Abstract
BACKGROUND AND PURPOSE: We assumed that patients with surgically treated aneurysmal subarachnoid hemorrhage (SAH) might have more lesions than those revealed by CT that could be visible on MR images.
METHODS: We conducted a retrospective study of a series of 147 patients with aneurysmal SAH who were treated surgically within 3 days of the onset of SAH. One hundred four patients (mean age, 48.8 years) underwent MR imaging studies 2.1 to 5.6 years (mean, 3.3 years) postoperatively.
RESULTS: Eighty-four (81%) patients presented a total of 152 areas of increased signal intensity on T2-weighted images, consistent with infarction; 48% of the patients had lesions in the frontal lobes. CT performed 3 months postoperatively revealed hypodense areas on the scans of only 57% of the patients and showed lesions in the frontal lobes of only 16% of the patients.
CONCLUSION: Patients who undergo early surgery for aneurysmal SAH have more lesions than are revealed by CT. The difference is remarkable, especially in the frontal lobes.
Aneurysmal subarachnoidal hemorrhage (SAH) is a devastating disease. The current overall mortality rate is 50% (1, 2). It is estimated that 10% to 60% of patients with SAH die before receiving medical attention (1–4). In addition to initial bleeding and subsequent bleeding, delayed cerebral arterial vasospasm is a major cause of morbidity and mortality (3, 5, 6). Vasospasm is observed on the angiograms of ≤70% to 90% of the patients during the first 2 weeks after the onset of SAH (7, 8). Moreover, ≤50% of the patients with angiographic vasospasm develop ischemic neurologic deficits (9).
MR imaging is superior to CT in revealing infarctions (10). This becomes even more evident in the basal regions of the brain, where artifacts from bony structures of the skull base compromise the ability of CT to detect lesions. Moreover, on MR images, the diameter of aneurysm clip artifacts is only 20 to 30 mm (11–15), whereas on CT scans, the basal region is often unreadable after clipping or coiling of an aneurysm (16). Furthermore, MR imaging is superior to CT in detecting small lacunar lesions, particularly those located deep within the cerebral hemispheres and in the brain stem and cerebellum (12, 17).
We assumed that patients with surgically treated aneurysmal SAH who undergo MR imaging may have more infarctions revealed than are seen on the CT scans. Also, patients suffering from SAH might harbor global and diffuse damage to the brain tissue that could present as leukoaraiosis on late MR images and could be non-visible on CT scans.
Methods
Patients
Of a total of 433 patients with aneurysmal SAH who were admitted to our hospital between April 1, 1992, and November 30, 1994, a consecutive series of 147 (34%) who had undergone early surgery (both sexes; age range, 16–70 years) was scrutinized. The diagnosis of SAH had been made based on CT findings. If no blood was visible on the CT scans, the patient was excluded from our study. The aneurysms had been verified by four-vessel angiography. Patients selected for the study were surgically treated within 72 hours after hemorrhage (early surgery). Patients who experienced subsequent bleeding, either before or after admission, were excluded from the study, as were patients with posterior circulation aneurysms and those who had undergone late surgery. Other exclusion criteria were intraparenchymal hematomas revealed by CT, pregnancy, severe complicating illness (hepatic or renal failure, recent myocardial infarction, terminal malignancy, AIDS), severe psychiatric illness, and coagulation disorders or anticoagulant medication. Moreover, the presence of severe vasospasm (>50% narrowing of the vessel lumen) on angiograms obtained at admission, indicating earlier SAH, excluded patients from the study. Ten patients had undergone surgery for unruptured aneurysms after a recovery period (1–5 months).
The patients were invited by telephone and letter to undergo follow-up MR imaging studies of the brain from October 1, 1994, to June 30, 1998, which was 2 to 6 years (mean, 3.3 years) postoperatively. A total of 14 patients died during the follow-up period. Twenty-six patients did not reply to the invitation, one patient was too heavy for our equipment, and MR imaging had to be interrupted for two patients who suffered from claustrophobia. A total of 104 patients (age range, 21–73 years; mean age, 48.8 years) underwent the MR imaging studies (Table 1). The study was approved by the ethical committee of the hospital.
