 16 hours, 17 hours, and 24 hours after onset), intraarterial signal was obscured partially because of cortical swelling from vasogenic edema (Fig 4). In the remaining patient with middle cerebral artery infarction, retrograde collateral circulation was suggested by contrast-enhanced MR angiography.
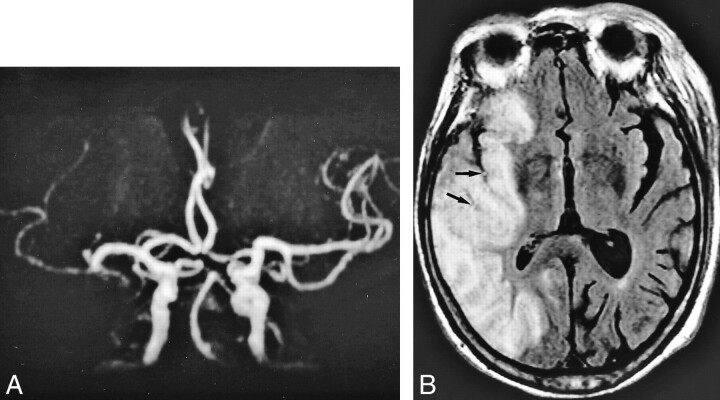
fig 2.
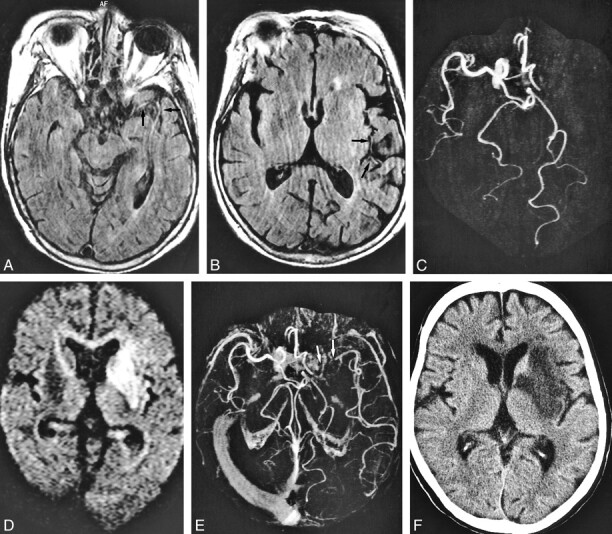
A 71-year-old woman who underwent imaging 7 hours after onset of right hemiparesis and aphasia.
A and B, FLAIR images (8000/10/1, TI = 2000) show intraarterial signal in M1, M2, and M3 segments of the left middle cerebral artery (arrows).
C, MR angiogram (32/6.8, flip angle = 15 degree) demonstrates lack of TOF in the left internal carotid and middle cerebral arteries.
Perfusion imaging (not shown) showed a hypoperfused area with increased time-to-peak and mean transit time values in the left middle cerebral artery territory as large as the area of intraarterial signal distribution and lack of TOF.
D, Diffusion-weighted image (500/123/1, b = 1200) shows hyperintense lesion in the left basal ganglia and insular cortex.
E, Postcontrast MR angiogram (32/6.8, flip angle = 15 degrees) shows intraluminal enhancement in the left middle cerebral artery except for in the M1 segment. Arrow indicates complete obstruction in the left M1 segment.
F, Final infarction is confirmed in the basal ganglia and insular cortex by CT performed 9 days after stroke symptom onset, corresponding to the initial lesion seen on diffusion-weighted images.
Note lesion in D is smaller than the area of intraarterial signal distribution. Intraarterial signal consists of not only complete obstruction but also slow collateral circulation. The area of final infarct is smaller than that of intraarterial signal distribution. Ischemic penumbra may be present in the mismatch between the intraarterial signal distribution and the diffusion-weighted lesion.
