Abstract
Buprenorphine is an essential component of analgesic protocols in common marmosets (Callithrix jacchus). The use of buprenorphine HCl (BUP) and sustained-release buprenorphine (BSR) formulations has become commonplace in this species, but the pharmacokinetics have not been evaluated. Healthy adult (age, 2.4 to 6.8 y; 6 female and 6 male) common marmosets were enrolled in this study to determine the pharmacokinetic parameters, plasma concentration–time curves, and any apparent adverse effects of these compounds. Equal numbers of each sex were randomly assigned to receive BUP (0.02 mg/kg IM) or BSR (0.2 mg/kg SC), resulting in peak plasma concentrations (mean ± 1 SD) of 15.2 ± 8.1 and 2.8 ± 1.2 ng/mL, terminal phase t1/2 of 2.2 ± 1.0 and 32.6 ± 9.6 h, and AUC0-last of 16.1 ± 3.7 and 98.6 ± 42.7 ng×h/mL. The plasma concentrations of buprenorphine exceeded the proposed minimal therapeutic threshold (0.1 ng/mL) at 5 and 15 min after BUP and BSR administration, showing that both compounds are rapid-acting, and remained above that threshold through the final time points of 8 and 72 h. Extrapolation of the terminal elimination phase of the mean concentration–time curves was used to develop the clinical dosing frequencies of 6 to 8 h for BUP and 3.0 to 3.5 d for BSR. Some adverse effects were observed after the administration of BUP to common marmosets in this study, thus mandating judicious use in clinical practice. BSR provided a safe, long-acting option for analgesia and therefore can be used to refine analgesic protocols in this species.
Abbreviations: BUP, buprenorphine HCl; BSR, sustained-release buprenorphine
Renewed interest in common marmosets (Callithrix jacchus) as models for biomedical research is being driven by the fields of gene-editing technology, neuroscience, and infectious disease.7,19,23,28,32,34 Research study aims as well as clinical interventions necessitate appropriate pain management protocols for these animals. Current recommendations for analgesia in common marmosets are extrapolated from other species or are based on anecdotal evidence. Dosage, duration of action, and potential adverse effects of analgesics used in this species require evaluation to refine guidelines for their use in clinical practice.
Buprenorphine is the most commonly used opioid analgesic in many NHP species, including common marmosets.6,24,33 It is a partial µ-opioid receptor agonist used for the treatment of moderate to severe pain, when included as part of a multimodal pain management approach. Buprenorphine's widespread use in laboratory animal medicine is attributed to a relatively long duration of action and favorable safety profile when compared with other available opioid agents. A considerable amount of data on the efficacy and recommended dosage of buprenorphine is available for various laboratory species, including mice, rats, rabbits, cats, dogs, and pigs.2,10,14,17,20,35,41
Formulations of sustained-release buprenorphine (BSR) have been developed and have become commercially available, providing a longer acting option than the standard buprenorphine HCl (BUP) formulation. Longer-acting compounds are preferable, because they have the potential to improve animal welfare by reducing handling and the number of injections per animal, reducing adverse effects associated with peak plasma buprenorphine concentrations, and avoiding repeated trough plasma buprenorphine concentrations, which may result in inadequate analgesia. The uses of both formulations of buprenorphine in several common laboratory animal species, including mice, rats, guinea pigs, dogs, cats, and macaques, have been described.5,9,10,16,30,39
The pharmacokinetics of BUP and BSR have already been described in both cynomolgus and rhesus macaques, the most commonly used NHP in biomedical research. The widely accepted dosage range for BUP in macaques is 0.01 to 0.03 mg/kg administered either intramuscularly or intravenously. Dosing recommendations provided in one study, using a plasma threshold of 0.1 ng/mL, suggest that for macaques, BUP at 0.01 mg/kg IM should be given every 6 to 8 h and at 0.03 mg/kg IM should be given every 12 h.30 The same study showed that a single subcutaneous dose of BSR at 0.2 mg/kg can be given every 5 d. A similar study demonstrated that BUP given to rhesus macaques at 0.03 mg/kg either intravenously or intramuscularly maintains plasma concentrations above 0.1 ng/mL for 24 and 12 h, respectively.18
BUP dosing recommendations for New World NHP, such as common marmosets, tend to be lower than those of Old World species, such as macaques. These recommendations are a result of the more profound adverse effects (for example, respiratory depression, apnea, and death) seen in common marmosets when higher doses of BUP are used or when BUP is used in combination with anesthetic agents, such as alfaxalone or isoflurane.1,4 Guidelines for BUP dosing in common marmosets range from 0.005 to 0.02 mg/kg IM.4,21,24
The purpose of the current study was to investigate the plasma concentration of buprenorphine over time after the administration of BUP and BSR in common marmosets. Observations were made to determine whether adverse effects occurred after the administration of BUP and BSR. We hypothesized that BUP dosed at 0.02 mg/kg IM would remain above a plasma threshold of 0.1 ng/mL for 6 to 8 h and that BSR dosed at 0.2 mg/kg SC would remain above the same threshold for 72 h.
Materials and Methods
Animals.
The animals used for this research study were housed at the Wisconsin National Primate Research Center at the University of Wisconsin–Madison, an AAALAC-accredited facility. The study was performed in strict accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the Public Health Service policy on the Humane Care and Use of Laboratory Animals.3,15,31 The protocol was approved by the University of Wisconsin–Madison's College of Letters and Sciences and Vice Chancellor for Research and Graduate Education Centers IACUC.
