Abstract
BACKGROUND AND PURPOSE: Perfusion and diffusion-weighted MR imaging are powerful new imaging techniques for evaluating tissue pathophysiology in association with many neurologic disorders, such as neurodegenerative diseases. The purpose of our study was to evaluate the sensitivity and specificity of dynamic susceptibility contrast-enhanced MR imaging and diffusion-weighted MR imaging in cases of Alzheimer's disease and to assess the role of atrophy in the quantification of cortical perfusion.
METHODS: Thirty-nine participants were studied: 18 patients with moderate cognitive impairment with probable Alzheimer's disease, 16 patients with mild impairment with possible or probable Alzheimer's disease, and 15 group-matched elderly healthy comparison volunteers. Relative values of temporoparietal, sensorimotor, and hippocampal regional cerebral blood volume (rCBV) were measured as a percentage of cerebellar rCBV, and group classification was assessed with logistic regression. Brain atrophy was used as a covariate to assess its role in rCBV quantification. Regions of interest placed on orientation-independent apparent diffusion coefficient maps allowed the calculation of apparent diffusion coefficient values and relative anisotropic indices of the head of the caudate nuclei, thalamus, parietal, frontal, and hippocampal cortices bilaterally, genu and splenium of corpus callosum, and anterior and posterior white matter in patients with Alzheimer's disease and in control volunteers.
RESULTS: Temporoparietal rCBV ratios were reduced bilaterally in the patients with Alzheimer's disease. Sensitivity was 91% in moderately affected patients with Alzheimer's disease and 90% in patients with mild cases. Specificity was 87% in healthy comparison volunteers. Lower values of sensitivity and specificity were obtained for sensorimotor (73%, 50%, and 67%, respectively) and hippocampal cortices (80%, 80%, and 65%, respectively). Using brain atrophy as a covariate, patients with Alzheimer's disease still showed a statistically significant reduction of rCBV compared with control volunteers. Diffusion-weighted MR imaging analysis only showed a trend, with no statistic significance, of reduction of anisotropy in posterior white matter.
CONCLUSION: Dynamic susceptibility contrast-enhanced MR imaging of rCBV may be an alternative to nuclear medicine imaging for the evaluation of patients with Alzheimer's disease. When brain atrophy is used as a covariate, differences in rCBV still persist between patients with Alzheimer's disease and control volunteers, suggesting that perfusion impairment is unrelated to atrophy. No significant results for either white or gray matter were obtained using diffusion-weighted MR imaging.
Alzheimer's disease is the most common cause of dementia. Alzheimer's disease is a neurodegenerative disorder that results in progressive memory loss and cognitive decline. Functional neuroimaging has been reported to be useful for the clinical assessment of cases with possible or probable Alzheimer's disease. Reduction in temporoparietal metabolism or blood flow has been found by using positron emission tomography (1–3) and single photon emission CT (4–7). More recently, perfusion MR imaging was successfully used to differentiate patients with possible or probable Alzheimer's disease (8). Reduced perfusion (regional cerebral blood volume [rCBV]) was found in the temporoparietal and sensorimotor cortices of patients with Alzheimer's disease compared with age-matched control volunteers. As with most functional brain imaging studies, the contribution of atrophy in measurements of brain perfusion was not considered. Diffusion imaging, based on thermal molecular movement, provides qualitative and quantitative parameters that are highly sensitive to the microstructural and pathophysiological alterations occurring in association with specific pathologic conditions. Sandson et al (9) reported diminished anisotropy in the posterior white matter and increased apparent diffusion coefficient (ADC) values in the hippocampi of patients with Alzheimer's disease when compared with a control group. This second observation was not confirmed by other authors (10).
In the present study, we used dynamic susceptibility contrast (DSC) MR imaging to show the presence of specific cortical hemodynamic deficits in patients with probable and possible or probable early Alzheimer's disease, comparing these groups with elderly healthy volunteers. The sensitivity and specificity of DSC MR imaging to distinguish patients with Alzheimer's disease from control volunteers was assessed. Brain atrophy was estimated for the same purpose and was used as a covariate to perfusion imaging data to account for any atrophy-related effect. Diffusivity and anisotropy of brain cortex, deep gray matter nuclei, and white matter were assessed to establish their role in discriminating patients from control volunteers.