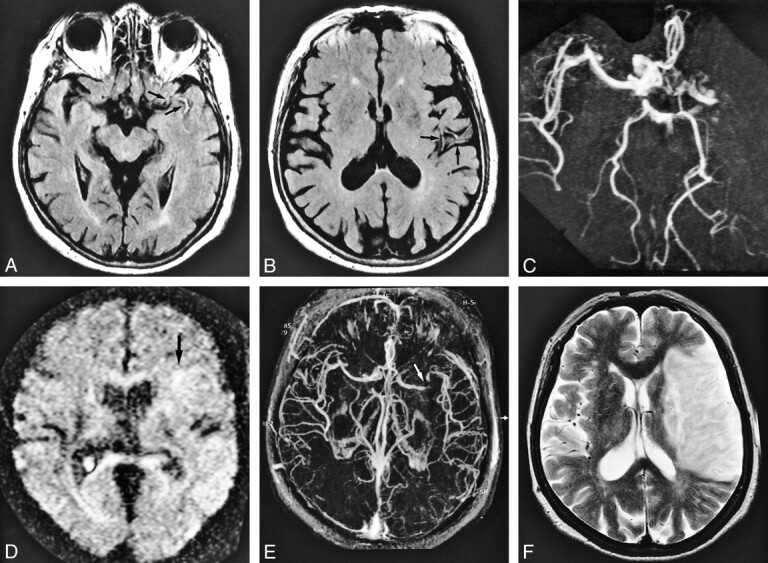
fig 3.
A 59-year-old man who presented with homonymous hemianopsia. MR imaging was performed 13 hours after stroke symptom onset.
A, MR angiogram (32/6.8, flip angle = 15 degrees) shows lack of TOF in the right posterior communicating artery.
B, On FLAIR images (8000/10/1, TI= 2000), intraarterial signal is visible in the right posterior cerebral artery (arrow); however, signal is discontinuous and isointense to cerebral parenchyma. Final infarction was confirmed in the posterior cerebral territory on T2-weighted image (not shown) obtained 14 days after stroke symptom onset. Note that FLAIR is inferior to MR angiography in detecting occluded artery.
fig 4.
An 83-year-old woman who underwent imaging 23.5 hours after onset of loss of consciousness.
A, MR angiogram (32/6.8, flip angle = 15 degree) shows occlusion in distal portion of M1 segment of the right middle cerebral artery.
B, FLAIR image (8000/10/1, TI = 2000) already shows hyperintense lesion and cortical swelling in the right middle cerebral artery territory. Intraarterial signal (arrows) is shown in the right middle cerebral artery corresponding to lack of TOF; however, signal is hampered by narrowed sulci owing to vasogenic edema.
In 41 (74.5%) of 55 patients who underwent fast T2-weighted imaging, intraarterial signals were better visualized than lack of flow void on T2-weighted images (Fig 5). Intraarterial signal coincided with lack of flow void in 12 patients (21.8%). Neither FLAIR nor T2-weighted imaging showed occluded arteries in two.
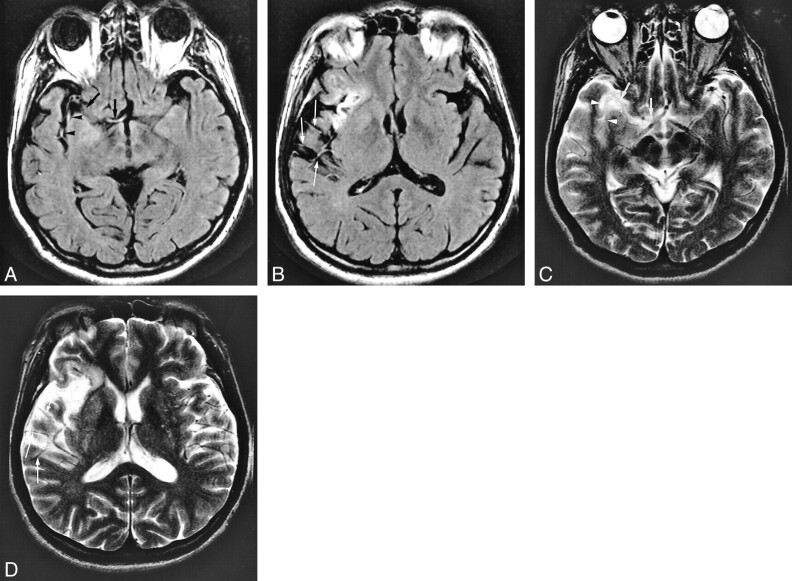
fig 5.
