Abstract
BACKGROUND AND PURPOSE: The clinical significance of microembolic signals (MESs) detected by transcranial Doppler sonography (TCD) in acute ischemic stroke remains unclear. The purpose of the present study was to assess the findings of diffusion-weighted MR imaging (DWI) and other clinical characteristics in patients with acute ischemic stroke and MESs.
METHODS: We performed TCD and DWI within 48 hours and 7 days, respectively, after stroke onset in 28 patients with acute brain infarction. The relationship between the number of MESs and DWI findings, risk factors for stroke, National Institutes of Health Stroke Scale (NIHSS) score on admission, and arterial disease was examined.
RESULTS: Ten patients had MESs detected by TCD (MES group) and 18 had no MESs (control group). The frequency of hypertension, diabetes mellitus, hyperlipidemia, and smoking; NIHSS score; blood-coagulation parameters; and interval between stroke onset and DWI study did not differ between the two groups. However, arterial disease was more frequent in the MES group than in the control group. Small, multifocal ischemic lesions (<10 mm in diameter) on DWI were more frequent in the MES group than in the control group. Conventional CT and MR imaging often failed to show these lesions.
CONCLUSION: Small, often asymptomatic DWI abnormalities were more frequent in patients with MESs detected by TCD and with large-vessel occlusive diseases than in stroke patients without MESs. TCD and DWI may provide early clues to the mechanism of stroke in the acute phase.
Microembolic signals (MESs) have been detected by transcranial Doppler sonography (TCD) in patients with carotid disease, atrial fibrillation, and prosthetic cardiac valves; at carotid angiography; and intraoperatively in patients undergoing cardiac or carotid surgery (1–9). In vitro and in vivo studies have shown that MESs represent microemboli made of air, fibrinogen, or atheromatous material (10, 11). Such MESs are detected in 11% to 12% of patients with acute stroke and are associated with an increased prevalence of prior ischemic stroke; they may also be a risk factor for subsequent ischemic stroke (12–14). Among patients who have undergone carotid endarterectomy, early postoperative detection of MESs may assist in identifying those at risk for postoperative brain ischemia (15–18). The frequency of MES detection may be an important determinant in risk prediction.
Diffusion-weighted MR imaging (DWI) detects hyperacute ischemic lesions and differentiates recent acute stroke from old ischemic lesions (19, 20). Compared with conventional CT and conventional MR imaging, DWI is more sensitive and specific in evaluating ischemic lesions (21). Some patients with transient ischemic attacks and no abnormal lesions on T2-weighted MR images have DWI abnormalities (22). Therefore, DWI can identify fresh ischemic lesions that cannot be detected by conventional CT and MR imaging.
The clinical significance of MESs in patients with acute ischemic stroke remains unclear. We speculated that acute ischemic stroke patients with MESs have additional, fresh, clinically silent brain infarcts. To address this notion, we studied the relationship between the frequency of MESs and DWI findings as well as other clinical characteristics.
Methods
We performed TCD and MR imaging in 28 patients (21 men and seven women, aged 67 ± 12 years) with acute carotid ischemic stroke who were admitted to our hospital within 48 hours after stroke onset between July 1998 and August 1999. Ten patients had MESs detected by TCD (MES group) and 18 patients had no MESs (control group).
The following clinical data were compiled for all patients: the number of MESs detected from the middle cerebral artery (MCA) by TCD, DWI findings, neurologic deficits, major risk factors for stroke, arterial or cardiac diseases that could be a potential source of embolic stroke, blood-coagulation factors, and the interval between stroke onset and MR study.
Within 24 hours after admission, TCD was performed using a DWL Multidop X unit with a 2.5-MHz probe. Patients were placed supine, and bilateral MCAs were insonated at a depth of between 45 and 55 mm for 30 minutes. The TCD probe was held in place with an elastic headband to reduce the occurrence of movement artifacts. The criteria for MESs included random occurrence, brief duration (<0.1 second), intensity greater than 6 dB above background, primarily unidirectional within the Doppler spectrum, a spike in the power/intensity trace, and an accompanying audible chirp, moan, or snap sound. All MESs were recorded on a hard disk. The MESs were counted during an off-line review of the Doppler recordings.
