Abstract
BACKGROUND AND PURPOSE: Functional MR (fMR) imaging is based on changes in regional blood flow. The purpose of this study was to evaluate the role of fMR imaging for detection of a vascular compromised status in the occipital lobe in patients with ischemia in the visual cortex.
METHODS: We performed fMR imaging in seven control subjects and seven patients with symptoms and signs of visual cortical transient ischemia and/or infarct. fMR imaging was performed using a gradient-echo sequence with the 2D fast low-angle shot technique. An axial slice including both visual cortices was selected, and stimulation of the visual cortex was performed using a red photostimulator. The number of activated pixels in each primary visual cortex area were counted and an asymmetry ratio [AR (%) = 100 × (R−L)/(R+L)/2] was calculated. Patients and control subjects underwent visual field examination, conventional MR imaging, and vascular imaging (MR angiography in all patients and control subjects, conventional catheter angiography in two patients). fMR imaging results were compared with the results of a visual field examination, conventional MR imaging, and vascular imaging.
RESULTS: fMR imaging of the patients showed significant activation asymmetry (P < .05) compared with that of control subjects. Vascular abnormalities in the posterior circulation were found in all seven patients. By conventional MR imaging, five patients were found to have infarction in the occipital lobe and the remaining two patients showed no abnormality. In visual field examination, six of the seven patients showed homonymous hemi- or quadrantanopsia suggesting postchiasmic abnormalities, and the remaining patient had normal findings. fMR imaging showed decreased activity in the visual cortices corresponding to vascular abnormalities (seven of seven patients), permanent infarction (five of seven patients), or visual field defect (six of seven patients). Two patients with normal conventional MR imaging had vascular lesions in the posterior circulation, and fMR imaging showed decreased activity in the corresponding visual cortices. One patient with normal visual field examination had multifocal stenosis in the posterior cerebral artery without infarction, and fMR imaging showed decreased activity in the corresponding visual cortex.
CONCLUSION: fMR imaging of the visual cortex may be a sensitive method for the detection of vascular-compromised status in the occipital lobe.
Functional MR (fMR) imaging is based on changes in regional blood flow in the cerebral cortex performing motor, sensory, or perception function. Previous studies using positron emission tomography (PET) revealed that the concentration of oxyhemoglobin was relatively high in venous blood of the activated cortex, because oxygen consumption was less than the increase in blood flow (1, 2). The blood oxygen level blood oxygenation level–dependent (BOLD) contrast technique, which is widely applied in fMR imaging, uses this property to differentiate activated from non-activated cortices (3). The BOLD effect is accompanied by a change in cerebral blood flow, cerebral blood volume, and the metabolic rate of cerebral oxygen consumption, and is highly sensitive to these changes (4–6). BOLD-based fMR imaging has been applied to healthy subjects to assess the cerebral blood flow reserve (7). If decreased perfusion exists in a certain cortical area, it may be depicted by fMR imaging using the BOLD effect as an area of decreased activation, even if the patient shows no symptomatic functional deficit. Therefore, fMR imaging with a specific task or stimulation can also provide information about regional perfusion or the vascular reserve of patients with suspected cerebral ischemia.
When a patient has a problem with vision, especially of the visual field, careful evaluation of the visual field defect pattern can localize the lesion site approximately along the optic pathway. For precise localization and characterization of the lesion, MR imaging is usually performed, and apparent lesions such as infarction, hemorrhage, or tumor can then be detected. However, in some cases, MR imaging may show no abnormal finding. In such cases, a vascular-compromised state, such as a transient ischemic attack, should be considered. Additional studies are also required for early therapeutic investigation, because stroke tends to occur in the same vascular territory as previous transient ischemic attack (8). Cerebral angiography and perfusion studies can provide important information about this possibility. However, we believe that fMR imaging might be effective in defining the area at risk of infarct because of its ability to localize regional perfusion differences. A small number of applications of functional activation in the visual cortex or pathway of stroke patients have been made using PET or fMR imaging (9, 10), and many studies concerning functional reorganization after stroke in other motor or sensory areas have been published (11–13). Although those studies centered on functional inactivation caused by perfusion deficit in the infarction area, there have been few reports about functional activation for detecting decreased vascular reserve.