A 62-year-old man who underwent imaging 2.5 hours after onset of loss of consciousness and left hemiparesis.
A and B, FLAIR images (8000/10/1, TI = 2000) show intraarterial signal in the M1 (arrows), M2 (arrowheads), and M3 (thin long arrows) segments of the right middle cerebral artery. Old infarction proceeds in the right insular cortex.
C and D (same level as in A and B), T2-weighted images (4500/96/1) show lack of flow void in M1 (arrows) and M2 (arrowheads) segments; however, evidence of flow void is visible in the M3 segment (thin long arrows).
MR angiogram (not shown) shows lack of TOF in the right middle cerebral artery. CT confirmed final infarction in both perforator and hemispheric-branch territories of the right middle cerebral artery.
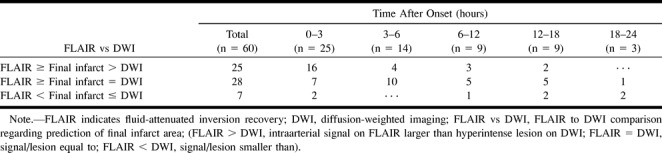
Table 2 compares intraarterial signal distribution on FLAIR images, lesions on diffusion-weighted images, and final infarction. In 25 (41.7%) of 60 patients, intraarterial signal distribution was larger than lesion measurements on diffusion-weighted images (Fig 1). The area of final infarction had a size between that of intraarterial signal distribution and lesion measurement on diffusion-weighted images. In 28 (46.7%), lesion measurement on diffusion-weighted images coincided with final infarction (Fig 2D and F), and intraarterial signal distribution area was larger than or equal to final infarction area (Fig 2A). In the remaining seven (11.7%), intraarterial signal distribution area was smaller than lesion measurement on diffusion-weighted images and final infarct. These seven patients comprised three with vasogenic edema, two with localized cortical infarction, one with posterior cerebral artery occlusion, and one with recanalization.
TABLE 2:
FLAIR to DWI comparison in predicting the area of final infarction
Forty patients underwent dynamic contrast-enhanced perfusion study. The area of intraarterial signal distribution was equal to that of the perfusion abnormality in 35 patients (87.5%), particularly, in 28 (96.6%) of 29 patients within 6 hours after stroke symptom onset. In six patients, the area of intraarterial signal distribution was smaller than that of the perfusion abnormality. In one patient who had no final infarction, FLAIR showed hyperintense intraarterial signal corresponding to a lack of TOF and perfusion abnormality.
Postcontrast MR angiography followed dynamic contrast-enhanced perfusion study in 11 patients. In 10 patients, intraluminal enhancement effect was visible in the distal portion of occluded arteries (Figs 1E and 2E). Of these 10, the area of final infarction was equal to that of intraarterial signal distribution in four (Fig 1F) and was smaller in six (Fig 2F).
CSF pulsation artifacts were occasionally seen in the inferiorly located sections and in the ventricles. CSF pulsation artifact was not falsely diagnosed as intraarterial signal in our series. In one patient with basilar artery occlusion, CSF pulsation artifact in the prepontine cistern partially hampered the detection of intraarterial signal.