Brain CT studies were performed on the day of admission and again 3 days after stroke onset to evaluate ischemic lesions. Within 7 days of stroke onset, MR imaging was performed using a 1.5-T system equipped with single-shot echo-planar imaging to obtain rapid diffusion images. MR studies included axial T1-weighted, axial T2-weighted, and DWI sequences (approximately 30 minutes of scanning time). The imaging parameters were 4000/103 (TR/TE), 128 × 128 matrix, 230-mm field of view, and 4-mm slice thickness with a 2-mm gap between slices. Two b values were used: 0 and 1000 s/mm2. Diffusion gradients were applied in successive scans in each of the x, y, and z directions, and DWI images were formed from the average of these values. The criterion for the diagnosis of acute infarcts on DWI was focal high intensity, judged not to be due to normal anisotropic diffusion or magnetic susceptibility artifacts. The diameter of hyperintense lesions on DWI was classified as small (<10 mm), medium (10–30 mm), or large (≥30 mm). These lesions were also categorized as cortical, subcortical, or lacunar infarcts according to their location. The number, size, and location of recent ischemic lesions on DWI were evaluated by two neurologists who had no knowledge of the clinical or laboratory data.
Neurologic deficits on admission were determined by using the National Institutes of Health Stroke Scale (NIHSS) score (23). Major risk factors for stroke included hypertension, diabetes mellitus, hypercholesterolemia, and cigarette smoking. Blood-coagulation parameters, including antithrombin III, thrombin-antithrombin III complex, and D-dimer, were measured on admission.
To detect potential cardiac sources of emboli, all patients were examined using a 12-lead electrocardiograph (ECG), by 24-hour ECG monitoring and by transthoracic echocardiography. Causes of emboligenic cardiac disorders included nonvalvular atrial fibrillation, acute myocardial infarction, previous myocardial infarction with intraventricular thrombus, mitral valve disease, prosthetic cardiac valve, and dilated cardiomyopathy. Potential aortogenic sources of emboli or patency of the foramen ovale were evaluated in 13 patients by transesophageal echocardiography (TEE). Localized raised lesions in the aorta with a maximal intimal-medial thickness of over 40 mm and an obviously irregular surface or broad acoustic shadow were defined as complicated lesions.
All patients underwent color-flow duplex carotid sonography on the day of admission. Conventional cerebral angiography was performed in 17 patients and the remaining 11 patients underwent MR angiography. Stenoses were graded according to the method used in the North American Symptomatic Carotid Endarterectomy Trial (24).
All patients in whom MESs were detected by TCD were treated immediately with an antiplatelet agent (ticlopidine,200 mg). A follow-up TCD study was performed after antiplatelet therapy to determine whether the frequency of MESs had changed.
The Mann-Whitney U test was applied to detect differences in age, number of lesions on DWI, NIHSS score, and interval between stroke onset and MR assessment. All other findings were assessed by the χ2 test between two groups. Differences were assumed to be significant at P ≤ .05.
Results
In the MES and control groups, one and two MCAs, respectively, ipsilateral to the side of hemiparesis could not be studied by TCD because of a lack of an acoustic window. Therefore, Doppler waves from 19 and 34 MCAs, respectively, were obtained.
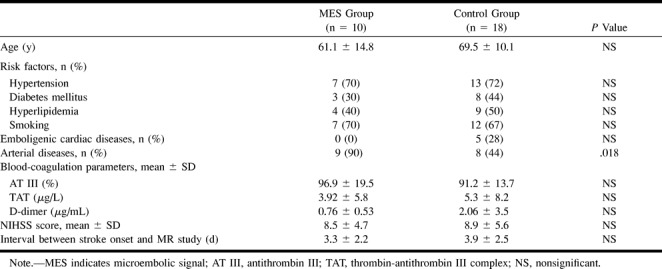
Table 1 lists the clinical features and background characteristics of the two groups. The frequency of hypertension, diabetes mellitus, hyperlipidemia, smoking, NIHSS score, blood-coagulation parameters, and interval between stroke onset and MR examination did not differ between the two groups.
TABLE 1:
Demographic features of patients in two groups
Significant arterial disease contralateral to the side of hemiparesis was observed in 17 patients (60%): MCA occlusion was found in four patients, M2 occlusion in two, internal carotid artery (ICA) occlusion in one, intra- or extracranial ICA stenosis of 50% or more in four and five, respectively, and ICA stenosis of less than 50% with ulceration in one. Arterial disease was more frequent in the MES group than in the control group (P = .02).