Therefore, we performed fMR imaging in patients with visual disturbances due to postchiasmic problems. The purpose of this study was to determine the role of fMR imaging as a sensitive method for the evaluation of the vascular-compromised status of the visual cortex.
Methods
We studied seven patients with symptoms and signs of visual cortical ischemia and/or infarct, and seven healthy control subjects with no previous history of visual disturbances. The patient group consisted of five men and two women, ranging in age from 29 to 74 years. The duration of symptoms for each patient is listed in Table 1. Normal control subjects consisted of three men and four women, ranging in age from 25 to 36 years. Complete Goldman perimeter mapping or other standard visual field examination was available in all patients and control subjects. Examinations were performed after informed consent had been obtained, as required by our institutional review board.
TABLE 1:
Summary of the clinical findings and results of fMRI, visual field examination, conventional MR imaging, and vascular imaging
Data Acquisition
MR examinations were performed using a 1.5-T system (Magnetom Vision; Siemens, Erlangen, Germany). Conventional MR images, including T1- and T2-weighted images, were acquired in all subjects. Diffusion-weighted images were available in three patients.
fMR imaging was performed on the same day as conventional MR imaging. A gradient-echo technique, 2D fast low-angle shot, was used. The parameters were 90/56 (TR/TE), 40° flip angle, 240 mm × 240-mm field of view (FOV), 64 × 128 matrix, 8.32-s acquisition time, and 8-mm slice thickness. Each subject was carefully positioned in the coil, and gross movement of the head was prevented by goggles that fit tightly between the coil and face. No further motion correction was performed, because the goggles and head coil fixed subjects' heads, and the patients were alert and cooperative. Cortical activation was achieved by subjects simply looking at light and remaining still, and image blurring was not an issue because of a relatively large pixel size. An axial slice parallel to the calcarine fissure was obtained by adjusting the localizer in the mid- and parasagittal scout image. To avoid asymmetrical positioning or inadequate sampling of the region of interest, we obtained a T1-weighted axial image under the same conditions prior to the functional imaging. Photic stimulation was performed using light-proof full-field photic stimulation goggles, designed by the authors, operating at 8 Hz. Diodes emitting red light were used as a light source. Images were acquired five times with photic stimulation and five times in darkness, and repeated until a total of 40 images were acquired. Imaging time was 5 minutes 32.8 seconds.
MR angiography was performed in all patients and control subjects by using the 3D time-of-flight with section interpolation technique by zero-filling the k space. The parameters were 30/6.4 (TR/TE), 25° flip angle, 150 mm × 200 mm FOV, 160 × 512 matrix, 7 minute 42 second scan time, 0.7-mm effective thickness, and 102.2-mm entire thickness. Maximum intensity projection was used for image analysis. Conventional catheter angiography of internal carotid and vertebral arteries was performed in two patients to investigate major vascular problems. fMR results were compared with the results of a visual field examination, conventional MR imaging, and vascular imaging.
Data Analysis
The fMR data were transferred to a workstation and processed by an interactive data language program (IDL; Research Systems, Boulder, CO) developed in-house to map the activated area by color. Statistical analysis was based on cross-correlation and 0.6 was chosen as the threshold value. The resultant image with activated signal was added to the anatomic T1-weighted image of the same area. Z score mapping was also performed to calculate the asymmetry ratio by counting the number of activated pixels, with a threshold of 1.0.
