Abstract
Mouse handling and restraint affect behavior, physiology, and animal welfare, yet little information is available on how various mouse restraint methods affect cardiovascular parameters. We validated the use of a smartphone-based ECG system in mice by performing simultaneous smartphone and telemetry ECG recordings in conscious, restrained mice and in anesthetized mice. We observed that mice held in standard immobilizing restraint (“scruffing”) experienced severe bradycardia. Mice of both sexes and 4 different strains (BALB/cJ, C57BL/6J, DBA/2J, and FVB/nJ) were restrained by 3 handlers using 3 different restraint methods: light restraint; 3-finger restraint, which creates a dorsal transverse fold of skin; and the standard immobilizing restraint, which creates a dorsal longitudinal fold of skin that results in a crease on the ventral neck. Regardless of the handler, immobilizing restraint, but not 3-finger restraint, produced severe bradycardia with irregular rhythm in all 4 strains and both sexes, with an average decrease in heart rate of 31%, or 211 bpm, and a maximal decrease of 79%, or 542 bpm. When evaluated using telemetry, immobilizing restraint produced severe arrhythmias such as junctional and ventricular escape rhythms, and second- and third-degree atrioventricular block. Sinus pauses were observed for an average of 4 min, but up to 6.8 min after release from immobilizing restraint. Atropine administration to C57BL/6J mice attenuated immobilizing restraint-induced bradycardia, supporting the hypothesis that pressure on cervical baroreceptors during stretching of the neck skin results in a vagally-mediated reflex bradycardia. Because of these profound cardiovascular effects, we recommend using the light or 3-finger restraint and avoiding or minimizing the use of immobilization restraint while handling mice.
Abbreviations: AV, atrioventricular; HR, heart rate; SP-ECG, smartphone-ECG; TEL-ECG, telemetry-ECG
Animals often must be handled and restrained to achieve research goals. Procedures requiring restraint have well documented effects on behavior and physiology, and thus may affect animal welfare. Many studies have now assessed how routine, seemingly innocuous procedures may affect research study results, and how subtle variations in handling and restraint can affect animal welfare and the reproducibility of experiments.
Handling can have a profound impact on physiologic and behavioral parameters and may influence study outcomes. Handling has been associated with alterations in metabolism and the immune system in mice and rat pups.1,25,35 C57BL/6 mice showed increases in heart rate (HR), systolic blood pressure, and core body temperature that persisted for a few hours after handling.50 However, all methods of handling mice do not have equivalent effects on behavior and physiology. Using a tunnel for handling, as compared with grasping the mouse by the base of the tail, increased exploratory behaviors and reduced anxiety and depressive-associated behaviors.6,11,17 In contrast, handling by the tail base was associated with relatively higher urination, defecation,17 blood glucose,11 and tumor growth,1 but did not change heart rate or central blood pressure.50 The effects of tail handling, as compared with tunnel handling, are long lasting despite efforts at habituation.13,50 Furthermore, when compared with handling by the tail, tunnel handling of ICR mice reduced the coefficient of variation in an elevated plus maze.33 The response to handling varies by strain, sex, duration of handling and method of animal transfer, contributing to variation across studies.1,17
Many researchers must manually restrain animals for basic procedures. This is despite the results of a study suggesting that manual restraint is aversive to mice,17 and that tail handling followed by manual restraint and abdominal palpation results in an acute heart rate increase of approximately 100 beats per minute that persists over half an hour.31 In the United States, mice are commonly manually immobilized by “scruffing”, which produces a longitudinal fold of skin from the occiput extending caudally along the dorsum.42 Depending on the strength of the grip, this technique will cause a crease on the ventral neck and abducted immobilization of the forelimbs. Training courses instruct students to avoid putting too much pressure on the neck when using this method, and to constantly monitor the mouse for dyspnea and cyanosis, as novice handlers may inadvertently cause mice to acutely die due to obstruction of the airway.42
To reduce the need for scruffing, alternative mouse restraint methods have been developed. Norecopa, Norway's 3R Center, has published a “three-finger” restraint method with instructional videos available online.34 This method produces a transverse fold of skin on the dorsum, but no crease on the ventral neck. This method is posited to reduce stress to the mouse. Different handling methods are likely to have measurable differences in how they affect the murine stress response and physiology. However, to our knowledge, no studies to date have evaluated the effects of different manual restraint methods on cardiac physiology.
Handling and restraint undoubtedly affect heart rate and central blood pressure, such that cardiac research emphasizes the use of unrestrained animals.31,50 Electrocardiograms (ECGs) measure the electrical activity of the heart and are valuable in assessing cardiac physiology. The gold standard for ECGs in conscious mice is the use of either intraperitoneally or subcutaneously implanted radiotelemetry devices.16,22 These devices transmit wirelessly to a receiver via radiofrequency waves and allow recording of high-quality, high resolution continuous waveforms in unrestrained mice for long periods of time.16 However, disadvantages are inherent in using radiotelemetry in mice. Implanting a large radiotelemetry device requires invasive surgery and a skilled surgeon, resulting in prolonged recovery time, single housing, and associated morbidity and mortality.16 In addition, radiotelemetry devices and associated equipment are not available at many institutions and may be prohibitively expensive. To address these disadvantages, several commercially available, noninvasive ECG options are marketed for use in conscious mice. These systems are expensive and produce short recordings (several seconds) that allow rapid screening of mice.16 Standard ECG equipment used for larger species is often unsuitable for small species, due to size limitations and the requirement of physical or chemical restraint, both of which may adversely affect physiologic parameters.16 Overall, ECG modalities requiring restraint can be useful if invasive modalities are not possible or necessary. Comparisons of these approaches can provide useful information on the effects of restraint and handling on cardiovascular parameters.
In this study, we used a commercially available, noninvasive smartphone-based ECG device in conscious, restrained mice to measure the cardiovascular effects of 3 manual restraint methods. The smartphone ECG (SP-ECG) used is a single lead ECG device that performs real-time recording via ultrasonic audio and proprietary wireless communication to a smartphone application. The company markets similar validated FDA-cleared products to human patients for at-home use, specifically for detection of atrial fibrillation.15 Due to its small size, affordable cost, and ease of use, the veterinary community has also taken an interest in the SP-ECG, and it has been validated in dogs and cats,23,46,48 horses,47 dairy water buffalo calves,43 and dairy cattle.2
Here, we validate the use of the SP-ECG against telemetry for assessing HR and rhythm in conscious, restrained mice. We hypothesized that SP-ECG can be used for screening of HR abnormalities in mice. By using SP-ECG, we found that manual restraint of mice by immobilization (scruffing), as compared with the less restrictive “three-finger” restraint method, produces severe, vagally-mediated bradyarrhythmias. We therefore advocate minimal handling and suggest the use of the “three-finger” restraint method when possible for the routine handling of laboratory mice.
Materials and Methods
All animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals,18 and all procedures were approved by the Cornell University IACUC. Mice were housed in an AAALAC-accredited facility. With the exception of C57BL/6J mice, all mice used for this investigation were scheduled to be culled or transferred from breeding protocols and were not purchased or bred specifically for this project. Mice were determined to be free of Sendai virus, mouse hepatitis virus, mouse parvovirus, minute virus of mice, epizootic diarrhea of infant mice, reovirus type 3, pneumonia virus of mice, ectromelia virus, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, mouse adenovirus, polyoma virus, Mycoplasma pulmonis, cilia-associated respiratory bacillus, murine pinworms (Aspiculuris and Syphacia spp.), and mouse ectoparasites by dirty bedding sentinel serology, and PCR from sentinel animals, colony animals, and exhaust air duct PCR. In addition, DBA/2J and BALB/cJ mice were free of mouse norovirus, Helicobacter species, Klebsiella species, and Rodentibacter species.