Twelve (6 female, 6 male) healthy adult (age, 2.4 to 6.8 y old) common marmosets (Callithrix jacchus) were used in the study. Each marmoset in the study underwent a routine physical exam by a veterinarian semiannually and prior to being enrolled in the study. All animals were socially housed in female–male pairs within enclosures measuring 0.6 × 0.9 × 1.8 m or 0.6 × 1.2 × 1.8 m. The marmosets were maintained on a 12:12-h light:dark schedule, temperature range of 24 to 30 °C, and a relative humidity range of 30% to 70%. Animals had free access to water and food (Mazuri Callitrichid High Fiber Diet no. 5MI6, Purina Mills International, St Louis, MO) and were provided various supplemental food items twice daily.
Drugs.
The animals were assigned randomly into 2 groups of 6, with equal numbers of each sex in both groups. Each marmoset was weighed prior to administration of the drug, to calculate an accurate dose. Unsedated animals were restrained by using a marmoset tube restraint device to which they had been acclimated previously for dose administration and sample collection. No sedatives or other pharmacologic agents were used during the study; the marmosets did not undergo a painful stimulus or procedure prior to administration of the drugs. The first group received a single IM injection of 0.02 mg/kg BUP (Reckitt Benckiser Healthcare, Hull, England) in the right quadriceps muscle group. The dose and route were chosen to determine the longest effective duration that can be achieved by the intramuscular route and to demonstrate potential adverse effects at the higher end of the dosage range. The second group of animals received a single subcutaneous injection of 0.2 mg/kg BSR (Buprenorphine SR-LAB 1 mg/mL polymeric formulation, Zoopharm, Fort Collins, CO) on the right ventral abdomen. This dose was chosen based on a previously published dose used in macaques30 and the experience of the authors.
Sample collection.
Blood samples (0.3 to 0.6 mL) were collected via femoral venipuncture by using 1-mL heparinized syringes. Samples were collected from the animals in the BUP group at 5, 20, and 30 min and 1, 2, 4, and 8 h after injection. The marmosets in the BSR group had samples collected at 15 and 30 min and at 1, 8, 24, 48, and 72 h after injection. The marmosets were returned to their nest box or home enclosure between sample-collection time points. Blood samples were immediately placed on wet ice after collection. The blood then was processed by using centrifugation (500 × g) for 20 to 30 min, and the plasma was separated and stored in cryogenic tubes at –20 °C. The plasma samples were shipped on dry ice to the analytic laboratory (Center for Human Toxicology, University of Utah, Salt Lake City, UT).
Observations.
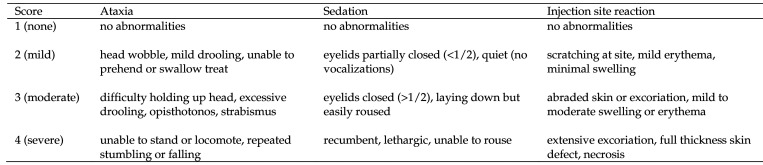
All marmosets were observed in the tube restraint device at each blood collection time point during the study. The marmosets were evaluated for general wellbeing and for potential adverse effects of buprenorphine after administration. Any abnormalities were recorded. Ataxia, sedation, and injection site reaction were rated at each time point using a numeric scoring system. Scores (1, none; 2, mild; 3, moderate; and 4, severe) were assigned according to inclusion criteria (Figure 1). The score defaulted to the highest number in which any criteria were met. Attempts were made to record respiratory rate but were discontinued due to the difficulty of obtaining accurate results.
Figure 1.
Criteria for ataxia score, sedation score, and injection site reaction score. The highest score for which any criteria were met was assigned to each corresponding time point.
Sample analysis.
The plasma samples were analyzed by using liquid chromatography–electrospray ionization–tandem mass spectrometry developed and validated at the University of Utah Center for Human Toxicology.8,27 Plasma samples (100 µL) were aliquoted and underwent pH adjustment to 10.0 with addition of 2 N NaOH. Liquid–liquid extraction was applied by the addition of 2 mL of n-butyl chloride:acetonitrile (4:1, v/v), mixing, and centrifugation (1200 × g). The organic phase was transferred, acidified, and dried down. Extracts were reconstituted in 50 μL 0.1% formic acid in water:acetonitrile (95:5, v/v) and transferred to autosampler vials. An Agilent 1100 series LC system coupled with a Thermo Scientific TSQ Quantum Access Triple-Stage Quadruple mass spectrometer was used for analysis. An ODS–AQ 2.0 × 100 mm, 5-μm column (YMC, Wilmington, NC) was used. The concentration of the sample was determined by the peak area ratio of the analyte to its international standard, with comparison against a calibration curve that was generated from blank plasma fortified with known concentrations of analyte and its internal standard. The assay has a lower limit of quantification of 0.1 ng/mL for each analyte.
Data analysis.
Statistic and pharmacokinetic analyses were performed by using Phoenix WinNonlin 8.1 (Certara, Princeton, NJ). Plasma concentrations–time curves for buprenorphine were evaluated by using noncompartmental analysis. Peak plasma concentration (Cmax) and time at peak concentration (Tmax) were determined based on direct observations. The rate constant of the terminal elimination phase (λz) was calculated by using the slope of the best-fit log–linear regression of the terminal phase using 3 or more time points. The log–linear trapezoidal rule was used to determine the observed area under the curve (AUC0-last), and the area under the curve to infinity (AUC0-inf) was determined by using λz. The time of last measurable concentration (Tlast) was decided at the time of the study design and was the same for each marmoset. The last measurable concentration (Clast) was directly observed at Tlast. The terminal phase half-life (t1/2) was determined by dividing λz by the natural log of 2. The volume of distribution was determined by dividing the dose by AUC0-inf × λz. Clearance was determined by dividing the dose by AUC0-inf. Mean residence time was determined by dividing area under the first moment curve (AUMC0-inf) by AUC0-inf.