To examine the degree of asymmetry in the number of activated pixels in the primary visual cortex area, the asymmetry ratio was calculated as:
 |
where R and L refer to the number of activated pixels in the right and left visual cortex areas, respectively. The primary visual cortex was identified as the medial surface of the occipital lobe along the calcarine fissure (9, 14, 15). Regions of interest were chosen in the gray matter along the calcarine fissure and included the primary visual cortex, but excluded the sagittal sinus. The asymmetry ratio of the patient group was compared with that of the control subjects. Statistical analysis was based on the Mann-Whitney U test, and a P value of less than .05 was considered statistically significant.
Results
In control subjects, there were no abnormal findings on visual field examination, conventional MR imaging, or vascular imaging. Gross distributions of the activated pixels on fMR images were symmetrical.
Numbers of activated pixels and asymmetry ratios of control subjects and patients are listed in Tables 2 and 3. Asymmetry ratios of control subjects ranged from 2.6% to 17.1% (mean ± SD, 10.0 ± 4.3), whereas the asymmetry ratios of patients ranged from 0% to 200% (mean ± SD, 137.3 ± 66.8). Significant asymmetry of activation was found in the patient group when compared with that of control subjects (P < .05). The relatively wide range of asymmetry ratios of patients was attributable to a patient (patient 7) who showed bilaterally decreased activity without apparent asymmetry of signals. MR angiography of this patient showed segmental stenosis of the basilar artery without sufficient collateral flow. For this reason, patient 7 was excluded from the comparative statistical analysis. The decision was made by consensus of two observers (Y-J.L. and T-S.C.).
TABLE 2:
Number of activated pixels and asymmetry ratios in the normal control group

fMR, visual field examination, conventional MR imaging, and vascular imaging results of the patients group are listed in Table 1. On conventional MR images, including diffusion-weighted images, five patients were found to have infarction in the occipital lobe, and the remaining two patients were normal. In these two patients, the symptom duration was long enough for signal change on T2-weighted images. In visual field examinations, six of the seven patients showed homonymous hemi- or quadrantanopsia, suggesting postchiasmic abnormalities. In all seven patients, vascular abnormalities in the posterior circulation were found on vascular images, which correlated well with the fMR findings. Although permanent infarction or visual field defect was not detected by conventional MR imaging (patients 2 and 3) or visual field examination (patient 2), fMR showed decreased cortical activity at the vascular lesion site.
One (patient 1) of the five patients with infarction had right homonymous quadrantanopsia (Fig 1A). The conventional T2-weighted image (Fig 1B) and the diffusion-weighted image of this patient showed infarction in the left-side posterolateral occipital lobe. The left primary visual cortex itself and the postchiasmic visual pathway were spared. A cerebral angiogram showed focal stenosis at the left posterior cerebral artery (Fig 1C), and fMR showed decreased activity in the left visual cortex (Fig 1D).
fig 1.
A 55-year-old man (patient 1) with a visual field defect.
A, Visual field examination shows right homonymous inferior quadrantanopsia.
B, T2-weighted image [4500/120 (TR/TE)] shows infarction in the left occipital lobe (arrow). The left primary visual cortex itself and the visual pathway are spared.
C, Digital subtraction angiogram shows focal stenosis at the left posterior cerebral artery (arrows).
D, fMR [90/56/40° (TR/TE/flip angle)] shows decreased activity in the left visual cortex.
On visual field examination, one patient (patient 2) did not show any visual field defect (Fig 2A). The T2-weighted image of this patient was also normal (Fig 2B). However, fMR showed decreased activity in the right visual cortex owing to multifocal stenosis that caused poor blood flow in the right posterior cerebral artery, which was found by MR angiography (Fig 2C and D).
fig 2.
A 52-year-old woman (patient 2) with several previous episodes of visual disturbance.
A and B, Visual field examination (A) and T2-weighted image [4500/120 (TR/TE)] (B) are normal.
C, MR angiogram [30/6.4/25° (TR/TE/flip angle)] shows poor blood flow (arrowheads) in the right posterior cerebral artery.
D, fMR [90/56/40° (TR/TE/flip angle)] shows decreased activity in the right visual cortex.