Emboligenic cardiac diseases were all NVAF and present in five patients, all of whom were in the control group. The frequency of emboligenic cardiac diseases between the two groups did not differ. Complicated lesions were revealed by TEE in two patients in the MES group and in two patients in the control group. A patent foramen ovale was observed in one patient in the MES group and in one in the control group.
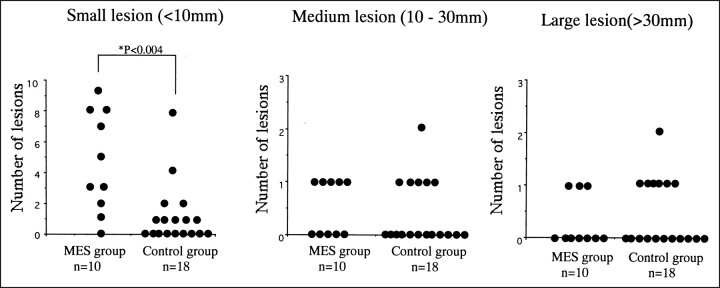
DWI showed not only hyperintense abnormalities corresponding to the lesions revealed by CT but also other small lesions that were not shown by CT. The small lesions on DWI were detected in the cerebral cortex or subcortex in 18 patients (Figs 1 and 2). T1- and T2-weighted MR images showed the abnormalities in the subcortex corresponding to DWI alterations, but not the small lesions in the cortex. Small hyperintense lesions on DWI were more frequent in the MES group than in the control group (P < .004); however, the frequency of medium and large hyperintense lesions did not differ between the two groups (Fig 3).
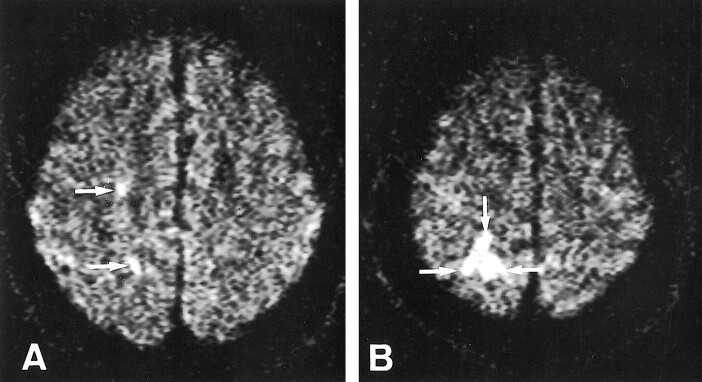
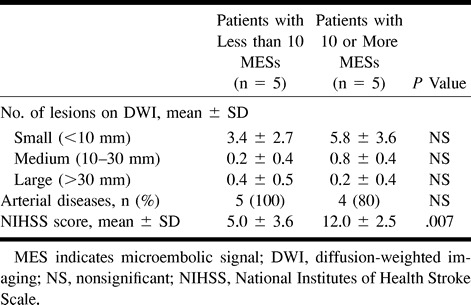
fig 1.
Patient 2.
A and B, Axial diffusion-weighted images 7 days after stroke onset show five spotty hyperintense lesions in the right hemisphere (arrows). Four MESs were recorded for 30 minutes from the right MCA. This patient had 90% stenosis at the origin of the right ICA.
fig 3.
Small lesions on DWI are more frequent in the MES group than in the control group (P < .004). However, the frequency of medium and large lesions does not differ between the two groups
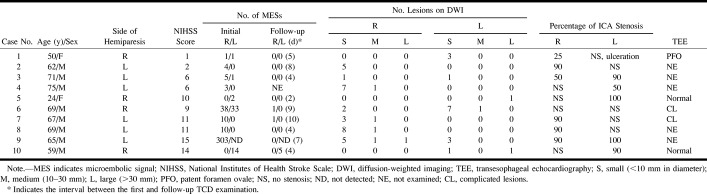
Table 2 shows the clinical characteristics of all patients in the MES group. The MES counts were recorded not only from 10 MCAs contralateral to the side of hemiparesis but also from three MCAs ipsilateral to the side of hemiparesis. The number of MESs per 30-minute insonation period varied from 1 to 303. Patient 1 had ICA stenosis of less than 50% with ulceration and a patent foramen ovale. Because MESs were documented bilaterally, the source of MESs was more likely to be the patent foramen ovale. Further examinations showed deep venous thrombosis in the lower legs; therefore, we determined that this patient had a paradoxical brain embolism through a patent foramen ovale. Patient 6 had MESs detected from bilateral MCAs and no significant cerebral arterial disease, but had complicated lesions in the aorta, suggesting an aortogenic brain embolism. Patient 9 had ICA stenosis contralateral to the side of hemiparesis and ICA occlusion ipsilateral to the side of hemiparesis. Spotty DWI lesions were observed in both hemispheres. Spotty brain infarctions ipsilateral to the side of hemiparesis may be caused by small emboli from the right ICA to the left MCA through the anterior communicating artery.