Results
Observations.
All of the common marmosets remained healthy throughout the entirety of the study. Adverse effects observed after BUP and BSR administration were temporary and resolved without intervention. All marmosets were released back into the Wisconsin National Primate Research Center marmoset colony after the study's completion.
All 6 marmosets in the BUP group received mild ataxia scores and 2 of the 6 received moderate ataxia scores for a least one time point during the study. All 6 marmosets in the BUP group received moderate sedation scores for 2 or more time points during the study. Marmosets that were in the BUP group had the greatest mean ataxia score (2.17) at the 4th time point (1.0 h) and the greatest mean sedation score (3.00) at the 4th (1.0 h) and 5th (2.0 h) time points (Table 1). No injection site reactions were associated with the BUP group.
Table 1.
Plasma concentration of buprenorphine, ataxia score, and sedation score at each time point following a single injection of BUP (n= 6)
| Time (h) | Plasma concentration (ng/mL) | Ataxia | Sedation |
| 0.08 | 13.8 ± 9.68 | 1.17 ± 0.41 | 1.50 ± 0.84 |
| 0.33 | 9.73 ± 4.05 | 1.67 ± 0.52 | 2.67 ± 0.52 |
| 0.50 | 8.08 ± 2.75 | 2.00 ± 0.63 | 2.83 ± 0.41 |
| 1.00 | 6.28 ± 2.24 | 2.17 ± 0.75 | 3.00 ± 0.00 |
| 2.00 | 1.74 ± 1.06 | 1.67 ± 0.52 | 3.00 ± 0.00 |
| 4.00 | 0.72 ± 0.38 | 1.67 ± 0.52 | 2.00 ± 0.00 |
| 8.00 | 0.30 ± 0.15 | 1.00 ± 0.00 | 1.83 ± 0.41 |
All data reported as mean ± 1 SD.
In contrast, 1 of the 6 marmosets in the BSR group received a mild ataxia score at a single time point. All 6 marmosets in the BSR group received mild sedation scores during the study, and none received a moderate score. Marmosets in the BSR group had the greatest mean ataxia score (1.17) at the 4th time point (8.0 h) and the greatest mean sedation score (2.00) at the 4th time point (Table 2). Injection-site reactions scores were elevated in 1 of the 6 marmosets in the BSR group. After administration of BSR, this marmoset was observed to be scratching at the site, resulting in mild localized erythema and swelling of the skin.
Table 2.
Plasma concentration of buprenorphine, ataxia score, and sedation score at each time point after a single injection of BSR (n= 6)
| Time (h) | Plasma concentration (ng/mL) | Ataxia | Sedation |
| 0.25 | 0.88 ± 0.29 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| 0.50 | 1.87 ± 0.56 | 1.00 ± 0.00 | 1.17 ± 0.41 |
| 1.00 | 2.56 ± 1.19 | 1.00 ± 0.00 | 1.67 ± 0.52 |
| 8.00 | 2.45 ± 1.10 | 1.17 ± 0.41 | 2.00 ± 0.00 |
| 24.0 | 1.56 ± 0.78 | 1.00 ± 0.00 | 1.33 ± 0.52 |
| 48.0 | 1.01 ± 0.52 | 1.00 ± 0.00 | 1.50 ± 0.55 |
| 72.0 | 0.55 ± 0.26 | 1.00 ± 0.00 | 1.17 ± 0.41 |
All data reported as mean ± 1 SD.
Pharmacokinetics.
Plasma concentration of buprenorphine over time varied between the individual marmosets in each group (Figures 2 and 3). All values for plasma concentration of buprenorphine measured throughout the study were above the lower limit of quantification (0.1 ng/mL) for the liquid chromatography-electrospray ionization-tandem mass spectrometry assay. Pharmacokinetic parameters for BUP and BSR in common marmosets are provided in Table 3.
Figure 2.
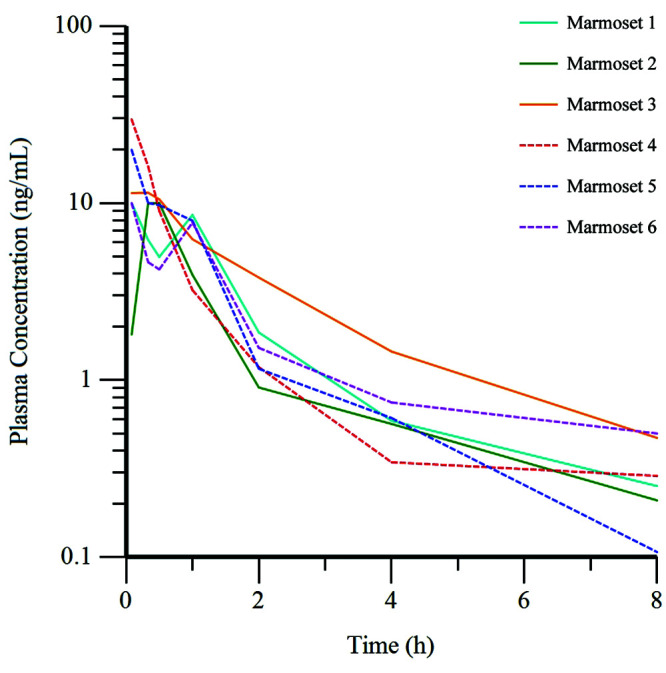
Plasma concentration of buprenorphine over time for each animal in the BUP group (n = 6) after the administration of BUP at 0.02 mg/kg IM. Dashed line, female marmosets; solid line, male marmosets.