Mice were acclimated to the room and other environmental conditions in which experimental procedures were conducted for at least 3 d prior to study initiation. Mice were housed in individually ventilated cages (Allentown MJU160MVSPSHR1×, Allentown, Allentown, NJ) containing 1/4 in autoclaved corncob bedding (Bed-O'Cobs 1/4”, The Andersons, Maumee, OH), a cardboard hut (Refuge XKA-2450-087, Ketchum Manufacturing, Brockville, Ontario), and a sterile nesting pad (Cotton squares, Ancare, Bellmore, NY), and received 60 air changes hourly. Mice were housed at a density of up to five mice per cage with the exception of post-surgical mice. Mice recovering from radiotelemeter implantation were singly housed on a paper-based bedding (Tech Fresh 7099, Envigo, Indianapolis, IN). Irradiated rodent diet (7912, Harlan Teklad, Madison, WI) and municipal chlorinated reverse osmosis water were provided without restriction. Non-implanted mice were maintained on a 1410-h light:dark cycle with transitions at 0500 (daybreak) and 1900 (nightfall), whereas mice with implanted radiotelemeters were housed on a 12:12-h light:dark cycle with transitions at 0600 (daybreak) and 1800 (nightfall). Room temperature and relative humidity were controlled between 20 and 22 °C and 30% to 70%, respectively.
The study population receiving ECG telemetry implantation was comprised of male and female FVB/nJ mice, aged 11 to 29 wk and bred inhouse. Four different strains of mice (C57BL/6J females, n = 9, aged 5 to 18 wk; DBA/2J males, n = 5, aged 24 wk; FVB/nJ females, n = 10, aged 14 to 22 wk bred inhouse; BALB/cJ males, n = 10, aged 9 wk) were used to compare restraint methods. An additional 5 FVB/nJ female mice (aged 14 wk) were implanted with radiotelemeters. Mice used for the atropine test were C57BL/6J females, aged 8 wk. All mice were purchased or bred from mice purchased from The Jackson Laboratory, Bar Harbor, ME.
Use of Smartphone-ECG.
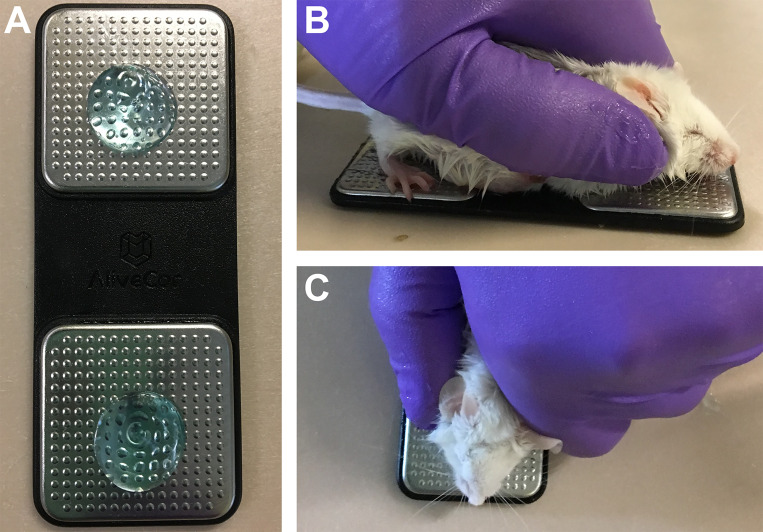
The smartphone-ECG (SP-ECG) is a single-lead, commercially available device (AliveCor, AliveCor, San Francisco, CA), with an associated app (AliveECG Vet) that can be downloaded for free from the Apple App Store onto a smartphone (iPhone 6s, Apple, Cupertino, CA). The app was set to a 50 mm/sec paper speed, 20 mm/mV amplitude, and 60 Hz mains filter. With the SP-ECG always in the same orientation with the lettering facing up, a small amount of ultrasound gel was applied to each lead (Figure 1) and spread into a thin film. An unanesthetized mouse was positioned in ventral recumbency with the front limbs and thorax on one sensor, and the hind limbs and abdomen on the opposite sensor. The size and restraint of the mice prevented a specific limb from being placed individually on a sensor. Anesthetized mice were placed in either ventral or dorsal recumbency. The SP-ECG displays as it is recording, but requires a minimum 10 s recording time for the ECG to be saved. Multiple recordings with a short break in between were performed to achieve a total recording time of 20 to 60 s per mouse. Recordings were exported from the smartphone as a pdf file and downloaded externally.
Figure 1.
Use of smartphone ECG device in mice. (A) Smartphone ECG device prepared with ultrasound gel. (B) Side and (C) top views of a mouse positioned on the smartphone ECG device for recording of ECGs.
Radiotelemeter implantation.
Mice were implanted with the HD-X11 radiotelemeter, weighing 2.2g (Data Sciences International, New Brighton, MN) as per the manufacturer's surgical manual for subcutaneous implantation in mice. Briefly, mice were induced with 5% isoflurane in 100% oxygen, maintained on 1.5% to 2.5% isoflurane via a nosecone, received pre-operative analgesia of ketoprofen at 5 mg/kg SC, and prepped for surgery. A 2 cm incision was created from the ventral neck to cranial thorax. A subcutaneous pouch was formed for placement of the transmitter along the animal's right flank. The ends of the leads were tunneled through the subcutaneous space and sutured to the pectoral muscles (cranial right or caudal left). The blood pressure sensor was coiled subcutaneously and not implanted into a vessel. Mice were singly housed after surgery with standard postoperative care, including analgesia, and were allowed 1 to 2 wk recovery prior to use on study.
Validation of Smartphone ECG.
Simultaneous ECG recordings were performed by SP-ECG and telemetry ECG (TEL-ECG) in 28 FVB/nJ mice instrumented with radiotelemetry devices. To create a wide range of heart rates over which to evaluate the SP-ECG, mice were recorded either consciously while restrained, or after 1 to 2 min of isoflurane anesthesia in either dorsal or ventral recumbency. Sixty-nine recordings were obtained over 49 different combinations of individual mouse, conscious or anesthetized, positioning, and restraint method. The same handler performed all the restraints.
Rhythm analysis of the ECG recordings was performed by a board-certified veterinary cardiologist who was blind to the animal treatment and assessed the recordings as in sinus rhythm or not. A rhythm diagnosis of non-sinus rhythms was provided whenever possible depending on the quality of recording, length of recording, and number of abnormal complexes. ECG tracings were also aligned by comparing time signatures of SP-ECG and TEL-ECG.
Heart rate was calculated independently for SP-ECG and TEL-ECG. For SP-ECG, heart rate was calculated manually. The first readable interval between R waves (R-R interval) without artifact, representing the interval between individual heart beats, was measured to the nearest 0.5 mm. This was done each second for the duration of the recording by an observer blind to animal treatment. The average R-R interval per recording was converted to beats per minute (bpm) based on the paper speed of 50 mm/sec (number of R-R intervals per 60 s = bpm). For TEL-ECG, the interbeat interval (equivalent to R-R interval) was calculated by the telemetry analysis software, Dataquest ART 4.31, to the nearest millisecond for every beat. For each of the 69 separate SP-ECG recordings obtained, the first interbeat interval per second was selected for the duration of the TEL-ECG recording that temporally aligned with the SP-ECG recording.
Comparison of restraint methods.