TABLE 1:
Demography of the patient material
Computed Tomography
CT (Philips, Tomoscan) was performed 3 months after the onset of SAH and surgery (127 patients) at the time of clinical follow-up. The CT scans were evaluated by an experienced neuroradiologist (A.S.) with no knowledge of the patient's clinical course or neurologic status. Hypodense areas, consistent with infarction, were found on the scans of 73 (57%) of the 127 patients.
MR Imaging
MR imaging studies were performed of a total of 104 patients. A Magnetom SP-42 imager (1.0 T; Siemens, Erlangen, Germany) was used for 40 patients, and a Magnetom Vision (1.5 T, Siemens) was used for 64 patients. Standard axial T1-weighted (570–600/15 [TR/TE]; matrix, 256 × 256) and T2-weighted (2000–3500/85–93; matrix, 190 × 256) sequences were obtained. Also, coronal T2-weighted and gadolinium- diethylenetriamine penta-acetic acid-enhanced (Magnevist, Schering, Berlin; Omniscan, Nycomed, Oslo) (0.2 mmol/kg of body weight) axial T1-weighted images were obtained. A fluid-attenuated inversion recovery sequence (9999/85; matrix, 154 × 256) was available and was used with the 1.5-T imager for 64 patients.
The images were analyzed by an experienced neuroradiologist (O.S.) who was not aware of the patient's clinical course, neurologic status, or previous CT findings. The number of areas of high signal on T2-weighted and fluid-attenuated inversion recovery images and low signal on T1-weighted images, indicating infarction, was counted and their size measured. Small high signal foci on T2- and proton density–weighted images were counted. Degree of leukoaraiosis was scored using a four-point scale: 0, normal; 1, mild; 2, moderate; 3, serious (21).
Measurements of signal intensity were obtained for 18 patients, each with a ruptured aneurysm of the middle cerebral artery as their only presenting aneurysm and each of whom underwent imaging at 1.0 T. The measured areas were basal ganglia (thalamus, head of caudate nucleus, and putamen), deep white matter on the levels of third ventricle and roof of lateral ventricles, centrum semiovale, and frontal cortex. Measurement was not included if a visible area of high signal intensity consistent with infarction was part of the region of interest. Measurements were obtained by two independent readers (R.K., T.A.).
Results
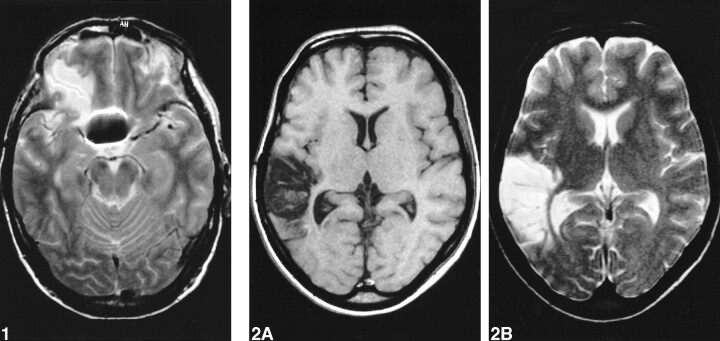
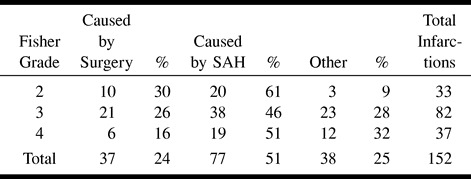
Eighty-four (81%) of the 104 patients presented a total of 152 larger areas (>5 mm) of increased signal intensity on T2-weighted and fluid-attenuated inversion recovery images and lowered signal intensity on T1-weighted images, consistent with infarction. Thirty-seven (24%) lesions were caused by surgery (22) (Fig 1); 115 lesions were considered to be vascular in origin, 77 (51%) of which were caused by SAH (Fig 2); the remaining 38 (25%) were not typically either surgically or SAH-related (Tables 2 and 3).
fig 1.
T2-weighted image shows lesion caused by surgery in the base of the right frontal lobe.fig 2. Images show large infarction caused by arterial vasospasm in the right temporal lobe.
A, T1-weighted image.
B, T2-weighted image.
