Abstract
BACKGROUND AND PURPOSE: The human brain exhibits a complex pattern of differential aging. The purpose of this study was to examine whether age differences in the volume of cerebellar regions and the ventral pons are differential or generalized, whether the age-related shrinkage is linear or exponential, and whether there are sex differences in the size of the cerebellum and pons.
METHODS: The volumes of the cerebellar hemispheres (excluding the vermis and the peduncles), the vermis, and the ventral pons were estimated from the prospectively acquired MR scans of 190 healthy volunteers (aged 18–81 years). The relation between regional volumes, age, and sex was assessed while taking into account differences in body size (height).
RESULTS: We found a moderate age-related reduction in the volume of the cerebellar hemispheres and the cerebellar vermis. In contrast to previous findings that suggested differential vulnerability of the posterior vermis, the age-related shrinkage of the vermian lobules was uniform—about 2% per decade. In accord with all reports in the literature, the size of the ventral pons was unrelated to age. The volume of the cerebellar hemispheres, the vermis, and the ventral pons were larger in men, even after adjustment for height. The magnitude of the sex difference was the largest in the hemispheres and the anterior vermis, and the smallest in the lobules VI–VII (declive-folium-tuber).
CONCLUSION: Moderate age-related shrinkage of the cerebellum and lack of age-related differences in the ventral pons are robust phenomena. However, in all likelihood, the effects of age on the cerebellum are not differential but uniform. The cerebellum and the pons are larger in men than in women and the difference is especially pronounced in the cerebellar hemispheres and the anterior vermis.
The human brain exhibits a complex pattern of differential aging. In the cerebral cortex, the basal ganglia, and the midbrain, age-related shrinkage of some regions co-exists with relative preservation of the others (1, 2). It is unclear, however, whether differential aging is observed in the posterior fossa structures as well. Moderate shrinkage of the cerebellar hemispheres and the vermis has been noted in postmortem studies (3, 4) and in some in vivo investigations (2, 5–9). However, other volumetric studies based on MR imaging yielded no effects of age on the size of the cerebellum (10, 11) or showed nonsignificant trends (12). A quantitative review that covered the in vivo literature until the year 1999 revealed inconclusive evidence if differential aging of the cerebellum occurred in healthy adults (2). According to that review, the median strength of association between the cerebellar volume and age as measured by Pearson product moment correlation was r = −0.32. Similar estimates for the cerebellar vermis varied across its lobules, reaching r = −0.40 for the lobules VI–VII (declive-folium-tuber, DFT), r = −0.35 for the lobules VIII–X (posterior vermis), and r = −0.31 for the anterior cerebellar vermis (lobules I–V). Variability across the reviewed samples was substantial, and the ranges of the estimated magnitude of age effect were −0.28 to −0.56 for the hemispheres, −0.34 to −0.76 for the DFT, −0.07 to −0.60 for the posterior vermis, and −0.07 to −0.57 for the anterior vermis. In contrast, there was clear agreement in the literature that the pons maintains its size throughout the life span: median correlation with age r = 0.07, with the range between −0.04 and 0.15.
Three studies that appeared since the completion of the quantitative review neither clarified the issue nor altered the estimates of the magnitude of age effect. The reported correlations between cerebellar volume and age covered a wide range: r = −0.02 (12), −0.27 (10), and −0.43 (13). The correlations between age and the size of the vermis were r = −0.07 (5) and −0.41 (13). Correlations between age and the adjusted volume of subdivisions of the vermian lobules were r = −0.35, −0.37, and −0.31 for anterior, DFT, and posterior, respectively (10), and −0.44, −0.34, −0.29, and −0.09 for anterior, DFT, lobule VIII, and lobules IX–X, respectively (13). Notably, when gray and white matter compartments of the cerebellum were measured separately, the negative effects of age were observed only in the gray matter, whereas the bulk of the white matter remained invariant across the age range (13). Studies in which the preponderance of the measured volume was gray matter also tended to produce larger effects (5).