Methods
Patient Selection
A group of 34 consecutive patients was prospectively recruited among the outpatients referred to the geriatric division of our hospital on the basis of cognitive and behavioral tests. The diagnosis of patients with probable or possible Alzheimer's disease was performed on the basis of criteria put forth by The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Associations. On the basis of the Mini-Mental State Examination (MMSE) scores, patients were divided into two groups. The first group consisted of 18 patients (nine men and nine women; mean age, 68 years) with a diagnosis of probable Alzheimer's disease with moderate impairment revealed by the MMSE, with a score of ≤23 (of a possible 30). The second group included 16 patients (seven men and nine women; mean age, 66 years) with a diagnosis of probable/possible Alzheimer's disease with mild impairment revealed by the MMSE, with a score of ≥24). A group of 15 volunteers (six men and nine women; mean age, 67 years), age-matched with the enrolled patients, was also recruited as a control group. All control participants were screened by medical history and physical examination. The exclusion criterion for control volunteers was any history of major psychiatric or CNS illness. The exclusion criteria for all groups included history of severe head injury, use of or dependency on illegal drugs, current alcohol abuse, cardiovascular risk factors, hypertension, smoking, diabetes mellitus, or loss of 25% of body weight during the last 12 months.
The groups did not differ significantly in age (one-way analysis of variance; F = 1.03, P = .212) or sex (χ2 = 1.1, df = 1, P = .298). Table 1 summarizes number, age, sex, and mean MMSE. After providing a complete description of the study, written informed consent was obtained; if a patient was not competent, consent was obtained from a spouse or guardian.
TABLE 1:
Demographic information of subject groups
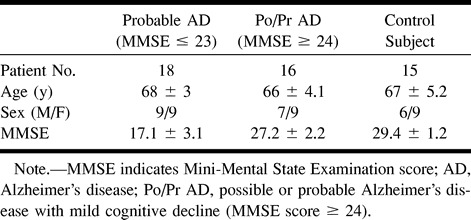
Image Acquisition
Conventional MR Images
A 1.5-T MR imager with a gradient strength of 23 mT/m, a rise time of 0.2 ms, and sinusoidal gradient profile and echo-planar capabilities was used to perform all examinations. A circularly polarized head coil with quadrature was also used. Turbo spin-echo T2-weighted and proton density images (2000/8.8, 110 [TR/TE]; echo train length, 16), fluid-attenuated inversion recovery images (6000/150; inversion time, 2000 ms; turbo factor, 21), and spin-echo T1-weighted images (560/14; flip angle, 90 degrees) were acquired in orthogonal planes. No sedation or anesthesia was used in any of our patients. Two patients could not undergo the complete diagnostic protocol and were excluded from further analysis.
DSC Images
A T2*-weighted echo-planar sequence (18/26; section thickness, 3.5 mm; flip angle, 8 degrees; matrix, 128 × 64; dynamic series, 40; number of signals acquired, 1; imaging time, 1 min 22 s) was used to obtain DSC images along the anteroposterior commissural plane. A dose of 0.4 mmol/kg of gadolinium-diethylenetriamine pentaacetic acid was injected via an antecubital vein, using a power injector at the rate of 5 mL/s. The bolus perfusion data were processed, converted into parameter maps for rCBV, and analyzed as previously described (11).
Diffusion-weighted Images
We used a single-shot axial spin-echo diffusion-weighted pulse sequence with the following parameters: 3682/114; section thickness, 6 mm; section gap, 1 mm; field of view, 230 mm; flip angle, 90 degrees; matrix, 128 × 256; echo-planar imaging factor, 63; acquisition time, 22.1 s; bmin, 0 mm2/s; bmax, 800 mm2/s; number of gradient directions, 3; number of signals acquired, 1; half imaging factor, 0.730.
Image Analysis
Perfusion Images
Regions of interest were drawn on rCBV maps. The cortex was delineated as a ring extending from the outer edge of the brain inward and was divided into five equiangular regions per hemisphere, as proposed by Harris et al (8). Analyses were performed on the images passing 10 mm superior to the anteroposterior commissural plane for temporoparietal cortex and 10 mm above that for sensorimotor cortex (posterior frontal and anteroparietal region). Circular regions of interest (50 pixels) were placed manually on the hippocampus on the section passing 30 mm below the anteroposterior commissural plane. Ratios of index regional mean value to cerebellum were analyzed as measures of relative rCBV. The cerebellum was chosen as a reference region because it is less affected than the cortex in cases of Alzheimer's disease (12). Pixels with signal intensity more than 2 SD above the brain mean value, probably representing blood vessels, were excluded from all regional analyses.