TABLE 2:
Number of infarctions according to Hunt and Hess Grade on arrival
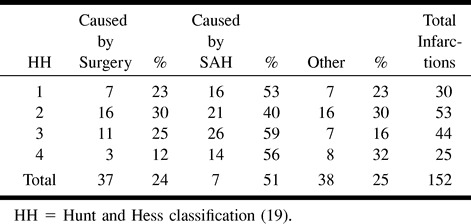
Ninety-seven patients underwent imaging with both techniques (CT at 3 months and MR imaging). Of these 97 patients, 77 (79%) had infarction revealed by MR imaging, whereas CT revealed hypodense areas in the brain for only 57 (59%) patients (χ2 test, P < .005). Sixteen patients presented with lesions in the frontal lobe as revealed by CT. Of the same 97 patients, 47 patients had lesions in the frontal lobe revealed by MR imaging (16 versus 48; χ2 test, P < .001).
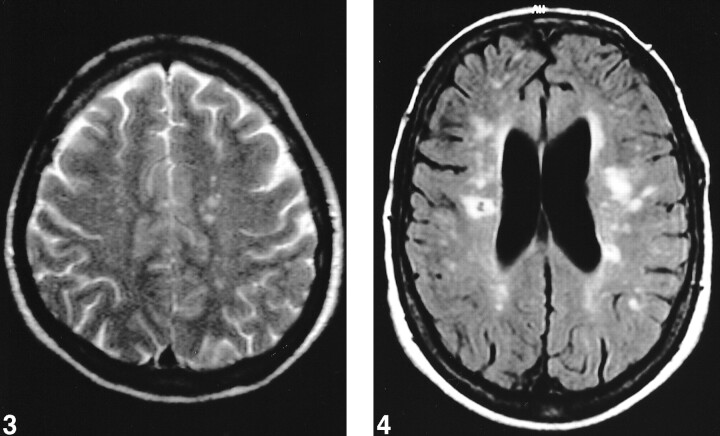
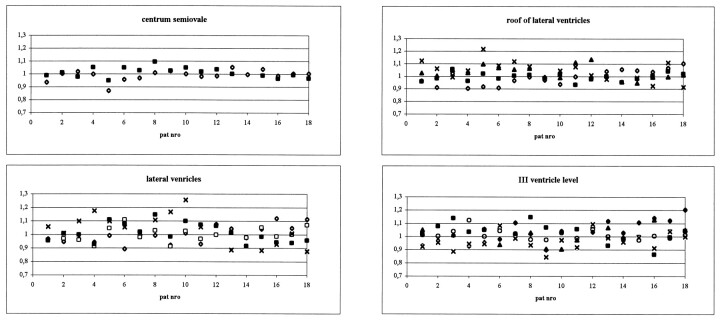
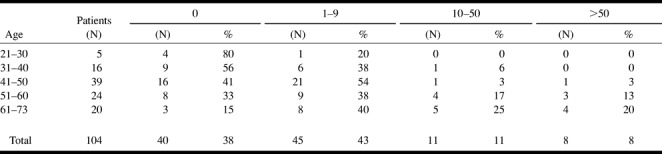
Signs of hemosiderin were present in eight (7%) of the areas of increased signal intensity. Sixty-four (62%) patients presented small high signal foci (<5 mm) on the T2-weighted images (Fig 3). Eight (8%) patients each had >50 of these lesions, 11 (11%) patients each had 10 to 50 of these lesions, and 45 (43%) patients each had one to nine of these lesions. Forty (38%) patients did not have any small high signal foci on the T2-weighted images. The number of these foci increased in association with age. Fifty percent of the patients having >50 foci were 61 years old or older. No one younger than 41 years had >50 foci (Table 4).
fig 3.
T2-weighted image shows small high signal foci in left centrum semiovale.fig 4. Fluid-attenuated inversion recovery image shows severe leukoaraiosis with increased periventricular signal intensity, numerous high signal foci, and a small infarction in the right centrum semiovale
TABLE 4:
The number of small high signal foci according to age
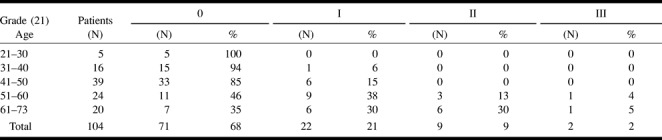
Leukoaraiosis was grade 0 in 71 (68%) of the patients, grade I in 22 (21%), grade II in nine (9%), and grade III in two (2%) (Table 5 and Fig 4). The length and depth of hypotension, measured as the time period of systolic arterial pressure <100 mm Hg and the lowest mean arterial pressure during surgery, did not affect the presence or severity of leukoaraiosis.