Normal conventional MR images were obtained in two patients (patients 2 and 3). One (patient 3) of these two patients (Fig 3A) had intermittent, left homonymous hemianopsia. A digital subtraction angiogram of this patient revealed dissection at the right vertebral artery, which was the dominant artery, with extension to the basilar artery (Fig 3B). The right posterior communicating artery was hypoplastic and the left posterior communicating artery showed good patency (Fig 3C). Visual clinical symptoms in this patient were believed to occur when blood flow from the basilar artery decreased. fMR of this patient showed decreased activity in the right visual cortex (Fig 3D).
fig 3.
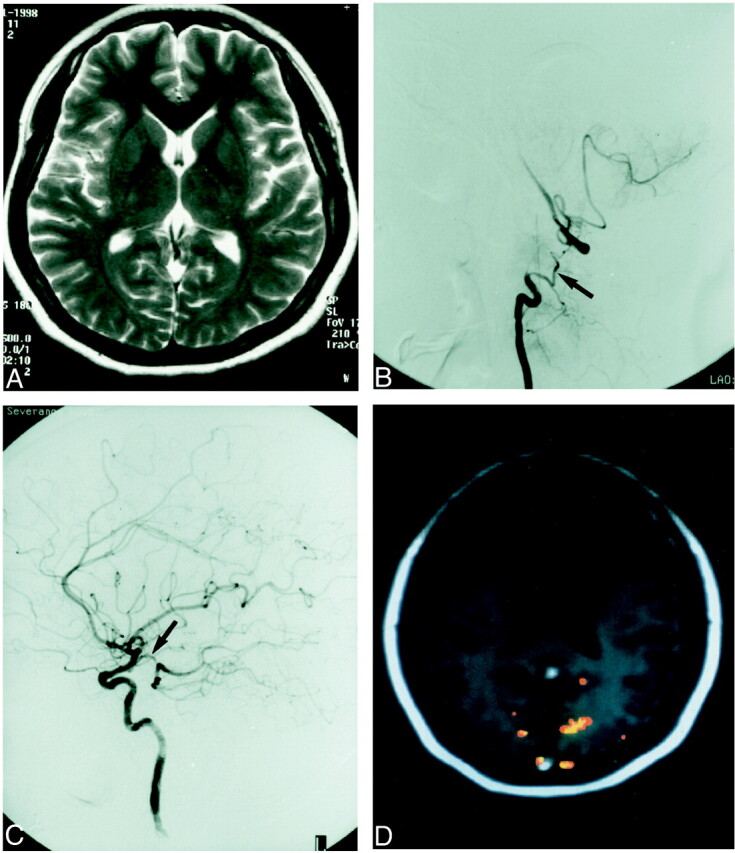
A 29-year-old woman (patient 3) with intermittent left homonymous hemianopsia.
A, T2-weighted image [4500/120 (TR/TE)] is normal.
B and C, Digital subtraction angiogram (B) shows discontinuity of the right vertebral artery and collateral vessels (arrow). The clinical and angiographic diagnosis was dissection of the vertebrobasilar artery. The right posterior communicating artery was hypoplastic (C), whereas the left posterior communicating artery (arrow) showed good patency. The clinical symptom of visual problem in this patient was believed to appear when blood flow from the basilar artery decreased.
D, fMR [90/56/40° (TR/TE/flip angle)] shows decreased activity in the right visual cortex.
Discussion
Careful evaluation of the visual field defect pattern can localize the lesion site approximately along the optic pathway. However, brain MR imaging may be necessary for the precise localization and characterization of the lesion site when ocular abnormalities are not depicted. Despite the presence of visual field defect, it is difficult to prove a functional problem of the visual cortex in a patient with transient ischemic attack if the brain MR imaging is completely normal. Moreover, visual field examination may not detect any abnormality unless it is performed when the symptom occurs. Even diffusion-weighted imaging, which is very sensitive to acute stroke, may show negative findings in acute stroke (16, 17). Since the same arterial territory is prone to subsequent stroke, and this is frequently more extensive, early detection and treatment are very important (8). Our study shows that fMR may be a sensitive method for the detection of such conditions.