Prior to restraint, all mice were handled by the base of the tail to mimic standard handling procedures. Three restraint methods were used. Standard immobilizing restraint (scruffing) (Figure 3 A) was characterized by a longitudinal skin fold on the dorsum, a crease on the ventral neck, and dorsal abduction of the forelimbs. This form of restraint is typically used at the authors’ institution for procedures that require complete mouse immobilization. Three-finger restraint (Figure 3 B) was characterized by a dorsal transverse skin fold, the absence of a crease on the ventral neck, and carriage of the forelimbs in a more natural position. This restraint method was learned by accessing an instructional video available on the Norecopa website.34 Light restraint was accomplished primarily by gently pressing the dorsum of the animal down and preventing sideways movement off of the SP-ECG without pulling on the skin of the dorsum and was characterized by a longitudinal skin fold that poorly immobilized the head.
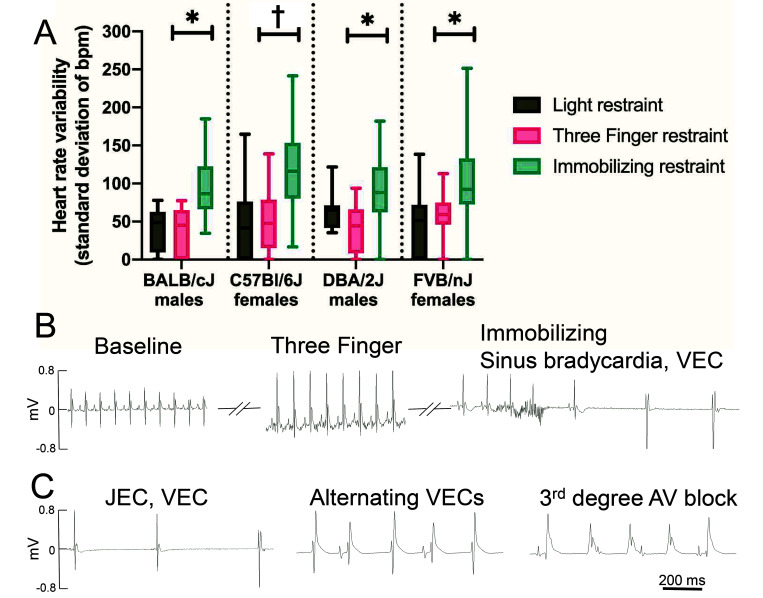
Figure 3.
Immobilizing restraint but not 3-finger restraint induces bradycardia in 4 strains and both sexes of mice. (A) Standard immobilizing restraint as characterized by a dorsal longitudinal skin fold, a crease on the ventral neck (arrow), and forelimbs abducted dorsally. (B) Three-finger restraint as characterized by a dorsal transverse skin fold, absence of crease on the ventral neck, and forelimbs in a natural position. (C) Heart rates measured by smartphone ECG for 3 handling methods (light minimal restraint, 3-finger restraint, and standard immobilizing restraint) in 4 strains of mice, both sexes, and 3 handlers. Data is presented as box and whisker plots with the whiskers representing minimum and maximum, the box the upper and lower quartiles, and the middle line the median, n = 10 (BALB/cJ); 9 (C57BL/6J), 5 (DBA/2J); and 15 (FVB/nJ). * P < 0.05, ‡ P < 0.001, Tukey HSD posthoc test between 3-finger and immobilizing restraints.
Four different strains (C57BL/6J females, n = 9; DBA/2J males, n = 5; FVB/nJ females, n = 10; BALB/cJ males, n = 10) were used to compare restraint methods. An additional 5 FVB/nJ female mice were implanted with radiotelemeters. C57Bl/6J females, DBA/2J males, and the unoperated FVB/nJ females were recorded by SP-ECG during all 3 handling methods. Mice were handled on 3 separate days by 3 experienced mouse handlers at the same approximate time of day. Restraint methods were performed in a randomized order with 10 to 20 min break between each method. The BALB/cJ males and the 5 FVB/nJ mice with implanted telemeters were only handled by one handler. Recordings were repeated if artifact levels were high or if the total recording time was less than 15 s. HR was calculated as above for validation of SP-ECG by an observer blind to animal treatment. The R-R intervals for all recordings for a mouse per condition and handler were averaged and converted to a bpm measurement. The individual R-R intervals for each recording were converted to a bpm measurement, and the standard deviation of these heart rates were calculated for each recording. Rhythm analysis was performed as above for validation of smartphone ECG. To assess the effect of immobilizing restraint after release in implanted FVB/nJ mice, the baseline HR prior to any handling was calculated from 15 R-R intervals during the first 15 s of the recording. Any R-R interval that exceeded 1.5 times the baseline R-R interval after release from restraint was identified.
Atropine response test.
Twelve female C57BL/6J mice, aged 8 wk, were randomly allocated to one of 2 treatments for a 2 period, 2 treatment crossover design. Mice received an intramuscular injection of 0.04 mg/kg atropine sulfate or equivalent volume of 0.9% normal saline (30 μL) in the quadriceps. Ten min after the injection, mice were recorded by SP-ECG while restrained by the light restraint method and then by the immobilizing restraint method. After a 5 h washout period, equivalent to approximately 3 half-lives of atropine,36 mice received the other treatment in the contralateral quadriceps muscle and the restraint and recording process was repeated. A reviewer who was blind to animal ID and experimental group measured the first complete R-R interval without artifact every one second to the nearest 0.5 mm. Averaged scores per treatment per mouse were converted to bpm by the calculation detailed above.
Statistical analysis.
Statistical analysis was performed using R version 3.5.1 “Feather Spray” (2018-07-02) on a ×86_64-w64-mingw32/x64 (64-bit) platform (The R Foundation for Statistical Computing, Vienna, Austria). Heart rate was compared between SP-ECG and TEL-ECG by Pearson correlation and Bland–Altman plots. Correlation was considered strong for Pearson r > 0.7, medium for Pearson r between 0.7 and 0.5, weak for Pearson r between 0.5 and 0.3, and negligible at r less than 0.3. Heart rate variability was assessed by calculating the standard deviation of all R-R intervals per mouse. The comparison of restraint methods on heart rate and variability was analyzed by a linear mixed effects model of heart rate and the standard deviation of heart rate: fixed effects were restraint method, strain, and the interaction between restraint method and strain, and random effects were handler and restraint method nested in handler. Arrhythmia (presence or absence) was assessed by logistic regression followed by posthoc pairwise comparisons using the Tukey method. The atropine response test was analyzed by a linear mixed-effects model of data transformed by rank, where fixed effects were treatment group and random effects were mouse ID. The assumptions of the linear mixed-effect model were checked by confirming normality of the residuals and plotting the predicted residuals compared with actual residuals to confirm the homogenous distribution of variance. The fixed effects of linear mixed-effects models were tested with F tests, using Satterthwaite correction for the degrees of freedom. Posthoc pairwise comparisons were made using Tukey HSD method to control for multiple comparisons. Significance was defined as α = 0.05.
Results
Smartphone ECG recording in mice.
The smartphone ECG (SP-ECG) device was simple to use with manual restraint alone and did not require anesthesia or fur removal (Figure 1). Better recordings were obtained when using ultrasound gel, although gel had not been required in other species.23 Mice were recorded for a minimum of 10 s, with a goal of 20 to 45 s, for an actual average of 27 countable s per recording.
Validation of smartphone ECG.
The SP-ECG reliably distinguished cardiac rhythm and determined HR in mice. Exact agreement between R waves was seen between SP-ECG and telemetry ECG (TEL-ECG) for both sinus rhythm and second-degree atrioventricular (AV) block, despite a highly irregular rhythm (Figure 2 A and B). P and T waves were not visible by SP-ECG at the normal sinus rhythm of the mouse, but P waves were visible and showed exact agreement with TEL-ECG at very low heart rates (Figure 2 B). Split QRS complexes, which indicate uncoordinated ventricular polarization in mice,42 were visible in the TEL-ECG but not the SP-ECG recording (Figure 2 B). TEL-ECG rhythm diagnosis of mice agreed with the respective SP-ECG diagnosis. As P waves are not visible on SP-ECG at normal heart rates, rhythm diagnosis was limited to regular or irregular, and the presence of ventricular escape complexes. As both systems are single lead ECGs and the polarity of the SP-ECG device is proprietary and therefore unknown, mean electrical axis was not considered.