Sex differences in gross cerebellar neuroanatomy have been observed in several studies. In some samples, in comparison to women, men had larger cerebellar hemispheres (5, 7, 12), cerebellar vermis (12), anterior vermis (5), and ventral pons (8). However, the differences do not always favor men. In one sample, women evidenced greater volume of medial cerebellar hemispheres and the DFT after adjustment for the total cerebellar size (10). One report suggested that women might show steeper age-related decline in the area of the vermis than do their male counterparts (12).
In sum, even though age-related shrinkage of the cerebellum appears established, the magnitude of the loss is unclear. In addition, one cannot resolve the issue of shrinkage being regional or generalized. Regarding sex differences, not only the magnitude, but also their direction and even mere existence, are far from being the matter of consensus. The studies summarized in the meta-analysis (2) differed in sample composition (not all screened for age-related diseases), measurement methods (not all used computer-assisted volumetry), and sample size (not all had enough statistical power to discover moderate and small effects). The latter bears on clinical interpretation of the findings as well, for whereas findings from small samples can be combined to make inferences about the existence of the phenomenon in question, they are characterized by relatively large standard errors and cannot help to establish its normative limits. Thus, additional prospective studies conducted on a sample of healthy volunteers using volumetric methods are needed to help clarify these issues.
In the prospective study reported here, we examined age and sex differences in the size of the cerebellum and the ventral pons. The main questions regarding the age differences were whether, in the healthy elderly, the cerebellar hemispheres and the lobules VI and VII of the vermis show age-related shrinkage, whereas the anterior vermis and pons do not. If age-related differences in the volume of posterior fossa structures were observed, we sought to establish whether the trend was linear or exponential, as suggested in some reports (10). In addition, we examined whether, with body size controlled, there are sex differences in size of the cerebellum and pons, and whether age-related trends differed between the sexes. To improve the methodology in comparison to most previous studies, including our own, we used a more precise method of cerebellar alignment following methodology described by Luft and colleagues (12), and used volumes rather than cross-sectional areas as indices of size for the vermian structures and the pons.
Methods
Subjects
The data for this study were collected in an ongoing investigation of neuroanatomic correlates of age-related differences in cognition. Subjects were recruited by advertising in local media and on the University of Memphis campus. They signed a consent form approved by the Committee for Protection of Human Subjects in Research of the University of Memphis and by the Baptist Memorial Hospital Patients Participation Committee, and were screened using an extensive health questionnaire. Persons who reported history of cardiovascular, neurologic, and psychiatric conditions, head trauma with loss of consciousness for more than 5 minutes, thyroid problems and diabetes, treatment for drug and alcohol problems, or a habit of taking more than three alcoholic drinks per day were excluded from the study. None of the participants used anti-seizure medication, anxiolytics, or antidepressants. Subjects who suffered from claustrophobia were explicitly advised against participation in the study. Eighteen subjects (seven men and eleven women, between the ages of 39 and 74 years) whose hypertension was successfully controlled by medication were also included in the sample. None of the subjects participated in our previous studies of neuroanatomy of aging (5, 6).
All subjects were screened for dementia and depression using a modified Blessed Information-Memory-Concentration Test (14) with cut-off of 30, and a Geriatric Depression Questionnaire (15) with a cut-off of 15. An experienced neuroradiologist (JDA) examined the MR scans for signs of space-occupying lesions and cerebrovascular disease. The final sample consisted of 190 subjects: 113 women (47.09 ± 16.17 years old) and 77 men (45.74 ± 16.90 years old). The age distribution was approximately rectangular, and there were no age differences between the sexes (t < 1). Average education among the subjects was 15.8 years (almost four years of college), suggesting a highly selected group of individuals, although there was a weak negative correlation between education and age (r = −0.19, P < .05). All subjects were consistent right-handers.