Figure 3.
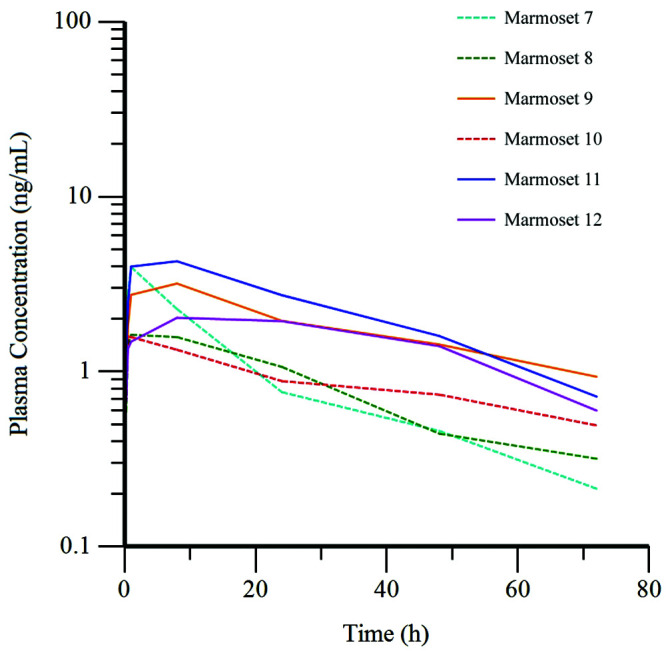
Plasma concentration of buprenorphine over time for each animal in the BSR (n = 6) group after the administration of BSR at 0.2 mg/kg SC. Dashed line, female marmosets; solid line, male marmosets.
Table 3.
Pharmacokinetic parameters of a single injection of BUP (n= 6) and BSR (n= 6) in common marmosets
| BUP | BSR | |
| Cmax (ng/mL) | 15.2 ± 8.10 | 2.78 ± 1.19 |
| Tmax (h) | 0.17 ± 0.13 | 4.42 ± 3.93 |
| λz (1/h) | 0.35 ± 0.12 | 0.02 ± 0.01 |
| t1/2 (h) | 2.23 ± 1.00 | 32.6 ± 9.57 |
| AUC0-last (ng×h/mL) | 16.1 ± 3.70 | 98.6 ± 42.7 |
| AUC0-inf (ng×h/mL) | 17.2 ± 3.78 | 125.8 ± 54.6 |
| Clast (ng/mL) | 0.31 ± 0.15 | 0.55 ± 0.26 |
| Tlast (h) | 8.00 ± 0.00 | 72.0 ± 0.00 |
| Volume (L/kg) | 0.20 ± 0.14 | 4.22 ± 2.07 |
| Clearance (L/h/kg) | 0.58 ± 0.21 | 0.90 ± 0.37 |
| MRT (h) | 2.27 ± 0.96 | 46.9 ± 14.5 |
All data reported as mean ± 1 SD.
Plasma concentrations did not reach the lower limit of quantification during the study; therefore, the time of last measurable concentration (Tlast) values were the same for all marmosets in either group (Table 3) as predetermined by the study design. Linear regression of the terminal phase of the mean concentration curves was performed and extrapolation was used to predict the times at which minimal therapeutic threshold concentrations would be met. The threshold of 0.1 ng/mL was reached at 8.3 h for the BUP group and 92.7 h for the BSR group. Raising the threshold to 0.3 ng/mL reduced the extrapolated times to 7.4 and 83.2 h, and raising it further to 0.5 ng/mL reduced the extrapolated times to 6.5 and 73.7 h.
A rapid and robust increase in the plasma concentration of buprenorphine occurred in marmosets after the intramuscular administration of BUP, with peak concentrations seen in 5 of the 6 marmosets at the first time point (5 min). Two marmosets in this group experienced a second peak in plasma concentration after an initial decline (Figure 2). The plasma concentration of buprenorphine in all marmosets in the BSR group reached peak levels more slowly after administration of this compound. The values increased until the third time point (1.0 h) for half of the marmosets and until the 4th time point (8.0 h) for the other half, with a decrease at subsequent time points for all marmosets (Figure 3).
Evaluation of the mean plasma concentrations at each time point for the BUP group show the greatest value (13.8 ng/mL) at the first time point (5 min) with a decrease at each subsequent time point (Table 1 and Figure 4). For the BSR group, the greatest and second greatest mean plasma concentration values (2.56 ng/mL and 2.45 ng/mL) were at the 3rd (1.0 h) and 4th (8.0 h) time points, respectively (Table 2 and Figure 5). Significant differences (P < 0.02, 0.05) were detected when comparing the mean Cmax for the BUP group (15.2 ng/mL) and BSR group (2.78 ng/mL) and the mean Tmax for the BUP group (0.17 h) and BSR group (4.42 h). Significant differences (P < 0.001, 0.01) were present between the mean terminal phase half-life of BUP (2.23 h) and BSR (32.6 h) and the mean AUC0-last of BUP (16.1 ng×h/mL) and BSR (98.6 ng×h /mL), as is expected due to chemical compositions of the different formulations (Table 3).