Diffusion-weighted Imaging
Orientation-independent ADC maps were electronically generated after image acquisition. Regions of interest (50 pixels) were drawn on the ADC maps within lateral ventricles, the head of the caudate nuclei, thalamus, parietal and frontal cortices bilaterally, genu and splenium of corpus callosum, and, bilaterally, on anterior and posterior white matter, avoiding areas of signal hyperintensity presented on previously acquired T2-weighted images. Anterior white matter regions of interest were drawn at one-third the distance between the frontal horn of the lateral ventricle and frontal cortex. Posterior white matter regions of interest were drawn at one-third the distance between the trigone of the lateral ventricle and parietal cortex. An anisotropy index value of anterior and posterior white matter was calculated as the ratio of the ADC values on the orthogonal planes (y, perpendicular to the predominant fibers direction; z, parallel to the predominant fibers direction) (y/z). The anisotropy index of the splenium and genu of the corpus callosum was calculated using the ratio of the ADC values on the orthogonal planes y and z (z/y). Being isotropic, the regions of interest in the gray matter and CSF were considered as absolute values.
Fluid-attenuated Inversion Recovery Images
Semiautomatic segmentation analysis was used to evaluate fluid-attenuated inversion recovery images. To measure the volume of both hemispheres, each surface of the sections above the bicommissural plane was multiplied for the section thickness.
Brain volume assessed as noted was related to the volume of the head. This was calculated using the formula of the semi-ellipsoid (v = 2/3πabc), where a and b represent the major diameters of the head at the level of the anteroposterior commissural plane and c represents the height of the head (distance from the anteroposterior commissural plane to the head vertex). The ratio between head and brain volumes was thus considered for further evaluation as a brain atrophy index, with the smaller values relating to more severe atrophy. The brain atrophy index was used to distinguish patients with Alzheimer's disease from control volunteers and as a covariate to perfusion data to account for any atrophy-related effect.
The segmentation technique was also used to measure the volume of the hyperintense lesions (lesion load) found in the supratentorial white matter of each hemisphere. This was expressed as percentage of cerebral volume (lesion load/brain volume).
Statistical Analysis
Logistic regression was used between healthy comparison volunteers and each of the two patient groups to determine the sensitivity for correctly classifying patients with possible or probable Alzheimer's disease and to determine the specificity of distinguishing both the healthy comparison volunteers from patients with Alzheimer's disease. Perfusion data were compared between groups by analysis of variance, with the brain atrophy index as a covariate to account for any atrophy-related effect. Perfusion data were also correlated to lesion load.
Results
In the patients with a diagnosis of probable Alzheimer's disease with moderate impairment revealed by the MMSE (score ≤23), we observed a statistically significant bilateral reduction of rCBV in the temporoparietal regions (P = .0001), whereas in sensorimotor areas, the reduction was slightly less significant (P = .0001 for left and .0004 for right) (Table 2).
TABLE 2:
rCBV values (normalized to cerebellum) in the TP and SM regions
A logistic regression test was applied to both patient groups and the control group to determine the ability of this technique to differentiate the patients on the basis of rCBV values. The use of this statistical approach allowed a correct group classification of 91% for moderately affected patients and 90% for mildly affected patients. Specificity to correctly classify the control volunteers was less satisfactory, at 87%. Logistic regression analysis of rCBV ratios in sensorimotor cortex provided poor group classification. Sensitivity in moderately affected patients was 73%, and sensitivity in mildly affected patients was 50%. Specificity for control volunteers was 67%.
The analysis of data concerning perfusion of hippocampus showed a significant difference between the groups of patients with probable/possible and probable Alzheimer's disease and the group of control volunteers (Table 3). Such difference was more evident when the left hippocampus was considered (P = .0012). Logistic regression analysis allowed a correct group classification of 80% for both moderately and mildly affected patients. Specificity to correctly classify the control volunteers was less satisfactory at 65%.