MR Image Acquisition and Processing
Imaging was performed on a 1.5-T system. All measurements were conducted off-line on the images acquired in the axial plane with T1-weighted 3D spoiled gradient–recalled acquisition sequence. The acquisition parameters were 24/5/1 (TR/TE/excitation); field of view, 22 cm; matrix, 256 × 192; number of slices, 124, slice thickness, 1.3 mm; and flip angle, 30°.
Volumetric Image Analysis
After acquisition, the MR data were reformatted off-line and corrected for undesirable effects of head tilt, pitch, and rotation by using proprietary software. The MR images were re-aligned on a Power Macintosh 8100 using BrainImage 2.3.3 public domain software (obtained from the World Wide Web URL http://sol.med.jhu.edu/Brainimage.html). The operator used standard neuroanatomic landmarks to bring each brain into a unified system of coordinates in which the sagittal plane cut through the middle of the vermis. In this situation, the cerebellum frequently appears misaligned with respect to the cerebrum (12). Therefore, we aligned the cerebellar hemispheres in the axial plane along the line drawn perpendicular to the vertical that ran tangential to the dorsal brainstem on the midsagittal slice (Fig 1). In addition, we aligned the brain along the middle of the vermis; in the coronal plane, the brains were aligned along the orbits. Reformatted images were cut into 0.86-mm coronal and sagittal sections. Reformatted coronal sections were sampled 1.5 mm apart, whereas the sagittal sections were sampled continuously. The midsagittal slice was selected as the slice on which the cerebral aqueduct became continuous with the fourth ventricle.
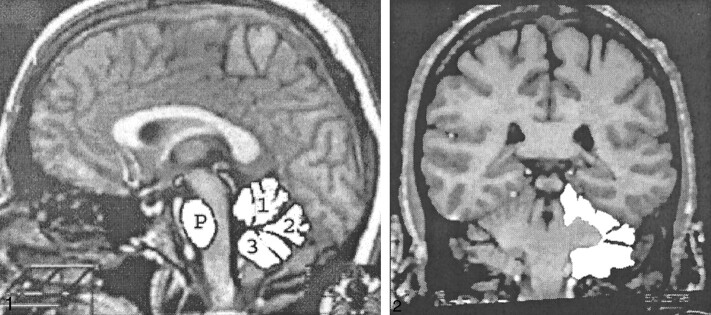
fig 1.
Demarcation of the ROIs on the midsagittal slice showing the vermis and the ventral pons: 1, anterior vermis; 2, declive, folium, and tuber; 3, posterior vermis; P, ventral pons. The white lines indicate the direction of alignment.fig 2. Demarcation of the ROIs on a coronal MR image showing cerebellar hemispheres. All regions were traced manually, and the filled-in regions are presented here for illustration only
The region of interest (ROI) areas were measured with NIH Image public domain software (Version 1.60, available at the World Wide Web URL http://rsb.info.nih.gov/nih-image). Images were displayed on a 21-inch monitor, and each ROI was traced manually using a digitizing tablet. Volumes were computed using the Cavalieri estimate (16). Reliability of measures was assessed by intraclass correlations presuming random selection of raters (ICC[2], ref. 17); all estimates of reliability exceeded 0.90. Trained operators, who were blind to the subjects' age and sex, manually traced all ROIs as outlined on the MR images in Figs 1 and 2. We resolved all questionable cases by consulting the correlative and general brain atlases (18, 19).
For the cerebellar hemispheres, the coronal slices were divided into two equal groups at random, and each half-sample was traced by a different operator. The areas of the hemispheres were measured on 32–40 coronal slices. The vermis, the cerebellar peduncles, and the fourth ventricle were excluded, whereas the hemispheric gray matter, the cerebellar tonsils, the vellum, and the corpus medullare were included in the tracing of each cerebellar hemisphere. The rostral border was defined as the first slice on which cerebellar gray matter became visible and distinguishable from the cerebellar peduncles. Distinguishing the point at which the cerebellar peduncles ended and the corpus medullare began was difficult in many cases. Because on the most anterior slices the bulk of the white matter belongs to the peduncles, we started inclusion of the cerebellar white matter at the most rostral slice that cut through the anterior vermis. The measurement proceeded until the cerebellar hemispheres were no longer observed or until they became indistinguishable from the occipital lobe of the cerebrum. Thus, the bulk of the measured volume consisted of the cerebellar gray matter from the lateral surfaces of the cerebellar hemispheres and the tonsils.