TABLE 5:
The degree of leukoaraiosis according to age
The images of all the patients had artifacts from the clips. Some of the patients each had numerous clips. The average size of the artifact was 23 × 19 × 26 mm for one clip.
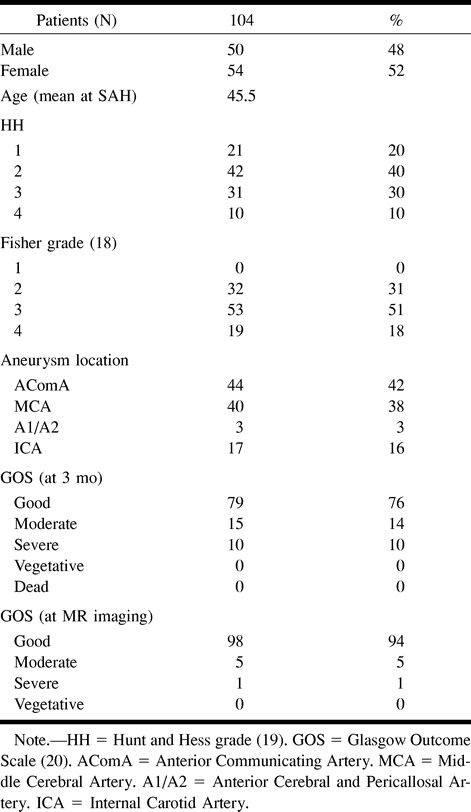
We measured the signal intensity of gray matter, deep white matter, and the basal ganglia of 18 patients. We compared the absolute values between the two hemispheres. No significant differences were found between the sides with the initial bleeding and the contralateral sides (Fig 5).
fig 5.
Ratio of signal intensities between the sides of initial bleeding and the contralateral sides in various regions of interest in 18 patients with ruptured aneurysms of the middle cerebral artery
Discussion
MR imaging has well-documented advantages over CT. Because of better resolution, MR imaging is more reliable in detecting ischemic lesions in brain tissue (12, 17). This becomes even more evident in the basal regions of the skull, where CT is compromised with artifacts, especially after surgery for intracranial arterial aneurysms when the aneurysm clip causes artifacts on CT scans (12, 13).
Many lesions that were visible on the MR images obtained 2 to 6 years after the surgical treatment of the SAH were either undetected on the CT scans obtained 3 months postoperatively or did not exist at that time. On the CT scans obtained 3 months postoperatively, no hypodense areas in the brain tissue of 41% of the 97 patients who underwent both imaging methods were shown. With MR imaging, the percentage of the same patients without tissue damage was as low as 21%. Especially remarkable is the increase of lesions detected in the frontal lobes, 16 versus 47, many of which might be related to surgery. This calls for further investigation for the significance of this finding. Some of the new lesions could be explained by the time interval between CT and MR imaging (range, 1.8–5.4 years; mean, 3.1 years), but considering the age of the patients (mean, 48.8.years), it is not likely. Infarctions seen on CT scans correlated well with cognitive deficits (23). However, there also have been reports of patients with severe cognitive deficits without CT findings (24). In these cases, MR imaging is evidently beneficial for the patient.
Small, high signal foci in the white matter are common incidental findings on T2-weighted images, particularly of older patients. These asymptomatic lesions have no known clinical importance (17, 25). In this study, the number of high signal foci was similar to the numbers in earlier reports, and the number increased with age, as the numbers did in earlier reports (26, 27).