Figure 4.
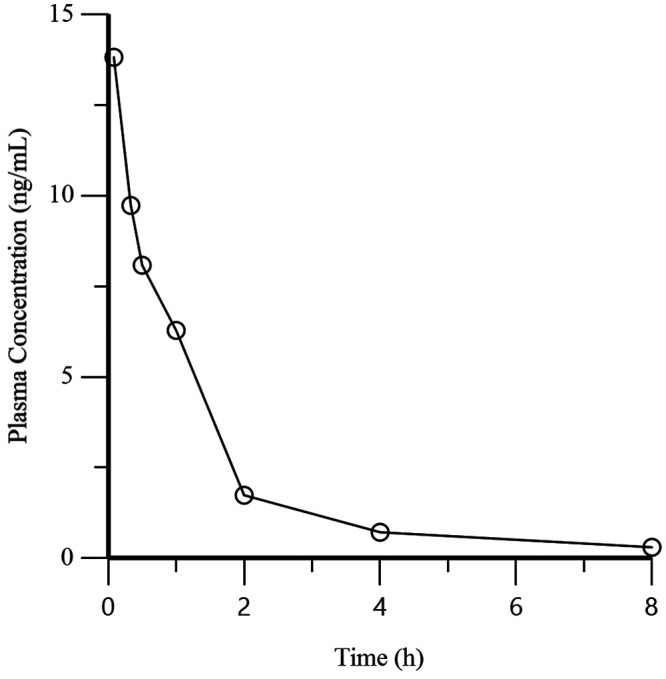
Mean plasma concentration of buprenorphine over time for the BUP (n = 6) group after the administration of BUP at 0.02 mg/kg IM.
Figure 5.
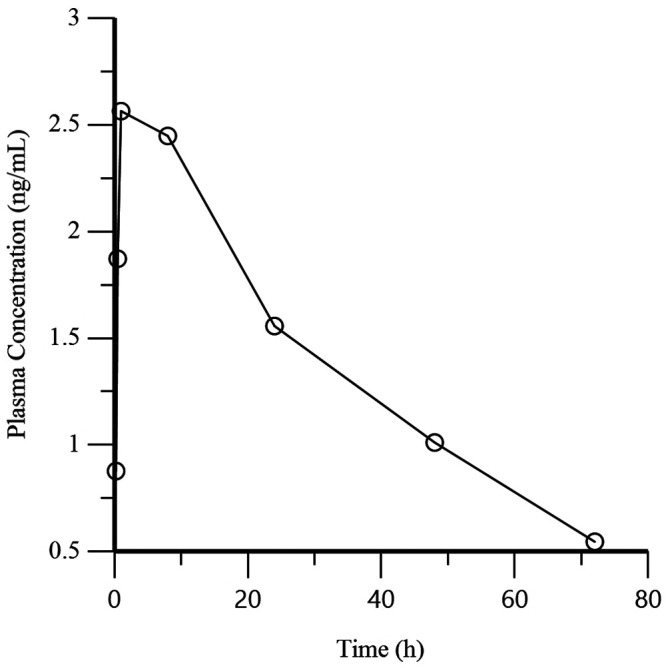
Mean plasma concentration of buprenorphine over time for the BSR group (n = 6) after the administration of BSR at 0.2 mg/kg IM.
Each group was further divided according to sex, body condition score, and weight to evaluate whether these variables affected the plasma concentration of buprenorphine. The BUP and BSR groups contained equal numbers of females and males (3 each); each group was divided according to lower (3.17 ± 0.58, 3.50 ± 0.50) and higher (4.5 ± 0, 4.50 ± 0.50) body condition scores (n = 3 per group) and again by lower (0.406 ± 0.052, 0.427 ± 0.047 kg) and higher (0.532 ± 0.047, 0.541 ± 0.025 kg) weights (n = 3 per group), for comparison. The t tests resulted in significant P values (P < 0.035) only when comparing female and male marmosets in the BSR group at the 24-, 48-, and 72-h time points. Female marmosets had lower plasma concentrations at each of these time points (Figure 3).
Discussion
This study reports the pharmacokinetic parameters and observed adverse effects of BUP and BSR administered to conscious adult common marmosets. The plasma concentration of buprenorphine measured over time after administration of BUP and BSR appears similar to those reported in other NHP species.18,22,30 Our results indicate that BUP dosed at 0.02 mg/kg IM in common marmosets achieved plasma levels above the proposed minimal therapeutic threshold of 0.1 ng/mL at all measured time points (5 min to 8 h). Similarly, BSR dosed at 0.2 mg/kg SC resulted in plasma concentrations of buprenorphine above 0.1 ng/mL for all measured time points (15 min to 72 h).
The plasma concentration–time curves presented in this study after the last measured time point for each group (8 and 72 h) were determined by linear regression and extrapolation using the previous time points. The true pharmacokinetic activity beyond the last measured time points cannot be determined by this study and is therefore a limitation. Ideally, additional data would have been collected at further time points for each group (for example, 12 and 96 h) to provide more precise data for the tail end of the plasma concentration–time curves. According to linear regression and extrapolation, the mean concentration curves will reach plasma levels of 0.1 ng/mL on average at 8.3 h for the BUP group and 92.7 h for the BSR group.