The cerebellar vermis was traced on three slices: the midsagittal slice and two parasagittal slices, one on each side of the midsagittal. The volume of the cerebellar vermis was estimated from three slices. Three ROIs were traced by following the vermian outline as faithfully as possible, entering all “coves” created by fissures and sulci. The vermian ROI1 (the anterior vermis) consisted of the lobules I–V: lingual, centralis, and culmen ROI 1 (anterior vermis). The anterior border of the ROI1 was the superior medullary velum, and the primary fissure served as its posterior border. The ROI2 consisted of the lobules VI and VII (the declive, the folium, and the tuber). Its anterior border was the primary fissure, and the prepyramidal fissure demarcated the posterior border. Finally, ROI3 (the posterior vermis) included the pyramis, the uvula, and the nodulus. The anterior border was the prepyramidal fissure and the posterior border was the velum. The tonsils were excluded from the vermian ROIs.
The ventral pons was traced on the same three slices (the midsagittal and two parasagittal on each side), as was the vermis. On those slices, the pons was traced, as it appeared—a smooth, ovoid contour without breaks. The dorsal border was the medial lemniscus—a strip of reduced signal intensity on a T1-weighted MR image. The volume of the pons was estimated from the three slices using Cavalieri estimate.
Results
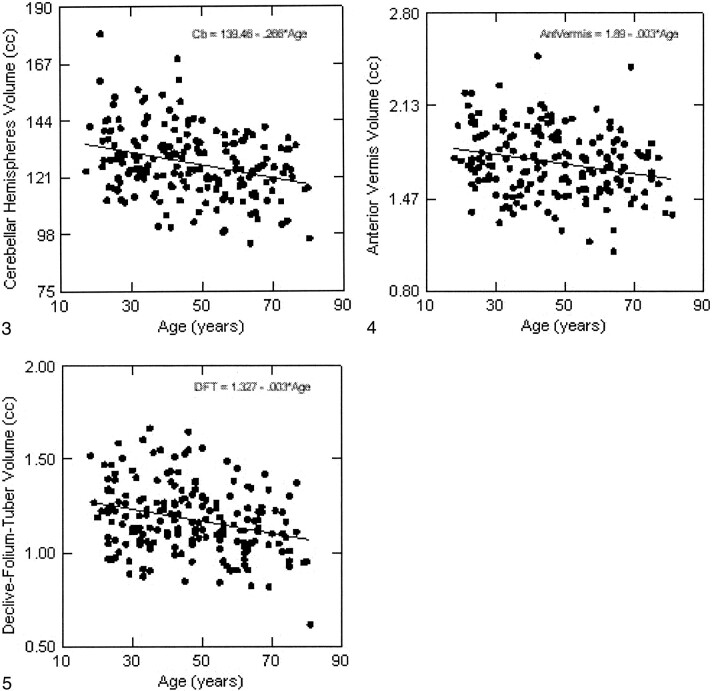
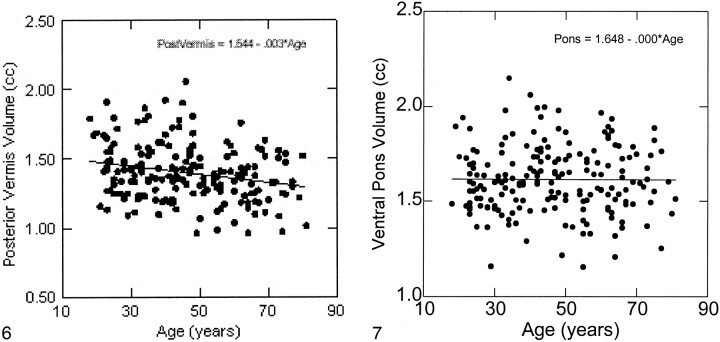
The volumes of the hemispheric gray matter and of all vermian lobules (but not of the ventral pons) correlated negatively with age (Table). The magnitude of negative correlations between the volumes and age was compared using Steiger's Z* test (20), and no significant differences were found (P > .25). The scatter plots and the age trends for all measures are depicted in Figures 3–7. Using the equation of linear regression of the regional size on age, we estimated that within the age span between 20 and 80 years, shrinkage of the vermian lobules and the cerebellar hemispheres was about 2% per decade and ranged between 1.9% to 2.2% per decade.