Leukoaraiosis occurs more often in patients with histories of stroke and in patients with cognitive deterioration of presumed vascular origin (11, 28–31). The risk factors associated with leukoaraiosis are aging, arterial hypertension, diabetes mellitus, and cardiac diseases (25, 27, 30, 32, 33). Moreover, a correlation of leukoaraiosis with hypotensive crises has been reported (34). Patients with normal pressure hydrocephalus have a high prevalence of alterations in the white matter that are detectable by either CT or MR imaging (35). In this study, leukoaraiosis was present in 33 (32%) of 104 patients. In 11 (11%) patients, leukoaraiosis was considered to be moderate or severe. In our study, the frequency of leukoaraiosis was greater among patients with histories of hypertension (36% versus 23%), although the difference was not statistically significant (χ2 test, P = .09).
Hemosiderin was visible on the images of eight (8%) patients only, compared with 23 (22%) patients with intracerebral hematoma revealed by postoperative CT (104 patients). It thus appears that surgery for anterior circulation aneurysms or SAH does not increase the frequency or severity of degenerative brain alterations seen on MR images.
Conclusion
Patients suffering from SAH because of a ruptured anterior aneurysm harbor more lesions in the brain tissue at 2 to 6 years from ictus than might be suspected on the basis of early CT studies, especially in the frontal lobes.
TABLE 3:
Number of infarctions according to the Fisher Grade on primary CT
Acknowledgments
The authors thank Professor Seppo Sarna for statistical assessment.
Footnotes
This study was supported by the Finnish Medical Foundation and Maire Taponen Foundation.
Address reprint requests to Riku Kivisaari, MD, Department of Neurosurgery, Helsinki University Central Hospital, Topeliuksenkatu 5, 00260 Helsinki, Finland.
References
- 1.Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage: a population-based study. Stroke 1993;24:1649-1654 [DOI] [PubMed] [Google Scholar]
- 2.Inagawa T, Tokuda Y, Ohbayashi N, Takaya M, Moritake K. Study of aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Stroke 1995;26:761-766 [DOI] [PubMed] [Google Scholar]
- 3.Hernesniemi J, Vapalahti M, Niskanen M, et al. One-year outcome in early aneurysm surgery: a 14 years experience. Acta Neurochir (Wien) 1993;122:1-10 [DOI] [PubMed] [Google Scholar]
- 4.Meyer FB, Morita A, Puumala MR, Michael R, Nichols DA. Medical and surgical management of intracranial aneurysms. Mayo Clin Proc 1995;70:153-172 [DOI] [PubMed] [Google Scholar]
- 5.Öhman J, Servo A, Heiskanen O. Risk factors for cerebral infarction in good-grade patients after aneurysmal subarachnoid hemorrhage and surgery: a prospective study. J Neurosurg 1991;74:14-20 [DOI] [PubMed] [Google Scholar]
- 6.Solenski NJ, Haley EC Jr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study: Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med 1995;23:1007-1017 [DOI] [PubMed] [Google Scholar]
- 7.Inagawa T. Effect of early operation on cerebral vasospasm. Surg Neurol 1990;33:239-246 [DOI] [PubMed] [Google Scholar]
- 8.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1985;16:562-572 [DOI] [PubMed] [Google Scholar]
- 9.Ljunggren B, Brandt L, Kagstrom E, Sundbarg G. Results of early operations for ruptured aneurysms. J Neurosurg 1981;54:473-479 [DOI] [PubMed] [Google Scholar]
- 10.Awad I, Modic M, Little JR, Furlan AJ, Weinstein M. Focal parenchymal lesions in transient ischemic attacks: correlation of computed tomography and magnetic resonance imaging. Stroke 1986;17:399-403 [DOI] [PubMed] [Google Scholar]
- 11.Burtscher M, Owman T, Romner B, Ståhlberg F, Holtås S. Aneurysm clip MR artifacts: titanium versus stainless steel and influence of imaging parameters. Acta Radiol 1998;39:70-76 [DOI] [PubMed] [Google Scholar]
- 12.Gilman S. Medical progress: imaging the brain: first of two parts. New Engl J Med 1998;338:812-820 [DOI] [PubMed] [Google Scholar]