In common with other studies, our fMR study showed reliable results on the functional status of activated cortices, and correlated well with the results of visual field examinations. fMR showed decreased activity in the visual cortices where infarction was found. However, there were several cases that did not coincide with the results of visual field examination or conventional MR imaging. fMR of two patients (patients 2 and 3) with transient ischemic attack showed decreased activity in the visual cortex where vascular problems were found on angiograms. Conventional brain MR imaging of these patients did not reveal any abnormality. Although the results of fMR were neither quantitative nor diagnostic, it could reasonably support the necessity for further investigations. fMR of other two patients (patients 1 and 7) with infarction in the occipital lobe revealed infarction in the occipital lobe, but the primary visual cortex and the visual pathway were not involved. We believe that the fMR results in these two patients were the result of decreased vascular reserve.
Generally, there is approximately a 30%–50% increase in regional blood flow, as well as increase of metabolism, in the activated cerebral cortex (18). Since fMR is based on increased regional blood flow in activated cortices, the signal cannot be detected if the cerebral cortex is in a condition of decreased vascular reserve. Therefore, the results of fMR may indicate regional perfusion or vascular reserve. In a previous study by Sorensen et al (10), cases were encountered with abnormal activation mapping, despite normal T2-weighted images and relative cerebral blood volume. This can be explained as the state of decreased vascular reserve. For the evaluation of cerebral perfusion and vascular reserve, 99mTc-hexamethyl-propyleneamine oxime brain single-photon emission CT or perfusion MR imaging using magnetic susceptibility of contrast agent has been used (19–22). The merits of fMR over these methods are that it is simple to use, easy to repeat, and does not use radionuclides or other contrast agents.
Unlike other cortical areas, the visual cortex is easy to stimulate and the stimulation is relatively strong. The functional neuroanatomy of the visual cortex is well established, and the accuracy of fMR has been proved in previous studies (10, 14, 15, 23). Therefore, if we confine fMR to the visual cortices, we believe that it can be effectively used for the evaluation of the functional status of visual cortices and the detection of a compromised state of blood flow. Although there have been many studies concerning the application of functional activation to stroke patients, functional study as a method of depicting patients with decreased vascular reserve has not been reported to date.
Unfortunately, echo-planar imaging was not available in our study owing to geometric distortion of images, presumably affected by the goggles we used. Although the conventional gradient-echo sequence has relatively good spatial resolution, it limits the number of slices to one or two because of its long acquisition time. For that reason, we could obtain only one image slice and could not evaluate the whole volume of the visual cortices. However, it symmetrically covered both sides of the primary visual cortex and, therefore, allowed evaluation of the difference in visual cortical activity on both sides.
Conclusion
fMR of the visual cortex demonstrated visual functional deficit due to potential vascular ischemia. fMR of the visual cortex may be a sensitive method for the detection of a vascularly compromised status in the primary visual cortex and can provide indications for further investigation.
TABLE 3:
Number of activated pixels and asymmetry ratios in the patient group
Footnotes
Address reprint request to Tae-Sub Chung, MD, Department of Diagnostic Radiology and the Research Institute of Radiological Science, Yonsei University College of Medicine, YongDong Severance Hospital, #146-92, Dokok-Dong, Kangnam-Ku, Seoul 135-270, Korea.