TABLE 3:
rCBV values (normalized to cerebellum) in right and left hippocampi

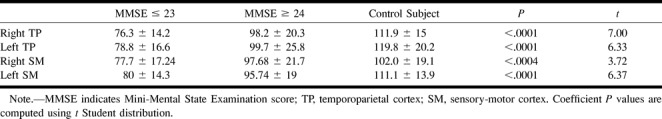
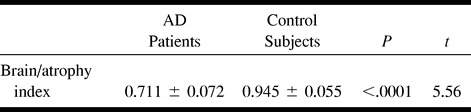
The results of diffusion ADC maps are summarized in Table 4. We have not found any statistically significant group difference in regions of interest sampled within frontal and parietal cortices, which showed slightly higher ADC values in healthy volunteers. Regions of interest sampled within the hippocampus did not provide any statistically significant group difference, neither for the right hippocampus (P = .217) nor for the left (P = .15). No statistically significant group differences were found in the head of caudate nuclei and thalami bilaterally. Calculation of the anisotropy index of posterior and anterior white matter did not provide any remarkable information or help in correct group classification of the participants. A higher anisotropy index (decreased anisotropy) was found only within the right posterior white matter (P = .072) of patients with moderate impairment in comparison with healthy volunteers. Measurements of anisotropy index within the genu (P = .215) and splenium (P = .19) of corpus callosum did not disclose any significant group difference. Measurements of atrophy, both for the whole brain and temporoparietal and sensorimotor cortices (Table 5), provided a remarkable group difference (P = .0001, t = 5.56).
TABLE 4:
Region-of-interest values on ADC trace maps for parietal, frontal cortex, hippocampi, head of caudate nuclei, thalami, anterior and posterior white matter and genu and splenium of corpus callosum
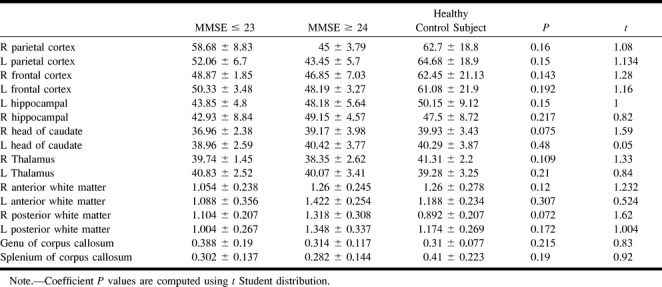
TABLE 5:
Brain-to-atrophy index (calculated as the ratio between skull volume and supratentorial brain volume) in AD patients and control subjects
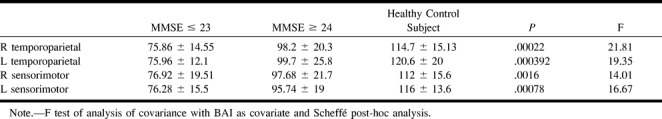
To assess the possible role of atrophy in the reduction of rCBV in temporoparietal and sensorimotor regions of patients with Alzheimer's disease, we used brain atrophy index values as a covariate to account for any atrophy-related effect. Although the statistical significance was slightly inferior, the normalized rCBV values still allowed group differentiation between patients with Alzheimer's disease and control volunteers (P = .0002 for right temporoparietal cortex, P = .00039 for left temporoparietal cortex, P = .0016 for right sensorimotor cortex, P = .00078 for left sensorimotor cortex) (Table 6).
TABLE 6:
rCBV values (with BAI as a covariate) in right and left TP and SM cortex
Among control volunteers, a 2% lesion load was found. In patients with probable/possible Alzheimer's disease (MMSE scores, 24–27), the mean lesion load was 0.035%. In those with probable Alzheimer's disease (MMSE scores, 7–23), the mean lesion load was 0.9%. We correlated perfusion data to the lesion load. The rCBV values assessed in patients with Alzheimer's disease and in control volunteers were shown to be independent of lesion load (P = .56).