Figure 2.
Validation of the smartphone ECG compared with the gold standard of ECGs obtained by radiofrequency telemeter. (A and B): Rhythms are equivalent between smartphone ECG (top) and telemetry ECG (bottom) in simultaneous recordings of mice in (A): Sinus rhythm and (B) Second-degree atrioventricular block with a ventricular escape complex on the second beat. (B) In addition shows that both R-S (green arrows) and P waves (pink arrows) are visible in the smartphone ECG tracing at lower heart rates. (C) Bland–Altman plot of heart rates obtained by simultaneous recordings of smartphone ECG and telemetry ECG of conscious restrained or anesthetized mice, n = 28 mice, 69 individual recordings. Heart rates are numerically similar, with the smartphone ECG occasionally over-estimating heart rate. Dashed lines represent 95% confidence intervals and average bias. SP-ECG: smartphone ECG. TEL-ECG: telemetry ECG.
A strong correlation (r = 0.8909, Pearson correlation, P < 2.2e-16) was found between SP-ECG and TEL-ECG methods for measurements of HR. A Bland–Altman plot displays the differences between TEL-ECG and SP-ECG heart rates as a function of the mean HR, showing good agreement (Figure 2 C). Most differences between methods clustered around 0, with an average -43.4 bpm difference for bias, as SP-ECG tended to overestimate HR with respect to TEL-ECG (95% Limits of Agreement for differences from -195 to 109).
The SP-ECG software has an automated counting function, but it was highly inaccurate at the high heart rates typically encountered in mice. HR calculated manually by measuring R-R intervals was poorly correlated with the automated counting average (r = 0.1521 by Pearson correlation, P = 0.1926). The mean HR for SP-ECG by manual counting was 560 bpm, in comparison with the automated counting average of 136 bpm (data not shown). The values for the SP-ECG automated counting did not agree with TEL-ECG values or manual counting. Moreover, a HR of 136 bpm is physiologically unlikely for mice.
Of the mice that had both conscious restrained and anesthetized recordings, the mean HR for conscious mice was 604 ± 32 bpm for TEL-ECG and 650 ± 24 bpm for SP-ECG (mean ± SEM). The mean HR for mice anesthetized with isoflurane was 440 ± 10 bpm for TEL-ECG and 450 ± 10 bpm for SP-ECG. Achieving good contact with the SP-ECG leads for anesthetized mice in dorsal recumbency was difficult, perhaps due to poor contact of the gel through the fur rather than through the footpads when in ventral recumbency, and produced low amplitude tracings (data not shown). One unanesthetized mouse died during immobilizing restraint and showed abnormal ECG findings (Figure 2 B, discussed below). This mouse appeared normal on gross necropsy. No other mice died during this study.
Change of heart rate and rhythm with different restraint conditions.
During the course of the study, we noticed anecdotally that restraint methods appeared to affect HR of mice measured by SP-ECG. HR was measured by SP-ECG in mice under light restraint, immobilizing restraint (Figure 3 A) and 3-finger restraint (Figure 3 B).
The linear mixed effect model accounted for random effects of handler and the interaction between handler and method (how each handler may perform a specific method differently). These random effects accounted for only 2.1% and 10.7% of the variability in the model, for a total of 12.8% of the variability. The rest of the model was accounted for by the fixed effects of mouse strain, restraint method, and the interaction between mouse strain and restraint method. Mouse sex and age were significant confounders, likely because available mice differed by both sex and age for each strain, and were not included in the model.
Regardless of handler, HR fell during immobilizing restraint as compared with the 3-finger restraint for all 4 strains of mice; this result occurred in both sexes (Figure 3 C). The method of restraint significantly affected mouse HR (F = 22.2829, df = 2/4.239, P = 0.0056). Immobilizing restraint greatly decreased both mean HR and the minimal observed HR (Figure 3 C). This effect was greatest for the C57BL/6J females (P = 0.001, average 49% decrease in HR, 79% maximal decrease in HR), followed by BALB/cJ males (P = 0.0115, 28% average decrease in HR, 62% maximal decrease in HR), and FVB/nJ females (P = 0.0151, 22% average decrease in HR, 78% maximal decrease in HR); the lowest observed effect was in DBA/2J males (P = 0.0523, 20% average decrease in HR, 62% maximal decrease in HR). The overall average decrease in HR for all mice was 31% or 211 bpm. Mouse strain and the interaction between restraint method and strain also had a significant effect on HR (F = 6.1145, df = 3/238, P = 0.0005 and F = 5.942, df = 6/240, P = 8.348e-6 respectively). Mice undergoing immobilizing restraint occasionally showed gasping behavior that resolved within a few seconds of release from restraint. However, the respiration rate was not quantified during restraint.
No significant difference was detected between light restraint and 3-finger restraint for any strain (P = 0.9549, 0.9666, 0.9267, and 0.9116 for C57BL/6J, BALB/cJ, DBA/2J, and FVB/nJ, respectively). The light restraint was intended to be as limited as possible. The mean HR of FVB/nJ females with light restraint was 715 bpm, which is comparable to the mean baseline HR of 708 bpm after opening the cage of 10 FVB/nJ males or females in the telemetry study (data not shown).
In addition to a bradycardia, mice undergoing immobilizing restraint showed a greater prevalence of arrhythmia. The variation in HR, as measured by the standard deviation of the sampled heart rates, was significantly higher for mice during immobilizing restraint than during 3-finger restraint for all strains and both sexes (F = 12.75, df = 2/4.241, P = 0.0161), indicating high irregularity in rhythm tested (Figure 4 A). The mean standard deviation of heart rates was higher than that of the 3-finger restraint method by 164% (P = 0.0485), 128% (P = 0.0036), 114% (P = 0.0425), and 81% (P = 0.0266) for BALB/cJ, C57BL/6J, DBA/2J, and FVB/nJ, respectively. Similar to the model for HR, random effects of handler and interaction between handler and method accounted for 6.1% and 8.0% of the variability in the model, for a total of 14.1% of the variability, with the remainder accounted for by the fixed effects of mouse strain, restraint method, and the interaction between mouse strain and restraint method. No difference was found in HR variability between light restraint and 3-finger restraint (P = 0.9933).
Figure 4.
Immobilizing restraint produces arrhythmias. (A) Heart rate variability measured by smartphone ECG for 3 handling methods (light minimal restraint, 3-finger restraint, and standard immobilizing restraint) in 4 strains of mice, both sexes, and 3 handlers. Heart rate variability was assessed by the standard deviation of R-R intervals per mouse and is a measure of irregular rhythm. Data is presented as box and whisker plots with the whiskers representing minimum and maximum, the box the upper and lower quartiles, and the middle line the median, n = 10 (BALB/cJ); 9 (C57BL/6J), 5 (DBA/2J); and 15 (FVB/nJ). * P < 0.05, † P < 0.01, Tukey HSD posthoc test between 3-finger and immobilizing restraints. (B) Representative TEL- ECGs from one FVB/nJ mouse during baseline recording, 3-finger restraint, and immobilizing restraint. Immobilizing restraint produced sinus bradycardia leading into ventricular escape beats. (C) Arrhythmias observed during immobilizing restraint in FVB/nJ mice: Junctional escape beats followed by ventricular escape beats, alternating sinus beats and ventricular escape complexes, and third-degree atrioventricular block.