fig 3.
Regression of the cerebellar hemispheres volume on age. The volume is not adjusted for height.fig 4. Regression of the anterior cerebellar vermis area (lingula, centralis, and culmen) on age. The volume is not adjusted for height.fig 5. Regression of the declive-folium-tuber volume on age. The volume is not adjusted for height
To examine sex differences in the aging of posterior fossa structures, we analyzed the volumes of the vermian lobules, the pons, and the cerebellar hemispheres in three general linear models. In each model, the volume of the ROI served as the dependent variable, sex was a grouping factor, age (re-centered at the sample mean) was a continuous independent variable, and height (also re-centered at the sample mean) served as a covariate. Because men were significantly taller than women (176.81 ± 7.66 cm versus 164.45 ± 7.39 cm, t[188]) = 11.18, P < .001), we introduced height into all models as a covariate to control for variations that could have been related to the differences in body size. Cerebellar hemispheres (right versus left) and three vermian ROIs were repeated measures in their respective models. The assumption of the homogeneity of regression slopes was met for all models (height × sex interaction Fs < 1).
The results of these analyses revealed significant main effects of age on the volume of both cerebellar hemispheres: F(1,186) = 24.12, P < .001. The age-related shrinkage of the vermis was also significant: F(1,186) = 22.67, P < .001. No significant age × lobule and age × hemisphere interactions were observed (both F < 1.15, not significant). The left cerebellar hemisphere was significantly larger than the right: F(1,186) = 6.79, P < .01, although the magnitude of the difference was only 0.45%. To examine for possible nonlinear effects, we added an age term to the models. None of the quadratic components reached statistical significance (all F < 1).
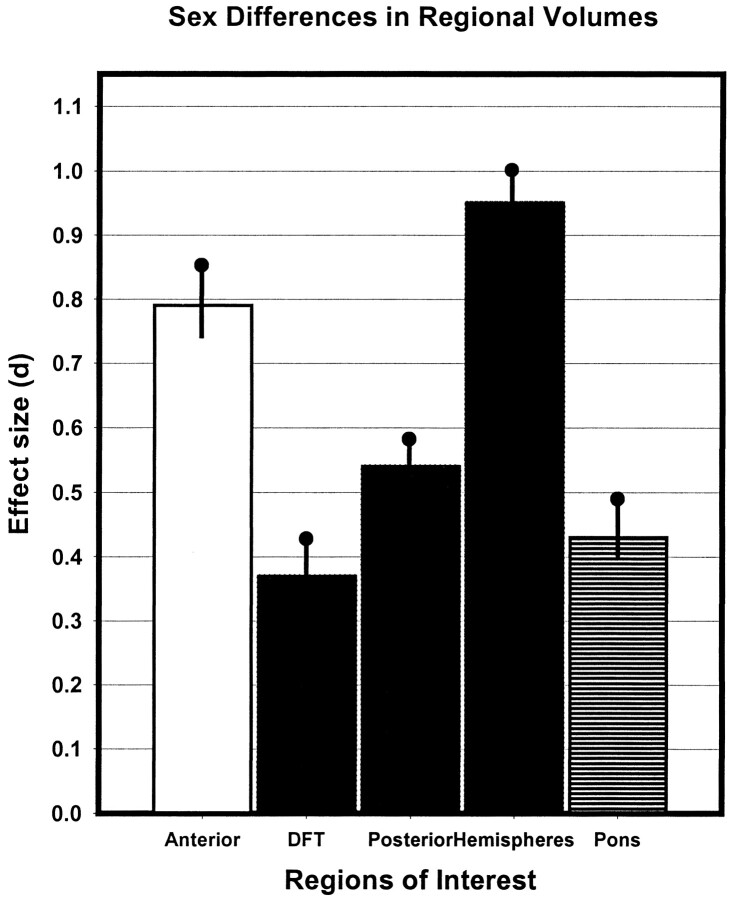
A significant effect of sex on the volume of cerebellar hemispheres and the vermis was observed (F[1,186] = 32.54, and 20.86, respectively, both P < .001). The vermis and the cerebellar hemispheres were larger in men in spite of adjustment for height. However, there was a significant lobule × sex interaction (F[2,372] = 4.49, P < 0.05 with Greenhouse-Geisser correction). The post-hoc comparisons among the ROIs were conducted using effect size (corrected Cohen's d) (21) computed from the means and standard deviations of the volume and area measures adjusted for height. Cohen's d estimates the magnitude of effect in standard deviation units, and as illustrated in Figure 8, the magnitude of sex differences in regional size was the greatest in the cerebellar hemispheres (d = 0.95, 95% confidence interval [CI95%] = 0.90 to 1.00 SD) and the anterior vermis (d = 0.79, CI95% = 0.74 to 0.83 SD), and the smallest in the declive-folium-tuber (lobules VI–VII) of the vermis (d = 0.37, CI95% = 0.32 to 0.41 SD). The differences in the posterior vermis and the ventral pons were moderate: d = 0.54 (CI95% = 0.49 to 0.58 SD) and d = 0.43 (CI95% = 0.39 to 0.48 SD), respectively. Lack of overlap between 95% confidence limits indicates statistical significance of the differences between the pairs of comparisons.