Discussion
Generalized atrophy and microscopic alterations that consist of “senile” plaques and neurofibrillary tangles represent neuropathologic features in cases of Alzheimer's disease. The most severe neuropathologic manifestations occur in the posterior temporal and inferior parietal lobes and limbic structures. Primary motor and sensory areas are relatively spared (13–16). Individual neuronal groups show selective susceptibility for degeneration. Specifically, the hippocampal formation is consistently and heavily involved in the pathology of Alzheimer's disease; subsequently focal symmetric or asymmetric enlargement of the temporal horn is frequently observed in association with Alzheimer's disease. Neuroimaging and gross pathology show diffuse cerebral atrophy, histologically attributed to neuronal loss. The decrease in global cerebral volume, measured by means of a registration and subtraction method of serial MR images, was found to correlate with MMSE score changes over time (17). MR imaging and CT studies have revealed white matter lesions in patients with Alzheimer's disease to be more common than in elderly control volunteers. The hyperintensities on long-TR images in cases of Alzheimer's disease have been found to be of intermediate severity, between the findings in normal elderly patients and those with vascular dementia. For this reason, patients with dementia and prominent subcortical lesions and periventricular hyperintensity (as well as basal ganglion and cortical infarct) are more likely to have vascular dementia rather than Alzheimer's disease (18, 19). No correlation was found between the severity of white matter lesions and the severity of dementia (19).
Until recently, hemodynamic and metabolic parameters affecting the brain in cases of neurodegenerative diseases were imaged primarily using single photon emission CT and positron emission tomography. Previous positron emission tomography and single photon emission CT studies have shown a significant reduction of perfusion parameters in sensorimotor and temporoparietal cortices in patients with Alzheimer's disease and a reduced glucose metabolism, especially within the temporoparietal cortex (20, 21). Recently, Harris et al (8) documented that DSC MR imaging is capable of detecting hemodynamic deficits with high sensitivity and specificity in patients with Alzheimer's disease. Severe deficits in temporoparietal hemodynamic parameters were shown in patients with Alzheimer's disease compared with matched elderly volunteers, with excellent group discrimination, even in patients with mild cognitive decline. Analysis of our data relative to temporoparietal cortex showed results with high statistical significance (P < .0001) in differentiating moderately affected patients with Alzheimer's disease from elderly control volunteers, with a specificity of 90%. In sensorimotor regions, the specificity of hemodynamic impairment was high, but not as high as in temporoparietal regions (87%). These results are similar to those reported by Harris et al (8). Sampling of rCBV of the hippocampus revealed a significant reduction of this value in patients with Alzheimer's disease compared with age-matched healthy volunteers as well. Although the statistical significance was lower than temporoparietal and sensorimotor cortex evaluation, both mild and moderately affected patients showed significant hypoperfusion within the hippocampi compared with control volunteers.
The significant reduction of rCBV in temporoparietal and sensorimotor cortex could be related to cortical atrophy. It is a logical suspicion that the reduction of rCBV results in a reduction of cerebral tissue and not only in a fall in blood demand. Many MR imaging studies have found an increase of CSF and a reduction of cerebral volume in patients with Alzheimer's disease in comparison with healthy volunteers, particularly if hippocampus is considered (23–25). Rusinek et al (26) reported a significant reduction of gray matter in affected patients in comparison with age-matched control volunteers, especially in the temporal lobes and basal ganglia; less prominent variations were evident in the frontal and occipital cortices, while CSF volume was increased. More recently, Tanabe et al (27), using a semiautomatic segmentation of MR images of the brains of patients with Alzheimer's disease, showed significant brain atrophy to be attributable to loss of cortical gray matter. Brain atrophy was confirmed to be a characteristic feature of Alzheimer's disease in our study as well. A highly statistically significant correlation was found for atrophy between patients and elderly volunteers (P ≤ .0001). To understand the role of atrophy in the quantification of cortical rCBV, we correlated perfusion data with brain atrophy. Based on the assumption presented by Tanabe et al that in Alzheimer's disease, brain atrophy is mostly related to cortical atrophy, we used brain atrophy as a covariate to rCBV. Using this correction, we found that in patients with Alzheimer's disease (both with MMSE scores ≤23 and with MMSE scores ≥24), there still is a significant reduction of rCBV, although slightly reduced in comparison to non-normalized values. On the basis of these data, it is possible to argue that perfusion impairment may be unrelated to atrophy, indicating a true functional and cognitive decline. This observation can be considered in agreement with the observations reported by Meltzer et al (28), who showed temporoparietal and sensorimotor hypometabolism assessed by positron emission tomography studies to persist even after volume correction.
We did not find any difference between patients with Alzheimer's disease and control volunteers concerning white matter signal alterations. Lesion load was higher in control volunteers (2%) than in patients with Alzheimer's disease (ranging from .035% to .9%). Many previous studies have semiquantitatively and quantitatively analyzed white matter signal alterations in patients with Alzheimer's disease and in elderly control volunteers, with conflicting results. Most investigators have reported increases in hyperintensity in patients with Alzheimer's disease (29–32).