Discussion
During the first 24 hours of symptom onset, perfusion abnormality tends toward diffusion abnormality and the final infarct. Enlargement of infarction gradually occurs if the area of perfusion abnormality is larger than that of diffusion abnormality (4). Diffusion-weighted imaging and perfusion study enables delineation of ischemic penumbra and a therapeutic window (8). Some previous reports emphasize the utility of FLAIR in diagnosing occluded arteries in hyperacute ischemic stroke (5–7). Cosnard et al (7) reported FLAIR's overall accuracy was 70%.
Our results also demonstrated that within 24 hours of onset, FLAIR was able to show occluded arteries as intraarterial signal that was hyperintense or isointense to brain parenchyma, and detectability was 100% within the first 6 hours. We identified intraarterial signal 35 minutes after onset. Although it was not ascertained how early FLAIR can detect arterial occlusion, intraarterial signal on FLAIR images is considered to be visible immediately after occlusion.
Intraarterial signal on FLAIR images was more conspicuous than lack of flow void on T2-weighted images, and was easy to identify. T2-weighted imaging shows normal intracranial major arteries (anterior, middle, posterior, vertebral, and basilar arteries) as flow voids within bright CSF. These are, however, invisible on FLAIR images because intraluminal flow void is indiscernible within dark CSF. Occlusion of intracranial major arteries is visible as a lack of flow void (ie, high signal intensity) within bright CSF on T2-weighted images, whereas occlusion is seen as intraarterial signal within a dark CSF background on the FLAIR image. In the clinical setting, we consider intraarterial signal on FLAIR images to be a more distinct marker than lack of flow void on T2-weighted imaging in diagnosing occluded arteries.
In our study, intraarterial signal on FLAIR images nearly corresponded to lack of TOF effect on MR angiograms, and its area of distribution tended to be larger than that of lesions on diffusion-weighted images and final infarct. The area of intraarterial signal was almost equal to that of perfusion abnormality, particularly in patients studied within 6 hours after onset. Intraarterial signal distribution predicts the area of perfusion abnormality well because of major artery occlusion. Ischemic penumbra may be present in the mismatched region between intraarterial signal distribution and the lesion measured on the diffusion-weighted image.
Previous reports have discussed causes of intraarterial signal of occluded arteries (5–7). Noguchi et al (5) explained that arterial high signal coincided with very slow retrograde collateral flow via leptomeningeal anastomoses shown on conventional angiograms. Cosnard et al (7) speculated that high signal from the vessels was due to slowly moving or stationary blood.
In our series, postcontrast MR angiography following perfusion study showed intraluminal enhancement in the distal portion of intraarterial signal in 10 of 11 patients. Intraarterial signal without intraluminal enhancement indicates complete obstruction due to embolus or thrombus, whereas intraarterial signal with enhancement suggests intraluminal patency and retrograde collateral flow via leptomeningeal anastomoses. Intraluminal enhancement does not always mean retrograde collateral circulation. Very slow and stationary flow that is not sufficient to maintain cerebral metabolism may also be enhanced on postcontrast MR angiograms. In four of our 10 patients, the area of intraarterial signal distribution developed into final infarction in spite of intraluminal enhancement. Hemodynamic examination by conventional angiography is needed to evaluate development of effective collateral circulation.
We consider that complete occlusion due to embolus and thrombus, retrograde collateral circulation, and stationary flow may contribute to intraarterial signal on FLAIR images. FLAIR tends to overestimate the extent of arterial occlusion, as does 3D-TOF MR angiography.