The rapid and robust increase in plasma concentrations of buprenorphine after intramuscular administration of BUP was expected. A rapid onset of analgesia is often indicated for common marmosets in the clinical setting and highlights one of the main advantages of this route when compared with the slower increase in plasma concentrations measured after SC administration of BSR. Correspondingly, BUP may have better analgesic efficacy than does BSR due to the higher initial plasma concentrations achieved after administration. Buprenorphine administration in other species has been shown to provide increasing efficacy with increasing dose up to a ceiling at which point increasing dose no longer results in increased efficacy.14,29,42 The dosage necessary to achieve a ceiling concentration in common marmosets is unknown. Theoretically, BUP administration may result in the plasma concentrations that reach the ceiling effect and BSR administration may not, suggesting that BUP may be more efficacious particularly at the earlier time points observed in this study. Although the response is not quite as robust as in the BUP group, all marmosets in the BSR group reached therapeutic concentrations by the first time point (15 min), indicating that it is also fairly rapid-acting.
The most notable advantage of BSR use in common marmosets is the long duration of action. In most cases requiring analgesia, a single dose of BSR given at 0.2 mg/kg will provide a sufficient duration of action (> 3.0 d) to make additional doses unnecessary. Using BSR in common marmosets can profoundly simplify an analgesic protocol. If BSR is unavailable or contraindicated, BUP dosed at 0.02 mg/kg can be considered an adequate alternative with a fairly long duration of action (6 to 8 h). A longer duration of action has numerous advantages, including decreased frequency of dosing, fewer injections, decreased handling of the animals, improved animal welfare, and decreased time commitments for staff.
Another important advantage of BSR is a considerably better safety profile compared with BUP in common marmosets. A roughly 5-fold decrease in mean peak plasma buprenorphine concentrations with BSR relative to BUP coincides with less ataxia and sedation (Tables 1 and 2). Thus, the use of subcutaneous BSR results in much less significant adverse effects than the use of intramuscular BUP in common marmosets for the dosages used in this study.
The adverse effects observed in this study after administration of intramuscular BUP show that common marmosets are likely more sensitive to its effects than other species, such as macaques. Dosing at 0.02 mg/kg resulted in more severe ataxia and sedation than did BSR, demonstrating the potential for serious complications and possible compromise of animal health. The adverse effects of BUP may be compounded when used together with other drugs that are metabolized by the hepatic cytochrome P450 sytem.24 Caution is advisable when using BUP in common marmosets and low doses should be used whenever possible, particularly if used in combination with other drugs. The authors do not recommend the concurrent use of isoflurane and intramuscular BUP unless the patient is intubated and mechanical ventilation is available; a lower dose, such as 0.005 mg/kg IM, may be advisable. Alternative multimodal analgesia such as NSAID and local anesthetics should be used until the patient is extubated; BUP can then be administered as the sole agent.
The variation noted in plasma concentrations among subjects after IM administration of BUP, which are more apparent at the early time points in this study, has been reported in other species.11,30,40 Variation seen by this dosing route may be due to various factors including lipophilicity of the compound, muscle mass, vascularity, or inadvertent injection into adipose or fascial tissue. This variation in plasma concentrations suggests variation in absorption from the injection site and may explain why some individuals experience more severe adverse effects to BUP than others. Variation in plasma concentrations between individuals may result in a variety of responses when using BUP by the IM route in a clinical setting.
Injection site reactions have been noted with the use of BSR in other species,10,13,25,30 therefore, observations of the sites were performed during the study. One out of six marmosets in the BSR group had irritated skin at the site of the injection. This was attributed to the marmoset scratching at the area resulting in self-excoriation. The cause of the scratching remained undetermined, but BSR may have been a potential contributing factor. Alternatively, the injection itself could have caused the scratching, perhaps by inadvertently passing the needle into nerve tissue. Another possibility is that the marmoset was experiencing pruritus associated with the buprenorphine. Despite the cause of the scratching, injection site reactions after BSR administration were not a concern during or after this study. The authors routinely administer BSR to common marmosets, as described in this study, and have not detected any injection site reactions similar to those reported in other species.
Females, as compared with males, had lower plasma concentrations of buprenorphine at the 24-, 48-, and 72-h time points in the BSR group. Although the P values were low (P < 0.035), the number of marmosets of each sex (n = 3) was a limitation of this study resulting in low statistical power and an inability to determine statistical significance. However, a difference was noted (Figure 3) in the plasma concentrations of buprenorphine between females and males at these 3 time points; sex-associated differences in buprenorphine pharmacokinetics have been previously described in humans.26 Similarly, in a study of highly concentrated buprenorphine solution administered to rhesus macaques, females had significantly lower plasma concentrations for several time points after administration of both a high and low dose.22 The cause of the sex differences remain unknown. Differences may be attributed to differing circulating hormones, variation in time of estrous cycle, body composition, or differing cytochrome P450 metabolism. All female marmosets’ pregnancy status in the colony is routinely determined by abdominal ultrasonography every 4 wk; the marmosets in this study were not pregnant. Despite the differences, plasma drug concentrations in all marmosets of either sex remained above the minimum therapeutic threshold of 0.1 ng/mL for the BSR group, so dosing guidelines are the same for both sexes. However, female marmosets may metabolize BSR more rapidly than males.