Because clinical inclusion criteria and age were similar in our population of patients and control volunteers and a highly sensitive sequence (fluid-attenuated inversion recovery) with thin sections was used, our data should not be influenced by significant bias. Based on our observations, signal alterations in patients with Alzheimer's disease should be considered incidental findings, mostly related to aging rather than to the disease.
No correlation was found between lesion load and perfusion deficit. This finding confirms the opinion presented by Brun and Englund (33) that white matter disease occurs independent of gray matter degeneration. Moreover, because cortical hypoperfusion is unrelated to white matter disease (which is mostly due to loss of myelin, axons, and oligodendroglia consequent to microvascular impairment), it is possible to argue that the perfusion impairment is not caused by a microvascular disease but, more reliably, by a fall in blood demand. This is also confirmed by many previous studies that did not find correlation between white matter hyperintensities and MMSE scores (32, 34–37).
Pathologic alterations occurring in cases of Alzheimer's disease might be expected to influence diffusivity. Amyloid deposition, neuritic degeneration, and cytoskeletal destabilization in the white matter and loss of myelin sheaths, axons, and oligodendroglial cell with gliosis (35), as well as oxidative membrane damage, alterations of ion, or fluid homeostasis and reduced axoplasmic flow related to cytoskeletal dysfunction in the white matter, may cause changes of ADC values (38–40). Sandson et al (9) described the changes observed on diffusion-weighted images of patients with Alzheimer's disease. The authors found a significant reduction of anisotropy in posterior white matter in patients with Alzheimer's disease (P = .0001) and a slight increase of ADC values within the hippocampus. Our results only confirm a trend, with no statistical significance, of reduced anisotropy in the posterior white matter. ADC maps, in our study, did not show any statistically significant change of diffusion coefficient of the temporoparietal and hippocampal cortex. These findings do not agree with the data presented by Sandson et al (9), who reported an increased diffusivity within the hippocampus. They are, however, in agreement with another study, which did not show significant differences of the ADC values in the hippocampus between patients with Alzheimer's disease and age-matched control volunteers (10).
DSC MR imaging can effectively assess perfusion abnormalities in specific regions of the brain cortex able to discriminate patients with early Alzheimer's disease from control volunteers with a sensitivity and specificity close to 90%. Our study indicates that these hemodynamic alterations may be unrelated to atrophy. Based on our data and considering previous results (8) that indicate similar functional information provided by DSC MR imaging compared with single photon emission CT and positron emission tomography, we suggest the use of perfusion MR imaging in addition to conventional sequences whenever evaluating patients suspected of having early Alzheimer's disease. The increasing cost of DSC in addition to conventional MR imaging is minimal, and it is mostly related to the use of contrast material (which is often used routinely). Additional important advantages of DSC MR imaging over nuclear medicine are the use of nonionizing radiation, reducing inconvenience (all information in a single session), and higher spatial resolution. The possibility to quantify the hemodynamic impairment could also be useful in longitudinal evaluation and therapeutic trials of patients with early Alzheimer's disease.
Conclusion
Our study has confirmed a possible clinical role of perfusion MR imaging in the diagnosis of Alzheimer's disease, even in the early stages of the disease. Statistically significant alterations of perfusion parameters were found in temporoparietal, sensorimotor, and hippocampal cortices. Perfusion impairment seems unrelated to atrophy, and the hemodynamic alterations could be an expression of functional and cognitive impairment. The limited role of microvascular pathology in the genesis of atrophy is confirmed, in our opinion, by the absence of correlation with subcortical white matter lesions.
Our study confirmed that atrophy is a characteristic feature of Alzheimer's disease. Data obtained from our study confirm that diffusion-weighted imaging has a limited role in the evaluation of cortical and white matter changes occurring in association with Alzheimer's disease. These considerations could change when tensor images become widely available.
Footnotes
Address reprint requests to Alessandro Bozzao, Istituto di Radiologia, Ospedale S. Eugenio, Piazzale dell'Umanesimo 10, 00144 Roma, Italy.