References
- 1.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 1986;83:1140-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988;241:462-464 [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance imaging of rodent brain at high magnetic fields. Magn Reson Med 1990;14:68-78 [DOI] [PubMed] [Google Scholar]
- 4.Scheffler K, Seifritz E, Haselhorst R, Bilecen D. Oxygenation changes in the human cerebral cortex during neuronal activation and ferumoxide infusion. Magn Reson Med 1999;42:829-836 [DOI] [PubMed] [Google Scholar]
- 5.Liu HL, Pu Y, Nickerson LD, Liu Y, Fox PT, Gao JH. Comparison of the temporal response in perfusion and BOLD-based event-related functional MRI. Magn Reson Med 2000;43:768-772 [DOI] [PubMed] [Google Scholar]
- 6.Scheffler K, Seifritz E, Haselhorst R, Bilecen D. Titration of the BOLD effect: separation and quantitation of blood volume and oxygenation changes in the human cerebral cortex during neuronal activation and ferumoxide infusion. Magn Reson Med 1999;42:829-836 [DOI] [PubMed] [Google Scholar]
- 7.Hedera P, Lai S, Lewin JS, et al. Assessment of cerebral blood flow reserve using functional magnetic resonance imaging. J Magn Reson Imaging 1996;6:718-725 [DOI] [PubMed] [Google Scholar]
- 8.Cillessen JP, Kappell LJ, van Swieten JC, Algra A, van Gijin J. Does cerebral infarction after a previous warning occur in the same vascular territory? Stroke 1993;24:351-354 [DOI] [PubMed] [Google Scholar]
- 9.Bosley TM, Rosenquist AC, Kushner M, et al. Ischemic lesions of the occipital cortex and optic radiations: positron emission tomography. Neurology 1985;35:470-484 [DOI] [PubMed] [Google Scholar]
- 10.Sorensen AG, Wray SH, Weisskoff RM, et al. Functional MR of brain activity and perfusion in patients with chronic cortical stroke. AJNR Am J Neuroradiol 1995;16:1753-1762 [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke 1999;30:2331-2340 [DOI] [PubMed] [Google Scholar]
- 12.Nelles G, Spiekermann G, Jueptner M, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke 1999;30:1510-1516 [DOI] [PubMed] [Google Scholar]
- 13.Silvestrini M, Troisi E, Matteis M, Razzano C, Caltagirone C. Correlations of flow velocity changes during mental activity and recovery from aphasia in ischemic stroke. Neurology 1998;50:191-195 [DOI] [PubMed] [Google Scholar]
- 14.Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 1995;268:889-893 [DOI] [PubMed] [Google Scholar]
- 15.Deyoe EA, Carman GJ, Bandettini P, et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A 1996;93:2382-2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang PY, Barker PB, Wityk RJ, Ulug AM, van Zijl PC, Beauchamp NJ. Diffusion-negative stroke: a report of two cases. AJNR Am J Neuroradiol 1999;20:1876-1880 [PMC free article] [PubMed] [Google Scholar]
- 17.Lefkowitz D, LaBenz M, Nudo SR, Steg RE, Bertoni JM. Hyperacute ischemic stroke missed by diffusion-weighted imaging. AJNR Am J Neuroradiol 1999;20:1871-1875 [PMC free article] [PubMed] [Google Scholar]
- 18.Belliveau JW, Kennedy DN, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 1991;254:716-719 [DOI] [PubMed] [Google Scholar]
- 19.Silverman IE, Galetta SL, Gray LG, et al. SPECT in patients with cortical visual loss. J Nucl Med 1993;34:1447-1451 [PubMed] [Google Scholar]
- 20.Rempp KA, Brix G, Wenz F, et al. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 1994;193:637-641 [DOI] [PubMed] [Google Scholar]
- 21.Hwang TL, Saenz A, Farrell JJ, Brannon WL. Brain SPECT with dipyridamole stress to evaluate cerebral blood flow reserve in carotid artery disease. J Nucl Med 1996;37:1595-1599 [PubMed] [Google Scholar]
- 22.Nighoghossian N, Berthezene Y, Meyer R, et al. Assessment of cerebrovascular reactivity by dynamic susceptibility contrast-enhanced MR imaging. J Neurol Science 1997;149:171-176 [DOI] [PubMed] [Google Scholar]
- 23.Tootell RB, Reppas JB, Kwong KK, et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neuroscience 1995;15:3215-3230 [DOI] [PMC free article] [PubMed] [Google Scholar]