TABLE 2:
Clinical characteristic of patients with MESs
In the MES group, five patients had 10 or more MESs per 30 minutes and the remaining five had less than 10 MESs per 30 minutes. The frequency of arterial disease and small or medium or large hyperintense lesions on DWI was not significantly different between these two groups. On the other hand, the NIHSS score was higher in the group with 10 or more MESs per 30 minutes than in the group with less than 10 MESs per 30 minutes (P = .007) (Table 3).
TABLE 3:
Demographic features of patients in MES group
In the MES group, no patient was started on antithrombotic therapy before TCD examination and all were treated with an antiplatelet agent immediately after the detection of MESs. Follow-up TCD studies revealed that the frequency of MESs decreased in nine patients 4 to 10 days after antiplatelet therapy was begun. However, transient dysarthria occurred in one patient (patient 3). In the control group, five patients with emboligenic cardiac disease were treated with heparin (10,000U/day) before TCD examination, but the remaining 13 patients were started on antithrombotic therapy after TCD.
Discussion
In our study, patients with acute ischemic stroke and MESs had a greater prevalence of arterial disease and spotty brain lesions on DWI than did patients with acute ischemic stroke and no MESs. It has been reported that the main sources of microemboli in ICA stenosis are ulceration and thrombus formation of the atheromatous plaque (25). Small emboli may occlude peripheral brain arteries and generate spotty cortical or subcortical infarcts that can be detected by DWI. However, microemboli detected by TCD are asymptomatic and not evident on DWI. Because spotty cortical lesions on DWI are often asymptomatic (26, 27), emboli larger than microemboli detected by TCD may produce asymptomatic spotty lesions on DWI. MESs may indicate the presence of an unstable plaque or platelet aggregation in arterial disease that promotes an artery-to-artery embolism.
In our study, most patients (82%) with acute stroke had no MESs or relatively few; however, five patients (18%) had 10 or more MESs per 30 minutes. We arbitrarily divided the MES group into patients with infrequent MESs (less than 10 per 30 minutes; patients 1–5) and those with frequent MESs (10 or more per 30 minutes; patients 6–10). No significant difference in the frequency of arterial disease or the number of spotty lesions on DWI was observed between these two groups. However, patients with 10 or more MESs per 30 minutes appeared to have more severe neurologic deficits than did those with less than 10 MESs per 30 minutes. We believe that a large number of microembolisms may diffusely damage the brain and cause these neurologic deficits. Therefore, it is worthwhile to distinguish patients with many MESs from those with no or just a few MESs.
Tegeler et al (14) found that patients with MESs had a higher rate of recurrent stroke. In the present study, MESs were associated with small lesions on DWI. All patients with MESs were treated with an antiplatelet agent soon after detection of MESs with TCD. Follow-up TCD studies showed that the frequency of MESs decreased in all patients who had been treated with antiplatelets. Certainly, it remains unclear whether treatment actually reduced MESs or if this was the natural history. Nevertheless, we think that treatment with antiplatelets should be started as soon as possible to prevent further ischemic stroke after MES is detected by TCD.
Although atherosclerotic lesions of the aorta are recognized as a potential source of emboli (28, 29), a diagnosis of aortogenic brain embolism cannot be readily established. Of the patients whose MESs were recorded from bilateral MCAs, the source was perhaps aortic lesions or cardiac disease. Therefore, if MESs are detected bilaterally in patients with acute stroke and complicated aortic lesions but without another potential source of emboli, a diagnosis of aortogenic embolic stroke should be considered.
Conclusion
Small, often asymptomatic, DWI abnormalities are more frequently seen in patients with acute stroke who have microemboli and large-vessel occlusive disease than in such patients without microemboli. TCD and DWI may provide early clues to the mechanism of stroke during the acute phase.
fig 2.