References
- 1.Smith GS, de Leon MJ, George AE, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease: pathophysiologic implications. Arch Neurol 1992;49:1142-1150 [DOI] [PubMed] [Google Scholar]
- 2.Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Clinical deterioration in probable Alzheimer's disease correlates with progressive metabolic impairment of association areas. Dementia 1994;5:36-41 [DOI] [PubMed] [Google Scholar]
- 3.Fazekas F, Alavi A, Chawluk JB, et al. Comparison of CT, MR and PET in Alzheimer's dementia and normal aging. J Nucl Med 1989;30:1607-1615 [PubMed] [Google Scholar]
- 4.Holman BL, Johnson KA, Gerada B, Carvalho PA, Satlin A. The scintigraphic appearance of Alzheimer's disease: a prospective study using technetium-99m-HMPAO SPECT. J Nucl Med 1992;33:181-185 [PubMed] [Google Scholar]
- 5.Reed BR, Jagust WJ, Sacb JP, Ober BA. Memory and regional cerebral blood flow in mildly symptomatic Alzheimer's disease. Neurology 1989;39:1537-1539 [DOI] [PubMed] [Google Scholar]
- 6.Pearlson GD, Harris GJ, Powers RE, et al. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry 1992;49:402-408 [DOI] [PubMed] [Google Scholar]
- 7.Harris GJ, Links JM, Pearlson GD, Camargo EE. Cortical circumferential profile of SPECT cerebral perfusion in Alzheimer's disease. Psychiatry Res 1991;40:167-180 [DOI] [PubMed] [Google Scholar]
- 8.Harris GJ, Lewis RF, Satlin A, et al. Dynamic susceptibility contrast MRI of regional cerebral blood volume in Alzheimer disease: a promising alternative to nuclear medicine. AJNR Am J Neuroradiol 1998;19:1727-1732 [PMC free article] [PubMed] [Google Scholar]
- 9.Sandson TA, Felician O, Edelman RR, Warach S. Diffusion-weighted magnetic resonance imaging in Alzheimer's disease. Dement Geriatr Cogn Disord 1999;10:166-171 [DOI] [PubMed] [Google Scholar]
- 10.Hanyu H, Sakurai H, Iwamoto T, Takesaki M, Shindo H, Abe K. Diffusion weighted MR imaging of the hippocampus and temporal white matter in Alzheimer disease. J Neurol Sci 1998;156:195-200 [DOI] [PubMed] [Google Scholar]
- 11.Flacke S, Keller E, Hartman A, et al. Improved diagnosis of early infarcts by the combined use of perfusion and diffusion-weighted imaging. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1998;168:493-5019617367 [Google Scholar]
- 12.Pickut BA, Dierckx RA, Dobbeleir A, et al. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res 1999;90:103-112 [DOI] [PubMed] [Google Scholar]
- 13.Hyman BT, Damasio H, Damasio AR, Van Hoessen GW. Alzheimer's disease. Annu Rev Public Health 1989;10:115-140 [DOI] [PubMed] [Google Scholar]
- 14.Perl DP, Pendlebury WW. Neuropathology of Alzheimer's disease and related dementias. In: Meltzer HY, ed. Psychopharmacology: A Third Generation of Progress. New York: Raven Press; 1987
- 15.Risse SC, Raskind MA, Nochlin D, et al. Neuropathological findings in patients with clinical diagnoses of probable Alzheimer's disease. Am J Psychiatry 1990;147:168-172 [DOI] [PubMed] [Google Scholar]
- 16.Brun A. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and hystopathological grading. Hystopathology 1981;5:549-564 [DOI] [PubMed] [Google Scholar]
- 17.Fox NC, Sacra RI, Crumb WR, Rosier MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 1999;52:1687-1689 [DOI] [PubMed] [Google Scholar]
- 18.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJNR Am J Neuroradiol 1987;149:351-356 [DOI] [PubMed] [Google Scholar]
- 19.Bowen BC, Barker WW, Loewenstein DA, Sheldon A, Duara R. MR signal abnormalities in memory disorder and dementia. AJNR Am J Neuroradiol 1990;11:283-290 [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe N, Bruce R, et al. Temporal lobe perfusion on single photon emission computed tomography predicts the rate of cognitive decline in Alzheimer's disease. Arch Neurol 1995;52:257-262 [DOI] [PubMed] [Google Scholar]