A board-certified veterinary cardiologist performed rhythm analysis of the ECGs and found a higher prevalence of arrhythmias with immobilizing restraint. Of 276 ECGs analyzed, 11 were not classified due to excess movement artifact, with no relationship between artifact and restraint method. Sixty-five out of 265 ECGs were abnormal (24.5%), which corresponded to 5% of the light restraint group (4 recordings), 8% of the 3-finger restraint group (7 recordings), and 58% of the immobilizing restraint group (55 recordings). The proportions of arrhythmias were statistically different by logistic regression (df = 261, P < 2.2e-16), with immobilizing restraint significantly higher than light and 3-finger restraint (P < 0.0001, both comparisons), and no difference between light and 3 fingers restraint (P = 0.6619) when compared by Tukey posthoc pairwise comparisons. The probability of arrhythmias varied by strain: 37% for C57BL/6J, 41% for FVB/nJ, 60% for BALB/cJ, and 50% for DBA/2J.
The immobilizing restraint arrhythmias were all classified as bradycardia with an irregular R-R interval. Six C57BL/6J mice experiencing immobilizing restraint also had probable ventricular escape complexes. In addition, 10 out of 11 C57BL/6J mice identified as having wide (>40 ms) QRS complexes received immobilizing restraint. The resolution and filtering of SP-ECG make it difficult to distinguish P or T waves, so a complete rhythm diagnosis could not be obtained from those recordings. Five FVB/nJ mice were also implanted with telemetry devices, which allowed more definitive rhythm analysis before, during and after the immobilizing restraint. During immobilizing restraint, one of the 5 mice had a normal rhythm, which corresponded with a mild bradycardia as compared with baseline of 540 bpm. The other 4 mice were bradycardic, with heart rates ranging from 121 to 250 bpm, and all 4 exhibited irregular R-R intervals and sinus bradycardia during immobilizing restraint but not during baseline measurement or 3-finger restraint (Figure 4 A). In 2 mice, the sinus bradycardia developed into a junctional escape rhythm as evidenced by narrow complex with absent P waves,44 and subsequently a ventricular escape rhythm (Figure 4 C). An additional mouse on the FVB/nJ background in the SP-ECG validation study developed a variety of arrhythmias during immobilizing restraint: a rhythm of sinus beats with alternating ventricular escape complexes, a second-degree AV block in which P waves occur without a corresponding QRS complex, and a third-degree AV block, in which the atrial and ventricular beats are completely dissociated (Figure 2 B and Figure 4 C).44 This mouse also developed ventriculophasic sinus arrhythmia (data not shown). ECG abnormalities associated with myocardial ischemia in the mouse, such as abnormal J waves or negative T waves,5 were not observed, though hypoxia secondary to asphyxiation cannot be completely ruled out.
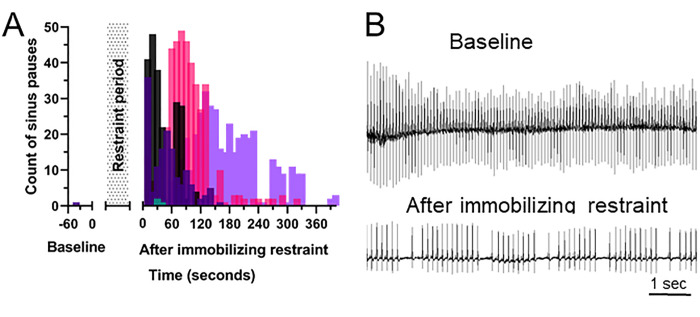
Radiotelemeter implanted FVB/nJ mice continued to have abnormal ECGs after release from an immobilizing restraint (Figure 5). Four out of 5 mice continued to have intermittent prolonged intervals (sinus pause) between beats, as defined by an R-R interval exceeding 1.5 times the baseline R-R interval. This occurred for 1.8 to 6.8 min, with an average of 4.1 min, after release from restraint. The sinus pauses clustered in approximately 1 to 2 second cycles of 1 to 2 slow beats after 3 to 6 normal beats in all 4 mice (Figure 5 B), consistent with a sinus arrhythmia without a respiratory component, based on the normal rapid respiratory rate of the mouse. The fifth mouse, which had a normal rhythm during immobilizing restraint, did not exhibit any abnormal beat intervals after release.
Figure 5.
Immobilizing restraint continues to produce arrhythmias after release from restraint. (A) Number of sinus pauses prior to and after release from immobilizing restraint in 5 individual FVB/n mice as measured by TEL-ECG, where sinus pause is defined as an R-R interval exceeding 1.5 times the average baseline R-R interval. (B) Representative TEL-ECGs at baseline prior to any handling and 4.5 min after release from immobilizing restraint, showing a non-respiratory sinus arrhythmia.
Atropine response test.
Restraint induced bradycardia was attenuated by prior atropine administration (Figure 6). Consistent with results in Figure 3 C, saline treated mice exhibited a significant immobilizing-restraint induced 29% decrease in HR (P < 0.0001). When the vagal response was inhibited by prior atropine administration, immobilizing restraint-associated bradycardia was attenuated, with the mean HR 160 bpm higher than in the saline-treated immobilized mice (P = 0.0494). Atropine rescued the majority but not all of the effects of handling-induced bradycardia. Heart rates during immobilizing restraint with atropine treatment were only 94% that of mice that experienced light restraint after atropine treatment (P = 0.01).
Figure 6.
Handling induced bradycardia is abrogated by atropine administration. Female C57BL/6J mice were treated with either saline or atropine and restrained by either a light minimal restraint or standard immobilizing restraint. Heart rate was assessed by smartphone ECG. Atropine rescued the majority of standard immobilizing restraint-associated bradycardia. Data is presented as box and whisker plots with the whiskers representing minimum and maximum, the box the upper and lower quartiles, and the middle line the median, n = 12. * P < 0.05, † P < 0.01, ‡ P < 0.001, Tukey HSD posthoc test between light and immobilizing restraints.
Discussion
This work demonstrates a restraint induced bradyarrhythmia in mice that was present across 4 inbred strains and both sexes, when restrained by 3 different experienced mouse handlers performing an immobilizing restraint typically used in the United States (scruffing), but not a 3-finger based alternative restraint method.
We screened numerous mice using both a smartphone-ECG that is inexpensive, small and portable (Figure 1) and telemetry-ECG. We validated the SP-ECG against TEL-ECG by performing simultaneous recordings, which demonstrated that the 2 methodologies produced equivalent rhythm analyses (Figure 2 A and B) and highly correlated heart rates (Figure 2 C). The SP-ECG has several disadvantages, including requiring frequent mouse and app manipulation, a 10 s minimum recording time before tracings are saved, such that the recording is lost if the mouse moves before the 10 s mark, and a 50 mm/sec maximal recording speed, which limits the resolution of the SP-ECG. This low speed, which is slower than the 100 mm/sec speed recommended for diagnostic quality ECGs in mice,40 proprietary filtering of the SP-ECG, and poor discrimination of P and T waves makes it impossible to discern P waves or T waves in normal mice using the SP-ECG (Figure 2 A), although P waves may be observed if the HR is abnormally slow (Figure 2 B). In addition, the SP-ECG's software provided with the app to calculate HR was highly inaccurate at high heart rates. Therefore, heart rates must be manually calculated when using the SP-ECG. Manual inspection of the TEL-ECG tracings indicate that the TEL software failed to detect low amplitude RS complexes on multiple occasions, leading to the reporting of R-R intervals in exact multiples of the true R-R interval. This may explain why the HR by TEL-ECG was lower than by SP-ECG (Figure 2 C). Due to these limitations, we do not recommend the SP-ECG for surgical monitoring, recording of HR in conscious, unrestrained mice, or high-definition HR recording in mice. Relevant to our study, the SP-ECG requires conscious mice to be restrained and is therefore well suited to assess variations in restraint technique.