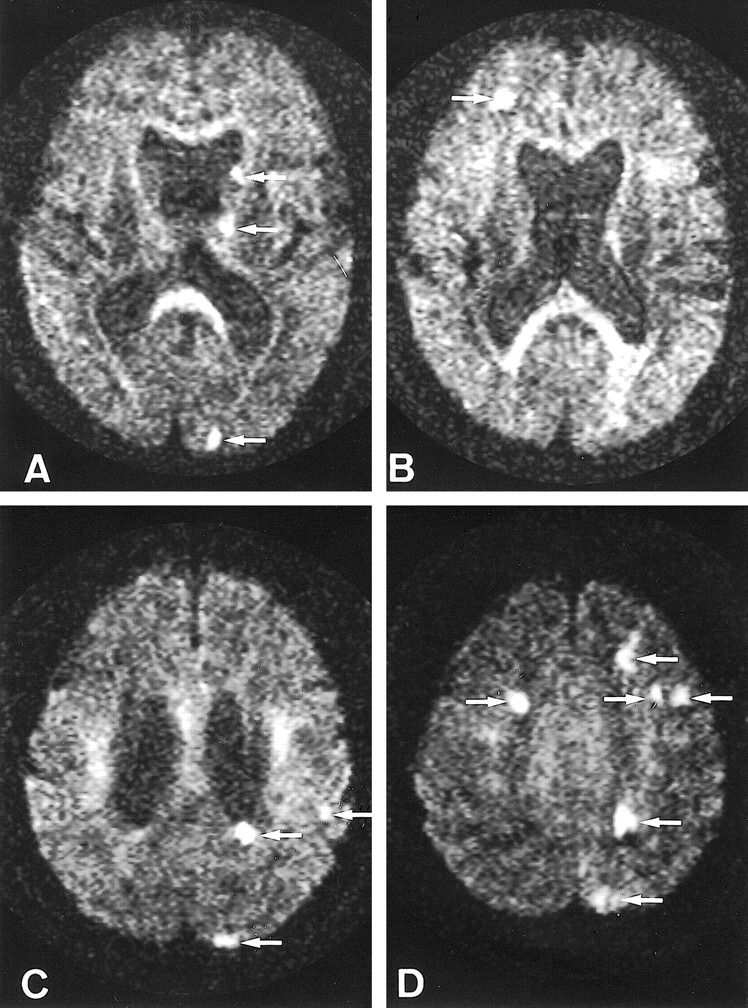
Patient 6.
A–D, Axial diffusion-weighted images 5 days after stroke onset show several spotty hyperintense abnormalities (arrows) in both hemispheres. Thirty-eight and thirty-three MESs were recorded with TCD for 30 minutes from the right and left MCAs, respectively. This patient had neither arterial nor cardiac disease but had complicated lesions in the aorta.
Acknowledgments
We thank Naoaki Yamada (Department of Diagnostic Radiology) for his valuable comments on the MR studies.
Footnotes
Supported in part by research grants for cardiovascular disease (9A–2, 9A–3, 9A–8) and for comprehensive research on aging and health from the Ministry of Health and Welfare of Japan, and by special coordination funds for promoting science and technology (Strategic Promotion System for Brain Science) from the Science and Technology Agency of Japan.
References
- 1.Pugsley W, Klinger L, Paschalis C, et al. Microemboli and cerebral impairment during cardiac surgery. Vasc Surg 1990;24:34-43 [Google Scholar]
- 2.Georgiadis D, Grosset D, Kelman A, Fairchney A, Lees K. Prevalence and characteristics of intracranial microemboli signals in patients with different types of prosthetic cardiac valves. Stroke 1994;25:587-592 [DOI] [PubMed] [Google Scholar]
- 3.Rams JJ, Davis AD, Lolley MD, Berger PM, Spencer MP. Detection of microemboli in patients with artificial heart valves using transcranial Doppler: preliminary observations. J Heart Valve Dis 1993;2:37-41 [PubMed] [Google Scholar]
- 4.Siebler M, Sitzer M, Steinmetz H. Detection of intracranial emboli in patients with symptomatic extracranial carotid stenosis. Stroke 1992;23:1652-1654 [DOI] [PubMed] [Google Scholar]
- 5.Seibler M, Sitzer M, Rose G, Bendfeldt D, Steinmetz H. Silent cerebral embolism caused by neurologically symptomatic high-grade carotid stenosis. Brain 1993;116:1005-1015 [DOI] [PubMed] [Google Scholar]
- 6.Babikian V, Hyde C, Pochay V, Winter M. Clinical correlates of high-intensity transient signals detected on transcranial Doppler sonography in patients with cerebrovascular disease. Stroke 1994;25:1570-1573 [DOI] [PubMed] [Google Scholar]
- 7.Tegeler CH, Hitchings LP, Eicke M, et al. Microemboli detection in stroke associated with atrial fibrillation (abstr). J Cardiovasc Tech 1990;9:283-284 [Google Scholar]
- 8.Grosset DG, Georgiadis D, Abdullah I, Bone I, Lees KR. Doppler emboli signals vary according to stroke subtype. Stroke 1994;25:382-384 [DOI] [PubMed] [Google Scholar]
- 9.Siebler M, Kleinschmidt A, Sitzer M, Steinmetz H, Freund HJ. Cerebral microembolism in symptomatic and asymptotic high-grade internal carotid artery stenosis. Neurology 1994;44:615-618 [DOI] [PubMed] [Google Scholar]
- 10.Russell D, Madden KP, Clark WM, Sandset PM, Zivin JA. Detection of arterial emboli using Doppler ultrasound in rabbits. Stroke 1991;22:253-258 [DOI] [PubMed] [Google Scholar]