This study did not evaluate the efficacy of buprenorphine on pain management in common marmosets. Thus, the use of 0.1 ng/mL as the minimal therapeutic threshold for plasma buprenorphine in common marmosets is a limitation of this study. Plasma concentrations of buprenorphine above this threshold are reported to be therapeutic in humans and are hypothesized to be therapeutic in macaques and were thus applied to the results of this study.12,18,22,30,37,38 Reliable analgesiometric tests (for example, tail flick latency, thermal withdrawal) for common marmosets and other NHP species have not been well established. Clinical postoperative pain management provides a more practical opportunity to evaluate analgesics. Alleviation of signs related to postoperative pain are reported to be appropriate for the evaluation of buprenorphine efficacy in other species.36 Further studies evaluating the efficacy of buprenorphine on pain management are needed in common marmosets and other NHP species to ensure that the reported pharmacokinetic parameters achieve adequate analgesia.
The current study did not test other commonly used doses of BUP for common marmosets, such as 0.005 mg/kg or 0.01 mg/kg.4,21,24 These doses are frequently used in clinical practice if higher doses are contraindicated. The duration of action of doses in the lower end of the range will be less than that reported for 0.02 mg/kg and will necessitate more frequent dosing and pain evaluation to ensure adequate pain management. Additional studies are needed in common marmosets to provide accurate duration of action for these dosages.
The current study provides information on the use of BUP and BSR in common marmosets. The pharmacokinetic parameters and plasma concentration curves reported in this study were used to provide guidelines for clinical dosing frequency based on a therapeutic threshold range of 0.1-0.5 ng/mL. BUP administered at 0.02 mg/kg IM and BSR administered at 0.2 mg/kg SC should be dosed at least every 6 to 8 h and 3.0 to 3.5 d, respectively. Plasma concentrations for BUP and BSR both surpassed the plasma therapeutic threshold at the first time points (5 and 15 min), thereby showing the both formulations are appropriate, rapid-acting analgesics in this species. These guidelines are based on mean and extrapolated values and may not be inclusive of all individual animals. Therefore, frequent and thorough evaluation of animals to recognize the need for additional or alternative analgesics remains the standard of care. Although BUP can have adverse effects, its judicious use in common marmosets is warranted and it can be used safely. Similarly, BSR provides a long-acting and safe option for analgesia in common marmosets, and its use can refine analgesic protocols and improve animal welfare.
Acknowledgments
We thank Megan Sosa, Jennifer Coonen, Michelle Harke, and the colony-management staff and veterinary staff of the Wisconsin National Primate Research Center. We acknowledge the statistical support provided by Nicholas Keuler. The authors acknowledge the financial support of NCRR/ORIP grant P51OD011106 to the Wisconsin National Primate Research Center (University of Wisconsin, Madison).
References
- 1.Allen PS, Nowland MH, Liechty E, Bergin I. 2013. Severe respiratory depression following buprenorphine administration in Callithrix jacchus. Abstract presented at the AALAS National Meeting, Baltimore, Maryland, 27–31 October 2013. J Am Assoc Lab Anim Sci 52:646. [Google Scholar]
- 2.Andaluz A, Moll X, Abellán R, Ventura R, Carbó M, Fresno L, García F. 2009. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet J 181:299–304. 10.1016/j.tvjl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Act and Anmal Welfare Regulations. 2020. USDA animal care. [Cited 17 December 2020]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/AC_BlueBook_AWA_508_comp_version.pdf. [Google Scholar]
- 4.Bakker J, Roubos S, Remarque EJ, Arndt SS, Kronen PW, Langermans JA. 2018. Effects of buprenorphine, butorphanol or tramadol premedication on anaesthetic induction with alfaxalone in common marmosets (Callithrix jacchus). Vet Anaesth Analg 45:309–319. 10.1016/j.vaa.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Barletta M, Ostenkamp SM, Taylor AC, Quandt J, Lascelles BDX, Messenger KM. 2018. The pharmacokinetics and analgesic effects of extended-release buprenorphine administered subcutaneously in healthy dogs. J Vet Pharmacol Ther 41:502–512. 10.1111/jvp.12497. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand HGMJ, Sandersen C, Flecknell PA. 2018. Reported analgesic and anaesthetic administration to non-human primates undergoing experimental surgical procedure: 2010–2015. J Med Primatol 47:217–225. [DOI] [PubMed] [Google Scholar]
- 7.Burns M, Wachtman L. 2019. Chapter 10. Physical examination, diagnosis, and common clinical procedures, p 145–175. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox JG. editors. The common marmoset in captivity and biomedical research. San Diego (CA): Academic Press. 10.1016/B978-0-12-811829-0.00010-8 [DOI] [Google Scholar]
- 8.Chang Y, Moody DE, McCance-Katz EF. 2005. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos 34:440–448. 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 9.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 10.Clark TS, Clark DD, Hoyt RF, Jr. 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JL, Messenger KM, LaFevers DH, Barlow BM, Posner LP. 2011. Pharmacokinetics of intravenous and intramuscular buprenorphine in the horse. J Vet Pharmacol Ther 35:52–58. 10.1111/j.1365-2885.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- 12.Escher M, Daali Y, Chabert J, Hopfgartner G, Dayer P, Desmeules J. 2007. Pharmacokinetic and pharmacodynamic properties of buprenorphine after a single intravenous administration in healthy volunteers: a randomized, double-blind, placebo-controlled, crossover study. Clin Ther 29:1620–1631. 10.1016/j.clinthera.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 14.Heavner JE, Cooper DM. 2008. Chapter 4. Pharmacology of analgesics, p 97–123. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. 10.1016/B978-012373898-1.50008-5 [DOI] [Google Scholar]