Immobilizing restraint, characterized by a longitudinal skin fold and a crease on the ventral neck (Figure 3 A), was shown to induce severe bradyarrhythmia in mice of 4 different inbred strains, in both sexes; this effect was observed when mice were restrained by 3 different experienced mouse handlers (Figure 3 C). However, this effect was not seen when mice were restrained using the 3-finger method or the light, minimal restraint method (Figure 3 B and C). Based on these findings, we conclude that the bradyarrhythmia observed is a common phenomenon of mice undergoing immobilizing restraint. Moreover, the fact that HR varied with alterations in restraint technique could have important implications on the reproducibility of studies. Immobilizing restraint decreased mouse heart rates by up to 79%, with an average 31% decrease, and produced an irregular rhythm of intermittent sinus pause which persisted for an average of over 4 min after the mice were released from their restraint. This indicates that the effects of immobilizing restraint persist beyond the period of restraint itself.
Immobilizing restraint appears to cause a bradycardia by activating the baroreceptor reflex. Stretch receptors, or baroreceptors, in the carotid sinus and aortic arch distend under pressure,3 such as from restraining a mouse so that a crease is produced on the ventral neck. Baroreceptor stimulation results in increased parasympathetic tone and decreased sympathetic tone to the heart, and therefore decreased heart rate. When we blocked the efferent vagal pathway of the baroreceptor reflex pharmacologically by administration of the anticholinergic competitive antagonist atropine during immobilizing restraint, the bradycardia was almost completely rescued (Figure 6). We did not pharmacologically alter sympathetic tone, so the small decrease in HR during immobilizing restraint, in comparison to light restraint, is possibly due to a centrally mediated decrease in sympathetic tone. Atropine alone is commonly used to increase HR;29 however, HR did not significantly increase in the light restraint group given atropine as compared with the saline group. Therefore, we conclude that atropine increased the HR in the immobilizing restraint group due to abrogation of the baroreceptor reflex, and not baseline anticholinergic effects.
High vagal tone produced by immobilizing restraint also appeared to have severe consequences on murine heart rhythm. The murine ECG during light restraint was regular and showed minimal variation (Figure 4 A). However, during immobilizing restraint, the standard deviation of HR approximately doubled (Figure 4 A). This was confirmed by rhythm analysis of the ECGs, which showed a significant increase and high prevalence of irregular R-R intervals during immobilizing restraint. Analysis of telemetry data from a subset of FVB/nJ mice during immobilizing restraint provided further insight to the mechanism of this irregular rhythm. Stimulation of parasympathetic receptors in the sinoatrial node decreases pacemaker firing,39 as was manifested by sinus bradycardia (Figure 4 B). Pacemaker activity was rescued by the AV node producing junctional escape complexes (Figure 4 C), indicating that the sinoatrial node had not fired quickly enough or was in sinus arrest.44 High vagal tone also prolongs refractory time and conduction through the AV node.39 Consistent with this explanation, we observed 2nd and 3rd degree AV blocks (Figure 2 B, Figure 4 C) and the takeover of pacemaker activity by the ventricles (Figure 4 C). Junctional escape and ventricular escape rates described previously in mice are consistent with the heart rates observed in this study (Figure 3 C).19 A ventriculophasic sinus arrhythmia also occurred during times of AV block. This rhythm refers to a shortened P-P interval during AV block, when a QRS complex is interpolated between 2 P waves. Ventriculophasic sinus arrhythmia is thought to be a function of the parasympathetic nervous system.7 Further, severe bradyarrhythmias such as AV block can lead to acute death.44 This may be a mechanism of sudden death in compromised mice that cannot tolerate bradycardia, or in mice restrained by novice handlers. Novice handlers may restrain a mouse with too much force, or fail to recognize a distressed mouse during restraint. However, this study used only experienced mouse handlers, and we did not investigate the role of experience on restraint.
The effects of immobilizing restraint on heart rhythm persisted beyond the period of restraint. Periods of sinus pause were present in 4 out of 5 mice for up to 6.8 min after release from restraint (Figure 5 A), indicating prolonged effect of vagal tone. In addition, the sinus pauses clustered in groups every 1 to 2 s (Figure 5 B), forming a sinus arrhythmia. Respiratory sinus arrhythmia, a vagally mediated pattern in which HR slows during expiration, is common in young people,28 dogs, rabbits, seals, and rats,4 but not in cats or mice.24,41 Respiratory sinus arrhythmia is associated with decreased rate and severity of metabolic and cardiovascular disease in both people and dogs26,28 Sinus arrhythmia also has a baroreceptor mediated component that is independent of respiration.37 Nonrespiratory sinus arrhythmia in humans occurs in individuals with pre-existing pathologies,8,14 as well as in apparently healthy adults.30 The sinus arrhythmia in our study does not appear to be associated with respiration, as the pauses occurred in cycles of every one to 2 s, which is much slower than the respiratory rate of mice.27 After release from immobilizing restraint, mice were active and appeared to return to normal behavior within seconds, although this was not quantified. The effects of a nonrespiratory sinus arrhythmia in mice are unknown, but the arrhythmia does indicate prolonged aberrations in cardiac function.
Other causative mechanisms for restraint-induced bradycardia in this study are less likely, but possible. Creases and pressure on the ventral neck may possibly compromise the trachea, resulting in obstructive apnea. We did not observe cyanosis or tracheal injury on gross necropsy, but we cannot rule out a partial tracheal obstruction. We also did not quantitatively monitor respiration. When the airway is completely obstructed by blocking the endotracheal tube, a strain dependent decrease in HR occurs.19 However, the mechanism of obstructive apnea in induced bradycardia is different than the proposed mechanism during immobilizing restraint, as mice with obstructive apnea displayed bradycardia in the presence of high doses of atropine,19 in contrast to the rescue of bradycardia seen with atropine administration in the present study. Hypoxia is associated with tachycardia, not bradycardia, in conscious dogs and humans.21 Bradycardia can also be produced during the tonic immobilization that occurs in association with predator attack in some species,12 yet that condition does not appear to occur during handling of mice. One group suggests that manual restraint by scruffing is not more stressful than the initial tail handling.17 The Bezold–Jarisch reflex produces bradycardia, but this reflex occurs during episodes of hypotension,49 and restrained mice show hypertension rather than hypotension.50 These causes of bradycardia are therefore less likely than the baroreceptor-mediated mechanism proposed above.
The effects of stress on the cardiovascular system during restraint must also be considered. Restraint in mice is often viewed as stressful. However, a catecholamine response in mice produces tachycardia and tachyarrhythmias,20 which were not observed during immobilizing restraint. In our study, HR during light restraint was similar to the HR measured by telemetry in mice that had been disturbed in their cage (median 715 compared with 708 bpm for FVB/nJ). These values are higher than heart rates typically associated with normal activity for these mice (approximately 600 to 650 bpm),20 indicating stress associated with disturbance by humans. A previous study observed tachycardia and elevated systolic blood pressure in mice during handling and restraint;50 however, those mice were handled by methods that would not be expected to place pressure on the ventral neck.