Intraarterial signal on FLAIR images may relate not only to lack of flow void but also to T1-shortening of thrombus, embolus, and clotted or stationary blood. In the FLAIR sequence, long TIs (2000 to 2500 msec) after a 180-degree inversion pulse null the long-T1 signal from CSF, whereas the longitudinal magnetization of brain tissue is almost fully recovered. Then the brain signal decays with time-constant T2, but the transverse magnetization of CSF remains zero. FLAIR is sensitive not only to the change of T2 but also to T1 (9, 10). The signal nulling of long-T2 tissue, such as CSF, enhances small differences of T1 recovery after the 90-degree excitation pulse. Then T1 contrast can be intensified and lesions with mild T1-shortening, such as acute subarachnoid hemorrhages, can be seen against a dark CSF background on FLAIR images (11). It is considered that T1-shortening of thrombus and clotted or stationary blood is discernible against a dark CSF background. How T1 and T2 relaxation times contribute to intraarterial signal on FLAIR images is not yet fully understood. Further experimental and clinical studies are needed to explain the exact mechanism for the signal changes on FLAIR images.
Methemoglobin causes T1-shortening through proton-electron dipole-dipole interaction. Subacute hematoma shows high signal on T1-weighted images. Conversion to methemoglobin, however, has not been reported on in the setting of hyperacute thrombus (7). In our series, hyperintense thrombus or embolus was not observed on images acquired by 3D gradient-echo T1-weighted sequences for MR angiography. Our results indicated that methemoglobin did not contribute to intraarterial signal.
In eight patients, MR angiography showed occluded arteries better than did FLAIR. Cortical swelling caused by vasogenic edema obscured the adjacent CSF spaces and interrupted the detection of intraarterial signal. This, however, is less problematic for diagnosing patients with acute ischemia, because vasogenic edema has already been irreversible and always develops into final infarction. Arterial occlusion must be diagnosed while cerebral parenchyma is still viable.
In three patients with posterior cerebral artery occlusion, MR angiography showed occluded arteries better than did FLAIR. The origin of posterior cerebral arteries varies depending on the bifurcation, and the course is tortuous from the peduncular segment to the ambient segment and then to the quadrigeminal segment. In addition, the main-branch diameter of the posterior cerebral artery is smaller than that of the middle cerebral artery. These anatomic features explain why intraarterial signal in cases with hyperacute stroke due to posterior cerebral artery occlusion was not recognized. Posterior cerebral occlusion may be a pitfall in diagnosing hyperacute ischemic stroke by intraarterial signal.
FLAIR missed intraarterial signal in two patients, although localized cortical infarct was confirmed in both. They underwent MR examination 10 and 22.5 hours after onset. The reason why FLAIR showed negative findings may have been recanalization and distal migration of an embolus that resulted from thrombolysis.
FLAIR is widely used to evaluate intracranial diseases; however, this technique has drawbacks (12). CSF flow artifact is one of the major disadvantages of FLAIR. Intense CSF pulsation causes inflow of uninverted spins into the slice of interest during the TI and results in high-signal jet. Those flow artifacts are usually seen in Monro's foramen, third and fourth ventricles, and the prepontine cistern (l3), but are rarely identified in the supratentrial subarachnoid spaces such as the suprasellar cistern, vallecular cistern, and sylvian fissure. In our series, intraventricular flow artifacts did not interrupt the detection of arterial occlusion in any cases; however, CSF inflow artifact in the prepontine cistern at an inferiorly located section hampered the depiction of intraarterial signal of the basilar artery. CSF flow artifact may present serious problems in detecting basilar and vertebral occlusion.
Although no patient with subarachnoid hemorrhage was included in our subjects, subarachnoid hemorrhage would affect the detectability of intraarterial signal on FLAIR images, because FLAIR shows acute and subacute subarachnoid hemorrhages as high signal against a dark CSF (11). Subarachnoid hemorrhage, however, does not usually coexist with hyperacute ischemic stroke, except when intracranial arterial dissection is present.
Another disadvantage of FLAIR in diagnosing hyperacute ischemic stroke is its long acquisition time. Although the fast spin-echo technique can reduce imaging time to approximately 3 to 5 minutes, fast-FLAIR requires more time than does T2-weighted imaging because of the required TI. FLAIR can cover a 104-mm thickness along the slice axis within a scanning time of 3 minutes 36 seconds, whereas 3D-TOF MR angiography covers a 72-mm slab thickness within a scanning time of 4 minutes 8 seconds. The FLAIR sequence based on EPI enables more rapid data acquisition; however, EPI-FLAIR is too sensitive to changes in magnetic susceptibility and too low in spatial resolution to evaluate intracranial arteries.