fig 8.
A bar diagram for comparison of the magnitude of sex differences in regional volumes of the cerebellum and pons. The vertical lines indicate half of the 95% confidence range of the effect size. The effects were estimated from means and standard deviations adjusted for height
To ensure that the differences among the regions were not an artifact of differential variability of the measures, we compared their coefficients of variation (CV, a ratio of standard deviation to the mean). The comparison yielded very similar values of CV ranging from 0.11 for the cerebellar hemispheres and the pons to 0.16 for lobules VI–VII.
Some reports in the literature indicated that history of hypertension can affect the appearance of the brain (22) and enhance age-related changes (23). Therefore, we repeated all the analyses after removing from the sample 18 participants (eight men and 10 women) who reported a history of medically controlled hypertension. As the results remained virtually unchanged, we decided to retain the participants with hypertension in the current sample.
Discussion
The results of this study confirm that aging affects the cerebellum and the ventral pons differentially. The cross-sectional age-related differences observed in this sample indicated that within the limits of statistical power provided by a sample of 190 participants with age range capped at 81 years, linear, not exponential time course, more parsimoniously describes the age-related shrinkage. However, we did not replicate differential aging within the vermis, as we previously reported. All vermian lobules appeared to suffer similar moderated effects of aging. The results showed that even successful (24) aging is accompanied by shrinkage of the cerebellum at an estimated rate of about 2% per decade. This rate is somewhat slower than estimated in our previous studies, at least with regard to the posterior vermis. As indicated in the introduction, there are several methodological improvements introduced in this study in comparison to our previous studies and most of the earlier studies in the literature. Thus, we believe that the obtained results may represent a more faithful reflection of the age and sex differences in the cerebellum and the pons than found in the previous studies (2). A longitudinal investigation will help to clarify the rate of age-related changes in the posterior fossa structures. Such a study is currently underway in our laboratory.