- 21.Johnson KA, Kijewski MF, Becker JA, Garada B, Satlin A, Holman BL. Quantitative brain SPECT in Alzheimer's disease and normal aging. J Nucl Med 1993;34:2044-2048 [PubMed] [Google Scholar]
- 22.O'Mahony D, Coffey J, Murphy J, et al. The discriminant value of semiquantitative SPECT data in mild Alzheimer's disease. J Nucl Med 1994;35:1450-1455 [PubMed] [Google Scholar]
- 23.Tanna N, Kohn M, Horwuch D, et al. Analysis of brain and cerebrospinal fluid volumes with MR imaging: part II. aging and Alzheimer dementia. Radiology 1991;178:123-130 [DOI] [PubMed] [Google Scholar]
- 24.Jack CR, Petersen RC, O'Brien PC, et al. MR- base hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 1992;42:183-188 [DOI] [PubMed] [Google Scholar]
- 25.Wahlund LO, Anderson-Lundman G, Basun H, et al. Cognitive functions and brain structures: a quantitative study of CSF volumes on Alzheimer patients and healthy control subjects. Magn Reson Imaging 1993;11:169-174 [DOI] [PubMed] [Google Scholar]
- 26.Rusinek H, de Leon MJ, George AE, et al. Alzheimer's disease: measuring loss of cerebral gray matter with MR imaging. Radiology 1991;178:109-114 [DOI] [PubMed] [Google Scholar]
- 27.Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol 1997;18:115-123 [PMC free article] [PubMed] [Google Scholar]
- 28.Meltzer CC, Zubieta JK, Brandt J, Tune LE, Mayberg HS, Frost JJ. Regional hypometabolism in Alzheimer's disease as measured by positron emission tomography after correction for effects of partial volume averaging. Neurology 1996;47:454-461 [DOI] [PubMed] [Google Scholar]
- 29.Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral structure on MRI: part II. specific changes in Alzheimer's and Huntington's diseases. Biol Psychiatry 1991;29:68-81 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R. Comparison of magnetic resonance imaging in Alzheimer's disease, vascular dementia and normal aging. Eur Neurol 1992;32:164-169 [DOI] [PubMed] [Google Scholar]
- 31.McDonald WM, Krishnan KR, Doraiswamy PM, et al. Magnetic resonance findings in patients with early-onset Alzheimer's disease. Biol Psychiatry 1991;29:699-710 [DOI] [PubMed] [Google Scholar]
- 32.Waldemar G, Christiansen P, Larsson HB, et al. White matter magnetic resonance hyperintensities in dementia of the Alzheimer type: morphological and regional cerebral blood flow correlates. J Neurol Neurosurg Psychiatry 1994;57:1458-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brun A, Englund EA. White matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol 1986;19:253-262 [DOI] [PubMed] [Google Scholar]
- 34.Leys D, Soetaert G, Petit H, Fauquette A, Pruvo JP, Steinling M. Periventricular and white matter MRI hyperintensities do not differ between Alzheimer's disease and normal aging. Arch Neurol 1990;47:524-527 [DOI] [PubMed] [Google Scholar]
- 35.Wahlund LO, Basun H, Almkvist O, Andersson-Lundman G, Julin P, Saaf J. White matter hyperintensities in dementia: does it matter? Magn Reson Imaging 1994;3:387-394 [DOI] [PubMed] [Google Scholar]
- 36.Bennett DA, Gilley DS, Wilson RS, Huckman MS, Fox JH. Clinical correlates of high signal lesions on magnetic resonance imaging in Alzheimer's disease. J Neurol 1992;239:186-190 [DOI] [PubMed] [Google Scholar]
- 37.Marder K, Richards M, Bello J, et al. Clinical correlates of Alzheimer's disease with and without silent radiographic abnormalities. Arch Neurol 1995;52:146-151 [DOI] [PubMed] [Google Scholar]
- 38.Mark RJ, Hedlund LW, Butterfield DA, Mattson MP. Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci 1995;15:6239-6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terry RD. The pathogenesis of Alzheimer disease: an alternative to amyloid hypothesis. J Neuropathol Exp Neurol 1996;55:1023-1025 [PubMed] [Google Scholar]
- 40.Pappella MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative stress in Alzheimer disease. Am J Pathol 1992;140:621-628 [PMC free article] [PubMed] [Google Scholar]