In an emergency, we first perform diffusion-weighted imaging for hyperacute ischemic stroke. Diffusion-weighted imaging is efficient for the early detection of tissue injury with cytotoxic edema that is likely to proceed to final infarction. It takes only 5 seconds for diffusion-weighted imaging to cover the whole brain. Next, FLAIR provides high detectability of major arterial occlusion. It actually takes about 6 minutes for scout imaging (diffusion-weighted imaging and FLAIR), including preparation, acquisition, and calculation time to cover the whole brain. The role of T2-weighted imaging is to delineate irreversible ischemia with vasogenic edema and chronic infarction (examination time approximately 3 minutes). MR angiography enables evaluation of degree of arteriosclerosis and detection of a vascular anomaly (examination time approximately 5 minutes). MR angiography can be omitted from this protocol, and fast T2-weighted imaging can be replaced by a single-shot EPI (examination time approximately 1 minute) or a single-shot fast spin-echo sequence (examination time approximately 1 minute), when the patient is unstable.
Diffusion-weighted imaging combined with perfusion studies is needed to predict ischemic penumbra; however, for socioeconomic restrictions, a bolus-tracking, contrast-enhanced perfusion study cannot be done in all patients with acute stroke symptoms. FLAIR plays an important role in the MR decision tree. First, when diffusion-weighted imaging shows a hyperintense lesion as large as the area of intraarterial signal disclosed by FLAIR imaging and the patient manifests neurologic deficits, the lesion, as shown on the diffusion-weighted image, is considered to correspond to the final infarct. Therefore, contrast-enhanced perfusion study is not needed. Second, when a diffusion-weighted finding still remains negative or subtle, no intraarterial signal is depicted, and neurologic deficits are mild, small perfusion deficits in perforating arteries are highly suspected. Therefore, dynamic perfusion study is no longer necessary. Finally, when FLAIR demonstrates intraarterial signal and diffusion-weighted findings are still negative or limited compared with neurologic deficits, dynamic-enhanced perfusion study should be applied for the early detection of perfusion abnormality and the evaluation of ischemic penumbra. The actual examination time for scout imaging (diffusion-weighted imaging, FLAIR, and T2-weighted imaging [fast spin-echo, single-shot EPI, or single-shot fast spin-echo]) ranges from 7 to 9 minutes. Total examination from scout imaging to perfusion analyses is usually completed within 30 minutes.
Postcontrast MR angiography following contrast-enhanced perfusion study is potentially efficient to predict development of leptomeningeal collateral circulation.
There are four limitations in our study. First, the evaluation of our series was performed retrospectively. Further prospective analysis is necessary to confirm the clinical utility of FLAIR. Cosnard et al (7) reported in their prospective study that the sensitivity and specificity of arterial occlusion by FLAIR images were 65% and 85%, respectively. Second, an optimal TI suitable for the depiction of intraarterial signal has not been investigated. We selected 2000 ms to shorten the scanning time. The theoretical TI is approximately 2500 ms (3600 ms × ln 2 [0.693]) to null CSF signal completely at 1. 5 T. However, it was impossible to compare various TIs in the same patient on an emergent basis. Third, we did not compare FLAIR with intermediate-weighted imaging that is sensitive to flow void and vessel occlusion. We used a single-echo fast spin-echo sequence with a 266 × 512 matrix for T2-weighted imaging to evaluate the lumen of internal carotid and vertebral arteries in cases of various cerebrovascular disease. An intermediate-weighted sequence was not included in our protocol for hyperacute ischemic stroke. When double-echo T2- and intermediate-weighted sequences with a 256 matrix are possible to perform on our MR unit, additional work will be required to determine which study (FLAIR or intermediate-weighted imaging) is practically adequate for the diagnosis of hyperacute ischemia. Fourth, we have to assess the specificity of intraarterial signal in hyperacute ischemic stroke. We did not assess how long intraarterial signal lasts. This study did not evaluate the utility of FLAIR from imaging chronic occlusion. In determining indication for thrombolysis, it is important to differentiate fresh thrombus from chronic occlusion, severe arteriosclerosis, and vasculitis. Chronic thrombus may show high signal on FLAIR images.