A descriptive study, such as the one reported here, cannot elucidate the mechanisms of differential aging. Several authors have speculated about the possible reasons for age-related shrinkage of the cerebellum, including differential strength of cerebrospinal fluid flow, watershed zone ischemia, genetic predisposition, and functional failure (5, 10, 12). Some researchers speculated that sexual dimorphism in cerebellar size can be attributed to the effects of sex hormones (10, 12), although empirical support for that supposition is still lacking. We have no data to strengthen or negate these speculations. It is possible, however, that the difference in age effects on the cerebellum and the pons is not a reflection of the differential neuronal shrinkage or attrition, but rather an artifact of differences in composition of the regions of interest: almost entirely gray matter in the cerebellum, and predominantly white matter in the pons. The majority of volumetric studies of the cerebrum in vivo reveal very little age-related shrinkage of the white matter, in contrast to significant declines in the gray matter volume (2). Direct comparisons of the age effects on the two compartments of the cerebellar tissue—the white and the gray matter—confirm that trend (13).
Our finding of larger cerebellar hemispheres in men mirrors analogous observations in the cerebral cortex (2). In addition, similar trends in the anterior vermis and the pons replicate our own findings in other samples (5, 6). In this sample, we also found similar sex differences in other cerebellar lobules. Notably, comparison of the magnitude of these differences across the regions reveals that the more frequently they are observed across samples, the stronger they are within the sample. Thus, on that basis, sexual dimorphism in the size of cerebellar hemispheres and the anterior vermis appears robust, whereas the differences in the posterior vermis are weaker and need to be replicated.
What are the clinical and functional implications of the findings reported here? The cerebellar shrinkage is rather mild and is unlikely to be noticed upon visual examination. Thus, to a clinician, volumetric examination of the brain in general, and of the posterior fossa in particular, brings no tangible benefits beyond heightening the awareness of individual differences associated with age and sex of the patient. Yet, in experimental studies of cognitive aging, cerebellar volume reduction has been shown to predict age-related declines in perceptual-motor skill acquisition (25) and classical conditioning (26) in healthy volunteers. For example, in one of these studies, age-related shrinkage of the cerebellar hemispheres predicted deficits in acquisition of a pursuit-rotor skill that would be otherwise attributed to age alone (25). Taking into account age-related differences in regional cerebellar volume allows for another step in elucidating the variance in cognitive performance that otherwise would be submerged under a vague rubric of calendar age. Finally, there is a practical implication of the reported findings. In functional neuroimaging studies, age-related shrinkage of the cerebellum must be taken into account in functional imaging studies of aging, when the cerebellum is considered as a suitable structure for reference and normalization (27, 28).
Descriptive statistics and correlations for the age, height, and regional volumes of the cerebellum and pons
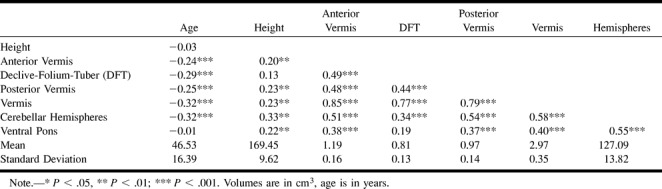
fig 6.
Regression of the posterior cerebellar vermis volume (pyramis, uvula, and nodulus) on age. The volume is no adjusted for height.fig 7. Regression of the ventral pons volume on age. The volume is not adjusted for height
Acknowledgments
Cooperation of the medical and technical staff of the Baptist MR Diagnostic Imaging Center, Memphis, TN is gratefully acknowledged.
Footnotes
This study was supported in part by a National Institute of Health grant AG-11230 to N.R. and by the Center of Excellence Grant from the State of Tennessee to the Department of Psychology of the University of Memphis.
Some of the findings reported herein were presented at the Annual Meeting of the Society for Neuroscience at New Orleans, November 2000.
Address reprint requests to Naftali Raz, Department of Psychology, 202 Psychology Building, The University of Memphis, Memphis, TN 38152-3230.