- 11.Markus HS, Brown NM. Differentiation between different pathological cerebral embolic materials using transcranial Doppler in an in vitro model. Stroke 1993;24:1-5 [DOI] [PubMed] [Google Scholar]
- 12.Settle MD, Angeli S, Stara I, Finocchi C, Gandolfo C. Microembolic signals with serial transcranial Doppler monitoring in acute focal ischemic deficit: a local phenomenon? Stroke 1997;28:1310-1313 [DOI] [PubMed] [Google Scholar]
- 13.Tong DC, Albers GW. Transcranial Doppler-detected microemboli in patients with acute stroke. Stroke 1995;26:1588-1592 [DOI] [PubMed] [Google Scholar]
- 14.Tegeler CH, Hitchings LP, Eicke M, et al. Carotid emboli predict poor outcome in stroke (abstr). Stroke 1993;24:186 [Google Scholar]
- 15.Levi CR, O'Malley HM, Fell G, et al. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy: high microembolic signal loads predict postoperative cerebral ischemia. Brain 1997;120:621-629 [DOI] [PubMed] [Google Scholar]
- 16.Tong DC, Bolger A, Albers G. Incidence of transcranial Doppler-detected cerebral microemboli in patients referred for echocardiography. Stroke 1994;25:2138-2141 [DOI] [PubMed] [Google Scholar]
- 17.Spencer MP, Thomas GL, Nicholls SC, Sauvage LR. Detection of middle cerebral artery emboli during carotid endarterectomy using transcranial Doppler ultrasonography. Stroke 1990;21:415-423 [DOI] [PubMed] [Google Scholar]
- 18.Spencer MP. Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke 1997;28:685-691 [DOI] [PubMed] [Google Scholar]
- 19.Baird A, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab 1998;18:583-609 [DOI] [PubMed] [Google Scholar]
- 20.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231-241 [DOI] [PubMed] [Google Scholar]
- 21.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423-429 [PMC free article] [PubMed] [Google Scholar]
- 22.Kidwell CS, Alger JR, Salle FD, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174-1180 [DOI] [PubMed] [Google Scholar]
- 23.Lyden P, Brott T, Tilley B, et al. NINDS TPA Stroke Study Group: improved reliability of the NIH stroke scale using video training. Stroke 1994;25:2220-2226 [DOI] [PubMed] [Google Scholar]
- 24. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 25.Sitzer M, Muller W, Siebler M, et al. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke 1995;26:1231-1233 [DOI] [PubMed] [Google Scholar]
- 26.Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999;354:1594-1597 [DOI] [PubMed] [Google Scholar]
- 27.Roh JK, Kang DW, Lee SH, Yoon BW, Chang KH. Significance of acute multiple brain infarction on diffusion-weighted imaging. Stroke 2000;31:688-694 [DOI] [PubMed] [Google Scholar]
- 28.Toyoda K, Yasaka M, Nagata S, Yamaguchi T. Aortogenic embolic stroke: a transesophageal echocardiographic approach. Stroke 1992;23:1056-1061 [DOI] [PubMed] [Google Scholar]
- 29.Amarenco P, Cohen A, Baudrimont M, Bousser MG. Transesophageal echocardiographic detection of aortic arch disease in patients with cerebral infection. Stroke 1992;23:1005-1009 [DOI] [PubMed] [Google Scholar]