Conclusion
We conclude that intraarterial signal on FLAIR images is an early sign of occlusion of major arteries. It is very likely to be seen immediately after occlusion. Combination of FLAIR images and diffusion-weighted images can be helpful for the detection of the area at risk of infarction (ischemic penumbra). FLAIR plays an important role for deciding whether perfusion study is necessary in the setting of hyperacute stroke. When the area of intraarterial signal distribution is larger than that of a lesion measured on diffusion-weighted images, hemodynamic evaluation by dynamic contrast-enhanced perfusion imaging should be performed to assess ischemic penumbra.
Footnotes
Presented at the 38th annual meeting of the American Society of Neuroradiology, Atlanta, GA, April 2000.
Address reprint requests to Keiko Toyoda, Department of Radiology, Jikei University School of Medicine, 3-25-8 Nishi-Shimbashi, Minato-ku, Tokyo, 105-8461 Japan.
References
- 1.Gonzalez RG, Shaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999;210:155-162 [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp Jr NJ, Barker PB, Wang PY, vanZijl PCM. Imaging of acute cerebral ischemia. Radiology 1999;212:307-324 [DOI] [PubMed] [Google Scholar]
- 3.Sorenson AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391-401 [DOI] [PubMed] [Google Scholar]
- 4.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;41:581-589 [DOI] [PubMed] [Google Scholar]
- 5.Noguchi K, Ogawa T, Inugami A, et al. MRI of acute cerebral infarction: a comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology 1997;39:406-410 [DOI] [PubMed] [Google Scholar]
- 6.Maeda M, Abe H, Yamada H, Ishii Y. Hyperacute infarction: a comparison of CT and MRI, including diffusion-weighted imaging. Neuroradiology 1999;41:175-178 [DOI] [PubMed] [Google Scholar]
- 7.Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology 1999;41:342-346 [DOI] [PubMed] [Google Scholar]
- 8.Sunshine JL, Tarr RW, Lanzieri CF, Landis DMD, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999;212:325-332 [DOI] [PubMed] [Google Scholar]
- 9.Mathews VP, Caldemeyer KS, Lowe MJ, Greenspan SL, Weber DM, Ulmer JL. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999;21:257-263 [DOI] [PubMed] [Google Scholar]
- 10.Hendrick RE, Raff U. Image contrast and noise. In: Stark DD, Bradley WG, eds. Magnetic Resonance Imaging. 2nd ed. St Louis: Mosby-Yearbook; 1992:123–129
- 11.Noguchi K, Ogawa T, Inugami A, et al. Acute subarachnoid hemorrhage: MR imaging with fluid attenuated inversion recovery pulse sequences. Radiology 1995;196:773-777 [DOI] [PubMed] [Google Scholar]
- 12.Okuda T, Korogi Y, Shigematsu Y, et al. Brain lesions: when should fluid- attenuated inversion-recovery sequences be used in MR evaluation? Radiology 1999;212:793-798 [DOI] [PubMed] [Google Scholar]
- 13.Baski R, Caruthers SD, Janardhan V, Wasay M. Intraventricular CSF pulsation artifact on fast fluid-attenuated inversion-recovery MR images. AJNR Am J Neuroradiol 2000;21:503-508 [PMC free article] [PubMed] [Google Scholar]