One of the 79 mice that underwent immobilizing restraint in this study died during restraint; this death occurred prior to our discovery about the cardiovascular effects of this handling method and the mouse inadvertently was restrained too long (over 1 min). The mouse experienced severe bradyarrhythmia (Figure 2 B, Figure 3 C middle and right panel) with concurrent agonal breathing prior to death. This mouse demonstrates the importance of limiting the duration of immobilizing restraint. TEL-ECG at the time of death did not show evidence of abnormal J waves or negative T waves, which can occur during ischemia;5 however the split QRS complex, defined in humans as notched R waves, may also indicate ischemia.32 Therefore, we speculate that the death of this animal was due to severe cardiac depression, secondary to vagally mediated bradycardia, rather than to respiratory compromise, although we cannot rule out a respiratory component. Although this mouse appeared normal on gross necropsy, we did not rule out presence of preexisting pathologies.
Ventral neck pressure-induced bradycardia has been documented previously. Suction on the neck in the region of the carotid baroreceptors in conscious humans decreases heart rate.37 In addition, the findings in our study mirror findings in rabbits restrained by the scruff of the neck: an average bpm decrease of 33%, maximal decrease of 82%, and a high prevalence of sinus arrhythmia and sinus arrest.12 In contrast, restraint of neck skin that does not affect the ventral neck, such as applying a binder clip on the scruff of a cat's neck, did not significantly change HR or blood pressure.38 In rodents, dorsal neck restraint occurs naturally under 2 conditions: by predators, and by the dam during transport. Restraint of 10 to 14 d old C57BL/6 mice by pinching the dorsal skin of the neck to mimic carrying by the mother also produced a crease on the ventral neck and resulted in immobilization, analgesia, and a decrease in HR.51 This effect was attenuated by administration of lidocaine and atropine, but not opioids.9,51 Immobilization and analgesia also occur during firm restraint by the scruff of the neck in adult Peromyscus maniculatus,45 and after placing an alligator clip on the dorsal neck of adult male ICR mice.10 These responses in ICR mice are rescued by administration of the anticholinergic drug scopolamine.10 These results provide additional evidence of a vagally-mediated bradycardia during neck restraint in rodents.
This work triggers many further questions. Future studies are required to determine the effects of immobilizing restraint on long term cardiac or autonomic function, or to rule out partial obstruction of the airway as a possible mechanism for the observed bradycardia. This study also did not address if the bradyarrhythmia has any effect on behavioral or stress associated outcomes. In addition, we have not further addressed effects of repetitive restraint or novice handlers or ruled out the contribution of a potential partial tracheal obstruction.
In conclusion, the SP-ECG is a validated method of assessing restraint-induced changes in murine cardiac physiology. Immobilizing restraint, a common method of restraint employed in the United States, has profound impacts on short-term cardiovascular status in the mouse. Mice undergo profound bradycardia with associated bradyarrhythmias, and effects can persist for several minutes after mice are released. These effects were observed with immobilizing restraint but not with an alternative 3-finger restraint method. Therefore, we recommend minimal restraint of mice, acclimation to new procedures, and potentially, the use of tunnel handling. We recommend incorporating the 3-finger restraint method into training programs for mouse users. If physical restraint is needed, we suggest using a light restraint or the 3-finger method and avoiding use of immobilizing restraint (scruffing) when possible.
Acknowledgments
The authors wish to thank Frances Chen for inviting us to investigate the SP-ECG, providing initial training on the device, and collaborating with us to share animals; and Lynn Johnson and Stephen Parry from the Cornell University Statistical Consulting Unit for invaluable advice on statistical analysis. We would also like to thank the Cornell University Transgenic Mouse Core for allowing us to borrow some of their mice for this study.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Bonelli F, Vezzosi T, Meylan M, Nocera I, Ferrulli V, Buralli C, Meucci V, Tognetti R. 2018. Comparison of smartphone-based and standard base-apex electrocardiography in healthy dairy cows. J Vet Intern Med 33:981–986. 10.1111/jvim.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boron WF, Boulpaep EL. 2009. Medical physiology: a cellular and molecular approach, 2nd ed. Philadelphia (PA): Saunders–Elsevier. [Google Scholar]
- 4.Bouairi E, Neff R, Evans C, Gold A, Andresen MC, Mendelowitz D. 2004. Respiratory sinus arrhythmia in freely moving and anesthetized rats. J Appl Physiol (1985) 97:1431–1436. 10.1152/japplphysiol.00277.2004. [DOI] [PubMed] [Google Scholar]
- 5.Boukens BJ, Rivaud MR, Rentschler S, Coronel R. 2014. Misinterpretation of the mouse ECG: ‘musing the waves of Mus musculus’. J Physiol 592:4613–4626. 10.1113/jphysiol.2014.279380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson JM, Dwyer DM, Flecknell PA, Leach MC, Rowe C. 2018. Handling method alters the hedonic value of reward in laboratory mice. Sci Rep 8:1–8. 10.1038/s41598-018-20716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadu RT, McPherson CA. 2013. The ventriculophasic response: relationship to sinus arrhythmia and the duration of interposed QRS complexes. Ann Noninvasive Electrocardiol 18:336–343. 10.1111/anec.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deboor SS, Pelter MM, Adams MG. 2005. Nonrespiratory sinus arrhythmia. Am J Crit Care 14:161–162. [PubMed] [Google Scholar]
- 9.Esposito G, Yoshida S, Ohnishi R, Tsuneoka Y, Rostagno Mdel C, Yokota S, Okabe S, Kamiya K, Hoshino M, Shimizu M, Venuti P, Kikusui T, Kato T, Kuroda KO. 2013. Infant calming responses during maternal carrying in humans and mice. Curr Biol 23:739–745. 10.1016/j.cub.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann A, Urca G. 1988. Clip-induced analgesia and immobility in the mouse: pharmacological characterization. Neuropharmacology 27:641–648. 10.1016/0028-3908(88)90187-6. [DOI] [PubMed] [Google Scholar]
- 11.Ghosal S, Nunley A, Mahbod P, Lewis AG, Smith EP, Tong J, D'Alessio DA, Herman JP. 2015. Mouse handling limits the impact of stress on metabolic endpoints. Physiol Behav 150:31–37. 10.1016/j.physbeh.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannico AT, Lima L, Lange RR, Froes TR, Montiani-Ferreira F. 2014. Proven cardiac changes during death-feigning (tonic immobility) in rabbits (Oryctolagus cuniculus). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200:305–310. 10.1007/s00359-014-0884-4. [DOI] [PubMed] [Google Scholar]
- 13.Gouveia K, Hurst JL. 2013. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One 8:1–8. 10.1371/journal.pone.0066401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratadour P, Cividjian A, Sagnard P, Parlow J, Viale JP, Quintin L. 2008. Unusual sinus arrhythmia. Int J Cardiol 127:e138–e141. 10.1016/j.ijcard.2007.04.138. [DOI] [PubMed] [Google Scholar]
- 15.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. 2017. Assessment of remote heart rhythm sampling using the Alivecor heart monitor to screen for atrial fibrillation. Circulation 136:1784–1794. 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]