- 13.Holtås S, Olsson M, Romner B, Larsson EM, Saveland H, Brandt L. Comparison of MR imaging and CT in patients with intracranial aneurysm clips. AJNR Am J Neuroradiol 1988;9:891-897 [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins A, Hadley DM, Teasdale GM, Condon B, Macpherson P, Patterson J. Magnetic resonance imaging of acute subarachnoid hemorrhage. J Neurosurg 1988;68:731-736 [DOI] [PubMed] [Google Scholar]
- 15.Romner B, Olsson M, Ljunggren B, et al. Magnetic resonance imaging and aneurysm clips: magnetic properties and image artifacts. J Neurosurg 1989;70:426-431 [DOI] [PubMed] [Google Scholar]
- 16.Matsumura K, Matsuda M, Handa J, Todo G. Magnetic resonance imaging with aneurysmal subarachnoid hemorrhage: comparison with computed tomography scan. Surg Neurol 1990;34:71-78 [DOI] [PubMed] [Google Scholar]
- 17.Edelman RR. Medical progress: magnetic resonance imaging (first of two parts). New Engl J Med 1993;328:708-716 [DOI] [PubMed] [Google Scholar]
- 18.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoidal hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980;6:1-9 [DOI] [PubMed] [Google Scholar]
- 19.Hunt WE, Hess RM. Surgical risks as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14-20 [DOI] [PubMed] [Google Scholar]
- 20.Jennet B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975;1:480-484 [DOI] [PubMed] [Google Scholar]
- 21.Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol 1993;50:818-824 [DOI] [PubMed] [Google Scholar]
- 22.Kivisaari RP, Salonen O, Öhman J. Basal brain injury in aneurysm surgery. Neurosurgery 2000;46:1070-1076 [DOI] [PubMed] [Google Scholar]
- 23.Vilkki J, Holst P, Öhman J, Servo A, Heiskanen O. Cognitive deficits related to computed tomographic findings after surgery for a ruptured intracranial aneurysm. Neurosurgery 1989;25:166-172 [DOI] [PubMed] [Google Scholar]
- 24.Vilkki J. Amnesic syndromes after surgery of anterior communicating artery aneurysms. Cortex 1985;21:431-444 [DOI] [PubMed] [Google Scholar]
- 25.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652-659 [DOI] [PubMed] [Google Scholar]
- 26.Salonen O, Autti T, Raininko R, Ylikoski A, Erkinjuntti T. MRI of the brain in neurologically healthy middle-aged and elderly individuals. Neuroradiology 1997;39:537-545 [DOI] [PubMed] [Google Scholar]
- 27.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 1994;44:1246-1252 [DOI] [PubMed] [Google Scholar]
- 28.Cadelo M, Inzitari D, Pracucci G, Mascalchi M. Predictors of leukoaraiosis in elderly neurological patients. Cerebrovasc Dis 1991;1:345-351 [Google Scholar]
- 29.Inzitari D, Diaz F, Fox A, et al. Vascular risk factors and leuko-araiosis. Arch Neurol 1987;44:42-47 [DOI] [PubMed] [Google Scholar]
- 30.Longstreth WT Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The Cardiovascular Health Study. Stroke 1996;27:1274-1282 [DOI] [PubMed] [Google Scholar]
- 31.Streifler JY, Eliasziw M, Benavente OR, Hachinski VC, Fox AJ, Barnett HJ, for the North American Symptomatic Carotid Endarterectomy Trial. Lack of relationship between leukoaraiosis and carotid artery disease. Arch Neurol 1995;52:21-24 [DOI] [PubMed] [Google Scholar]
- 32.Erkinjuntti T, Gao F, Lee DH, Eliasziw M, Merskey H, Hachinski VC. Lack of difference in brain hyperintensities between patients with early Alzheimer's disease and control subjects. Arch Neurol 1994;51:260-268 [DOI] [PubMed] [Google Scholar]
- 33.Ljindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. Stroke 1994;25:929-934 [DOI] [PubMed] [Google Scholar]
- 34.McQuinn BA, O'Leary DH. White matter lucencies on computed tomography, subacute arteriosclerotic encephalopathy (Binswanger's disease), and blood pressure. Stroke 1987;18:900-905 [DOI] [PubMed] [Google Scholar]
- 35.Bradley WG, Whittemore AR, Watanabe AS, Davis SJ, Teresi LM, Homyak M. Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible origin of normal-pressure hydrocephalus. AJNR Am J Neuroradiol 1991;12:31-39 [PMC free article] [PubMed] [Google Scholar]