Abstract
Rats commonly undergo surgery for research purposes. However, the effects of different methods of hair removal on wound healing and surgical site infections (SSI) in rats has not been evaluated. The current study evaluated 2 hair removal methods, clipping with an electric clipper and using a depilatory agent, and their effect on wound healing and SSI. Swabs for bacterial culture were obtained on Day 0 just after hair removal, after aseptic skin preparation, and on Days 1 and 3 before conducting skin biopsies to assess bacterial load and recolonization. Full-thickness punch biopsies were taken for histopathologic evaluation on Days 0, 1, 3, 7, and 10. The surgical incisions were assigned an ASEPSIS score on Days 1 and 3. The data revealed that the bacterial load was significantly higher with the depilatory method as compared with the clipper method, but only on Day 1. The histopathologic evaluation found no significant difference in wound healing between the 2 methods. Although the ASEPSIS score was significantly higher for the clipping method than for the depilatory method on Day 1, both techniques were equivalent by Day 3. We conclude that both hair removal methods are safe and efficacious components of aseptic technique in rats.
Abbreviations: Buprenorphine-SR, Buprenorphine-Sustained Release; SSI, Surgical Site Infections
Sprague–Dawley rats are often used in surgical procedures for biomedical research and training. The Guide for the Care and Use of Laboratory Animals20 requires the use of aseptic technique when performing survival surgery on any species. One purpose of aseptic technique is to reduce or eliminate the bacterial load on the animal prior to the start of surgery to prevent the introduction of bacteria into the sterile surface below the skin.2,30,42 Insufficient or inappropriate skin preparation may result in surgical site infections (SSI). SSI can delay or compromise wound healing.1,34 Aseptic technique requires the preparation of the surgical site on the animal by removing the hair such that skin damage, abrasions or other dermal injuries are avoided, followed by cleaning the skin with topical antiseptic compound.6,30
Traditionally, hair is removed from surgical sites because it harbors bacteria and prevents thorough cleansing of the incision site. Hair removal also facilitates visibility of the surgery site and removes a potential foreign-body that may result in SSI.10,22,43 The 3 most common hair removal methods are shaving with a razor, clipping the hair with an electric razor, and using a depilatory agent. In human patients, recommendations are that the hair not be removed unless visualization is needed or the hair would interfere with the surgical site or postsurgical bandaging.5,43 If hair removal is necessary for humans, the recommendation is to use either clippers or a depilatory agent. Using a razor has been shown to traumatize the skin, resulting in higher rates of SSI.22,28,35,36 Some research studies indicate that a depilatory agent is a better method, as it is efficient, atraumatic, and safe to use on or around wounds. However, it can cause a transitory lymphocytic reaction, and some individuals may have a sensitivity reaction.1,17,22,24 In mice, hair removal with either clipping or depilatory agent resulted in acceptable healing.25 In Wistar rats, the use of a depilatory agent did not affect the healing of a dorsal flap.3 Despite these findings, no studies have compared the effects of clipping and a depilatory agent on the prevalence of SSI and on wound healing in rats.
Our facility's standard practice is to remove hair with clippers using a no. 40 blade. However, this approach leaves a short stubble of approximately 1 mm.22 For surgical procedures that require a smooth skin, using a depilatory agent appears reasonable; however, little information is available on the effects of a depilatory agent on rat skin, SSI, and wound healing. We hypothesize that using a depilatory agent as a hair removal method in rats will reduce bacterial counts, dermal trauma, and SSI as compared with using clippers.
Materials and Methods
Animals.
All procedures were approved by the Uniformed Services University IACUC and were performed in accordance with the Animal Welfare Regulations4 and the Guide for the Care and Use of Laboratory Animals.20 Uniformed Services University vivarium is an AAALAC International accredited facility. All rats were included in a routine health surveillance program and were negative for all pathogens that were excluded from the rat colony in the facility: rat parvoviruses (RPV, KRV, H-1, RMV, and NS-1), rat theilovirus, sialodacryoadenitis virus, Pneumocystis carinii, Sendai virus, reovirus, lymphocytic choriomeningitis virus, cilia-associated respiratory bacillus, pneumonia virus of mice, Mycoplasma pulmonis, adenovirus (MAV), Salmonella, Helicobacter, Giardia, Pasteurella, Streptococcus, pinworms, Spironucleus, and fur mites.