- 16.Ho D, Zhao X, Gao S, Hong C, Vatner DE, Vatner SF. 2011. Heart rate and electrocardiography monitoring in mice. Curr Protoc Mouse Biol 1:123–139. 10.1002/9780470942390.mo100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826. 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Iiyori N, Shirahata M, O'Donnell CP. 2005. Genetic background affects cardiovascular responses to obstructive and simulated apnea. Physiol Genomics 24:65–72. 10.1152/physiolgenomics.00203.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jelinek M, Wallach C, Ehmke H, Schwoerer AP. 2018. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of β-adrenergic stimulation. Sci Rep 8:1–10. 10.1038/s41598-018-20792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato H, Menon AS, Slutsky AS. 1988. Mechanisms mediating the heart rate response to hypoxemia. Circulation 77:407–414. 10.1161/01.CIR.77.2.407. [DOI] [PubMed] [Google Scholar]
- 22.Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. 1993. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods 30:209–215. 10.1016/1056-8719(93)90019-B. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MS, Gelzer AR, Rishniw M. 2016. Detection of heart rate and rhythm with a smartphone-based electrocardiograph versus a reference standard electrocardiograph in dogs and cats. J Am Vet Med Assoc 249:189–194. 10.2460/javma.249.2.189. [DOI] [PubMed] [Google Scholar]
- 24.Lewis KA, Scansen BA, Aarnes TK. 2013. ECG of the month. J Am Vet Med Assoc 242:623–625. 10.2460/javma.242.5.623. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Harada M, Tamada K, Abe K, Nomoto K. 1997. Repeated restraint stress impairs the antitumor T cell response through its suppressive effect on Th1-type CD4+ T cells. Anticancer Res 17:4259–4268. [PubMed] [Google Scholar]
- 26.López-Alvarez J, Elliott J, Pfeiffer D, Chang YM, Mattin M, Moonarmart W, Hezzell MJ, Boswood A. 2015. Clinical severity score system in dogs with degenerative mitral valve disease. J Vet Intern Med 29:575–581. 10.1111/jvim.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mailhot-Larouche S, Deschênes L, Lortie K, Gazzola M, Marsolais D, Brunet D, Robichaud A, Bossé Y. 2018. Assessment of respiratory function in conscious mice by double-chamber plethysmography. J Vis Exp 2018 (137):1–11. 10.3791/57778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. 2007. Respiratory sinus arrhythmia and diseases of aging: obesity, diabetes mellitus, and hypertension. Biol Psychol 74:212–223. 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer J, Mans C. 2018. Rodents, p 459–493. Chapter 9. In: Carpenter JW, editor. Exotic animal formulary. St Louis (MO): Elsevier. [Google Scholar]
- 30.McMullen MK, Whitehouse JM, Shine G, Towell A. 2011. Respiratory and non-respiratory sinus arrhythmia: implications for heart rate variability. J Clin Monit Comput 26:21–28. 10.1007/s10877-011-9327-8. [DOI] [PubMed] [Google Scholar]
- 31.Meijer MK, Sommer R, Spruijt BM, van Zutphen LF, Baumans V. 2007. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Lab Anim 41:161–173. 10.1258/002367707780378168. [DOI] [PubMed] [Google Scholar]
- 32.Mittal SR. 2016. Fragmented QRS: A simple electrocardiographic prognostic marker in cardiovascular disease. J Clin Prev Cardiol 5:94–98. 10.4103/2250-3528.191100. [DOI] [Google Scholar]
- 33.Nakamura Y, Suzuki K. 2018. Tunnel use facilitates handling of ICR mice and decreases experimental variation. J Vet Med Sci 80:886–892. 10.1292/jvms.18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norecopa. [Internet]. 2018. Refined technique for scruffing animals. Education & training: films and slide shows. [Cited 27 April 2020]. Available at: https://norecopa.no/scruff.
- 35.Núñez JF, Ferré P, Escorihuela RM, Tobeña A, Fernández-Teruel A. 1996. Effects of postnatal handling of rats on emotional, HPA-axis, and prolactin reactivity to novelty and conflict. Physiol Behav 60:1355–1359. 10.1016/S0031-9384(96)00225-9. [DOI] [PubMed] [Google Scholar]
- 36.Palmér L, Edgar J, Lundgren G, Karlén B, Hermansson J. 1981. Atropine in mouse brain and plasma quantified by mass fragmentography. Acta Pharmacol Toxicol (Copenh) 49:72–76. 10.1111/j.1600-0773.1981.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 37.Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, Johnston J, Bernardi L. 1997. Origin of respiratory sinus arrhythmia in conscious humans. An important role for arterial carotid baroreceptors. Circulation 95:1813–1821. 10.1161/01.CIR.95.7.1813. [DOI] [PubMed] [Google Scholar]
- 38.Pozza ME, Stella JL, Chappuis-Gagnon A-C, Wagner SO, Buffington CAT. 2008. Pinch-induced behavioral inhibition (‘clipnosis’) in domestic cats. J Feline Med Surg 10:82–87. 10.1016/j.jfms.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G. 2012. Cholinergic transmission. p 151–173. Section 2: Chemical mediators. Rang & Dale's Pharmacology E-Book. Philadelphia (PA): Churchill Livingstone. [Google Scholar]
- 40.Richig JW, Sleeper MM. 2019. Electrocardiography of rodents, p 21–27. Chapter 3. In: Richig JW, Sleeper MM, editor. Electrocardiography of laboratory animals, 2nd ed. San Diego (CA): Academic Press. 10.1016/B978-0-12-809469-3.00003-7 [DOI] [Google Scholar]
- 41.Sato M, Matsumoto N, Noguchi A, Okonogi T, Sasaki T, Ikegaya Y. 2018. Simultaneous monitoring of mouse respiratory and cardiac rates through a single precordial electrode. J Pharmacol Sci 137:177–186. 10.1016/j.jphs.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Simon AM, Goodenough DA, Paul DL. 1998. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol 8:295–298. 10.1016/S0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 43.Smith J, Ward J, Urbano T, Mueller M. 2016. Use of AliveCor heart monitor for rate and rhythm evaluation in dairy water buffalo calves (Bubalis bubalis). J Dairy Vet Anim Res 4:1–4. doi:10.15406/jdvar.2016.04.00113 [Google Scholar]
- 44.Tilley LP, Smith FWK, Jr. 2016. Electrocardiography, p 49–76. Section 1, Chapter 3. In: Smith FWK, Jr, Oyama MA, Tilley LP, Sleeper MM, editors. Manual of canine and feline cardiology. St Louis (MO): Elsevier. [Google Scholar]
- 45.Vestal BM. 1975. Development of the immobility response (animal hypnosis) in two species of deermice (Peromyscus). Anim Learn Behav 3:11–15. 10.3758/BF03209091. [DOI] [Google Scholar]
- 46.Vezzosi T, Buralli C, Marchesotti F, Porporato F, Tognetti R, Zini E, Domenech O. 2016. Diagnostic accuracy of a smartphone electrocardiograph in dogs: Comparison with standard 6-lead electrocardiography. Vet J 216:33–37. 10.1016/j.tvjl.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Vezzosi T, Sgorbini M, Bonelli F, Buralli C, Pillotti M, Meucci V, Tognetti R. 2018. Evaluation of a smartphone electrocardiograph in healthy horses: comparison with standard base-apex electrocardiography. J Equine Vet Sci 67:61–65. 10.1016/j.jevs.2018.03.006 [DOI] [Google Scholar]
- 48.Vezzosi T, Tognetti R, Buralli C, Marchesotti F, Patata V, Zini E, Domenech O. 2018. Home monitoring of heart rate and heart rhythm with a smartphone-based ECG in dogs. Vet Rec 184:96. 10.1136/vr.104917. [DOI] [PubMed] [Google Scholar]
- 49.Warltier DC, Campagna JA, Carter C. 2003. Clinical relevance of the Bezold–Jarisch reflex. Anesthesiology 98:1250–1260. 10.1097/00000542-200305000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Wilde E, Aubdool AA, Thakore P, Baldissera L, Jr, Alawi KM, Keeble J, Nandi M, Brain SD. 2017. Tail-cuff technique and its influence on central blood pressure in the mouse. J Am Heart Assoc 6:6. 10.1161/JAHA.116.005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida S, Esposito G, Ohnishi R, Tsuneoka Y, Okabe S, Kikusui T, Kato T, Kuroda KO. 2013. Transport response is a filial-specific behavioral response to maternal carrying in C57BL/6 mice. Front Zool 10:1–11. 10.1186/1742-9994-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]