References
- 1.Kemper TL. Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE, eds. Clinical Neurology of Aging. 2nd ed. New York: Oxford University Press;1994:3–67
- 2.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, eds. Handbook of Aging and Cognition II. Mahwah, NJ: Erlbaum;2000:1–90
- 3.Ellis RS. Norms for some structural changes in human cerebellum from birth to old age. J Comp Neurol 1920;32:1-33 [Google Scholar]
- 4.Torvik A, Torp S, Lindboe CF. Atrophy of the cerebellar vermis in ageing: a morphometric and histological study. J Neurol Sci 1986;76:283-294 [DOI] [PubMed] [Google Scholar]
- 5.Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol 1998;19:65-71 [PMC free article] [PubMed] [Google Scholar]
- 6.Raz N, Torres IJ, Spencer WD, White K, Acker JD. Age-related regional differences in cerebellar vermis observed in vivo. Arch Neurol 1992;49:412-416 [DOI] [PubMed] [Google Scholar]
- 7.Escalona PR, McDonald WM, Doraiswamy PM, et al. In vivo stereological assessment of human cerebellar volume: effects of gender and age. AJNR Am J Neuroradiol 1991;12:927-929 [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SA, Doraiswamy PM, Husain MM, et al. Assessment of posterior fossa structures with midsagittal MRI: the effects of age. Neurobiol Aging 1991;12:371-374 [DOI] [PubMed] [Google Scholar]
- 9.Luft AR, Skalej M, Wette D, Voigt K, Klockgether T. Age and sex do not affect cerebellar volume in humans. AJNR Am J Neuroradiol 1997;18:593-596 [PMC free article] [PubMed] [Google Scholar]
- 10.Rhyu IJ, Cho TH, Lee NJ, Uhm C-S, Kim H, Suh Y-S. Magnetic reasonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett 1999;270:149-152 [DOI] [PubMed] [Google Scholar]
- 11.Salat D, Ward A, Kaye JA, Janowsky JS. Sex differences in the corpus callosum with aging. Neurobiol Aging 1997;18:191-197 [DOI] [PubMed] [Google Scholar]
- 12.Luft AR, Skalej M, Schultz JB, Welte D, Kolb R, Bürk K, Klockgether T, Voigt K. Patterns of age-related shrinkage in the cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex 1999;9:712-721 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology 2000;14:in press [DOI] [PubMed]
- 14.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797-811 [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. App Psychol Measur 1977;1:385-401 [Google Scholar]
- 16.Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods 1990;35:115-124 [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing raters reliability. Psychol Bull 1979;86:420-428 [DOI] [PubMed] [Google Scholar]
- 18.Courchesne E, Press GA, Murakami J, et al. The cerebellum in sagittal plane—anatomic-MR correlation: 1. The vermis. AJR Am J Roentgenol 1989;153:829-835 [DOI] [PubMed] [Google Scholar]
- 19.Nieuwenhuys R, Voogd J, Huijzen C. The Human Central Nervous System: A Synopsis and Atlas.. 3rd ed. Berlin: Springer;1988
- 20.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull 1980;87:245-251 [Google Scholar]
- 21.Hedges L, Olkin I. Statistical Methods for Meta-Analysis.. Orlando: Academic Press;1985
- 22.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57-62 [DOI] [PubMed] [Google Scholar]
- 23.Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke 1997;28:1410-1417 [DOI] [PubMed] [Google Scholar]
- 24.Rowe JW, Kahn RL. Human aging: usual and successful. Science 1987;237:143-149 [DOI] [PubMed] [Google Scholar]
- 25.Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech 2000;51:85-93 [DOI] [PubMed] [Google Scholar]
- 26.Kushner M, Tobin M, Alavi A, et al. Cerebellar glucose consumption in normal and pathologic states using fluorine-FDG and PET. J Nucl Med 1987;28:1667-1670 [PubMed] [Google Scholar]
- 27.Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE. Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with age. J Nucl Med 1995;36:1141-1149 [PubMed] [Google Scholar]
- 28.Moeller JR, Ishikawa T, Dhawan V, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab 1996;16:385-398 [DOI] [PubMed] [Google Scholar]