- 15.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 16.Johnson RJ, Kerr CL, Enouri SS, Modi P, Lascelles BDX, Del Castillo JRE. 2016. Pharmacokinetics of liposomal encapsulated buprenorphine suspension following subcutaneous administration to cats. J Vet Pharmacol Ther 40:256–269. 10.1111/jvp.12357. [DOI] [PubMed] [Google Scholar]
- 17.Joshi A, Parris B, Liu Y, Heidbreder C, Gerk PM, Halquist M. 2017. Quantitative determination of buprenorphine, naloxone and their metabolites in rat plasma using hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Biomed Chromatogr 31:e3785. [DOI] [PubMed] [Google Scholar]
- 18.Kelly KR, Pypendop BH, Christe KL. 2014. Pharmacokinetics of buprenorphine following intravenous and intramuscular administration in male rhesus macaques (Macaca mulatta). J Vet Pharmacol Ther 37:480–485. 10.1111/jvp.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishi N, Sato K, Sasaki E, Okano H. 2014. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ 56:53–62. 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- 20.Liu SY, Liu KS, Kuei CH, Tzeng JI, Ho ST, Wang JJ. 2005. Simultaneous determination of buprenorphine and its prodrug, buprenorphine propionate, by high-performance liquid chromatography with fluorescence detection: application to pharmacokinetic studies in rabbits. J Chromatogr B Analyt Technol Biomed Life Sci 818:233–239. 10.1016/j.jchromb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Ludlage E, Mansfield K. 2003. Clinical care and diseases of common marmosets (Callithrix jacchus). Comp Med 53:369–382. [PubMed] [Google Scholar]
- 22.Mackiewicz AL, Salyards GW, Knych HK, Hill AE, Christe KL. 2019. Pharmacokinetics of a long-lasting, highly concentrated buprenorphine solution after subcutaneous administration in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 58:501–509. 10.30802/AALAS-JAALAS-18-000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansfield K. 2003. Marmoset models commonly used in biomedical research. Comp Med 53:383–392. [PubMed] [Google Scholar]
- 24.Marini RP, Haupt J. 2019. Chapter 11. Anesthesia and select surgical procedures, p 177–194. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. San Diego (CA): Academic Press. 10.1016/B978-0-12-811829-0.00011-X [DOI] [Google Scholar]
- 25.Molter CM, Barbosa L, Johnson S, Knych HK, Chinnadurai SK, Wack RF. 2015. Pharmacokinetics of a single subcutaneous dose of sustained release buprenorphine in northern elephant seals (Mirounga angustirostris). J Zoo Wildl Med 46:52–61. 10.1638/2014-0115R.1. [DOI] [PubMed] [Google Scholar]
- 26.Moody DE, Fang WB, Morrison J, McCance-Katz E. 2011. Gender differences in pharmacokinetics of maintenance dosed buprenorphine. Drug Alcohol Depend 118:479–483. 10.1016/j.drugalcdep.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. 2002. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem 306:31–39. 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 28.National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Roundtable on Science and Welfare in Laboratory Animal Use. 2019. Care, use, and welfare of marmosets as animal models for gene editing-based biomedical research: proceedings of a workshop. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 29.Nielsen S, Rivas C, Demirkol A, Lintzeris N. 2019. Effects of ascending buprenorphine doses on measures of experimental pain: a pilot study. J Subst Abuse Treat 104:128–134. 10.1016/j.jsat.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 31.Office of Laboratory Animal Welfare (OLAW). 2002. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): National Institutes of Health, Department of Health and Human Services. [Google Scholar]
- 32.Patterson JL, Lanford RE. 2019. Chapter 28. Experimental infections of the common marmoset (Callithrix jacchus), p 515–523. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. San Diego (CA): Academic Press. 10.1016/B978-0-12-811829-0.00028-5 [DOI] [Google Scholar]
- 33.Popilskis SJ, Lee DR, Elmore DB. 2008. Chapter 12. Anesthesia and analgesia in nonhuman primates, p 335–363. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. 10.1016/B978-012373898-1.50016-4 [DOI] [Google Scholar]
- 34.Preuss TM. 2019. Critique of pure marmoset. Brain Behav Evol 93:92–107. 10.1159/000500500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson SA, Lascelles BDX, Taylor PM, Sear JW. 2005. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 28:453–460. 10.1111/j.1365-2885.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 36.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim 36:322–343. 10.1258/002367702320162423. [DOI] [PubMed] [Google Scholar]
- 37.Sittl R, Griessinger N, Likar R. 2003. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther 25:150–168. 10.1016/S0149-2918(03)90019-1. [DOI] [PubMed] [Google Scholar]
- 38.Smith AA, Halliday LC, Lindeblad MO, Fortman JD. 2019. Evaluation of analgesic patches in Cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 58:356–361. 10.30802/AALAS-JAALAS-18-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BJ, Wegenast DJ, Hansen RJ, Hess AM, Kendall LV. 2016. Pharmacokinetics and paw withdrawal pressure in female guinea pigs (Cavia porcellus) treated with sustained-release buprenorphine and buprenorphine hydrochloride. J Am Assoc Lab Anim Sci 55:789–793. [PMC free article] [PubMed] [Google Scholar]
- 40.Steagall PV, Monteiro-Steagall BP, Taylor PM. 2014. A review of the studies using buprenorphine in cats. J Vet Intern Med 28:762–770. 10.1111/jvim.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiede AJ, Garcia KD, Stolarik DF, Ma J, Jenkins GJ, Nunamaker EA. 2014. Pharmacokinetics of sustained-release and transdermal buprenorphine in Göttingen minipigs (Sus scrofa domestica). J Am Assoc Lab Anim Sci 53:692–699. [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. 1994. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 55:569–580. 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]