Male Sprague–Dawley rats (Rattus norvegicus) (n = 33), with ages ranging from 4.5 mo up to 23 mo and a weight range from 488 g to 955 g. The rats were transferred from another IACUC approved protocol in which they had not participated in any experimental procedures. They were then assigned to 1 of 2 experimental groups: clipping (Arco, Wahl Clipper, Sterling, IL) or depilatory agent (Nair Hair Removal Lotion Softening Baby Oil, Ewing, NJ). Half of each of these 2 experimental groups were then assigned to be used in either the first or second cohort of the experiment. Rats were pair-housed until the initial surgery date and then individually housed for the duration of the study. Housing consisted of a static polycarbonate shoebox-type cage with a filter top (Allentown, Allentown, NJ) and rodent hardwood bedding (catalog number 7090M, Laboratory-Grade Teklad Maple SaniChips, Harlan Teklad, Madison, WI). After the surgery, the bedding was switched to Shepard Specialty Paper (catalog no. SHE1009, Alpha-dri, Watertown, TN). While housed on the Shepard Specialty Paper, cage changes occurred twice weekly. Enrichment was provided daily in the form Fat Rat Huts (high-temperature polycarbonate, Bio-Serv, Flemington, NJ), nylabones (pure virgin nylon, Bio-Serv, Flemington, NJ) and cotton squares (iso-PAD: The Ultra Enrichment Media, Omni BioResources, Cherry Hill, NJ). The rats were fed a pelleted rodent food (Envigo Teklad Global 18% protein T2018.15 Rodent Diet) and filtered domestic water ad libitum. The room was kept on a 12:12-h light:dark cycle (lights on, 0600; lights off, 1800) with temperature maintained between 68 °F and 79 °F (20 °C to 26 °C). The relative humidity was maintained at 30% to 70%.
Anesthetic Procedure.
Our initial plan was to use injectable anesthetics. However, rats anesthetized with an intraperitoneal injection of ketamine hydrochloride (75 mg/kg; Ketathesia, Henry Schein, Dublin, OH) and xylazine (7.5 mg/kg; AnaSed Injection, AKORN Animal Health, Lake Forest, IL) on Days 0 and 1 of the study had prolonged recovery, with a few rats requiring supplemental oxygen and many needing reversal with atipamezole hydrochloride (0.5 mg/kg subcutaneously; Antisedan, Zoetis, Orion Corporation, Espoo, Finland) to assist in the recovery. The next day these rats were still very sedated, perhaps due to their age, and yet they required anesthesia for experimental use on that day. We decided that switching to inhalational anesthesia as safer for the rats. Therefore, all subsequent anesthetic events were conducted using inhaled isoflurane (Isothesia, Henry Schein, Dublin, OH) administered with a vaporizer. For the inhaled anesthesia, induction was performed using 5% isoflurane with 100% oxygen. Once loss of pedal reflex occurred, the rats were maintained via a nose cone at between 1% to 3% isoflurane at an appropriate anesthetic depth, as monitored with toe pinch and respiratory rate. Full recovery occurred as expected. All rats received thermal support during anesthesia (HotDog Patient Warming, Eden Prairie, MN).
Skin Preparation.
After confirmation of appropriate anesthetic depth, hair was removed using 1 of 2 methods (clipping or depilatory agent) from the thoracolumbar region in an approximately 3 in. by 2 in. rectangular shape. For the clipping method, the clippers were disinfected (Oster Professional Products, Spray Disinfectant, no.76300 to 102 part 110969, McMinnville, TN) before and after each use. The blade was placed parallel to the skin and advanced in the direction of the hair growth. A second pass clipping against the hair growth was performed to ensure a close clip. The area was wiped down with sterile water (Sterile 0.9% Normal Saline, USP, 100 mL, Mundelein, IL) and gauze to remove any loose hair. For the depilatory method, a gloved hand or tongue depressor was used to part the hair and expose the base of the hair shaft so that the product could be applied at the base of the hair shaft. Then, in a circular motion that moved the hair shaft from the direction of growth to away from the direction of growth, the depilatory agent was applied until the entire selected area was covered. The depilatory agent remained on the skin for 3 min, at which time a test section was gently cleared with a gauze covered gloved finger or tongue depressor. If the hair came off easily, the rest of the depilatory agent was removed. If not, the depilatory agent was reapplied in that section and another test was conducted 2 min later. All depilatory agent was removed no more than 10 min after the first application. Once removed, the area was generously cleansed with sterile water and gauze to ensure complete removal of the depilatory agent.
Bacterial Sampling, Culture, and Identification.
Bacterial samples were taken to determine the antimicrobial activity of each of the hair removal methods on the skin. On Day 0, samples were collected at 2 time points: once the skin had dried after the hair removal, and after the skin was aseptically prepped. On Days 1 and 3, the samples were collected prior to the skin being aseptically prepped for that day's biopsy. Samples were collected by defining a 1 × 1 in. area within the region from which the hair had been removed. Starting in the center of the area and rolling the swab (BD BBL Culture Swab Plus, Item 220118, Copan Italia, Brescia, Italy), it was moved in a spiraling outward motion toward the outer edges, so as to not to exceed the defined 1 × 1 in. area.
The samples were submitted to IDEXX BioAnalytics for aerobic culture, bacterial identification, and colony counts. An aliquot of approximately 1 mL of the BD Eswabs (BD Cat# 220245) transport medium was vortexed, and a sterile calibrated 10 µL loop (Fisherbrand 220363 to 600) was used to inoculate 10 µL of the liquid onto BBL Trypticase Soy Agar with 5% sheep blood (TSA II; Becton Dickinson). Sample plates were incubated at 35 °C (95 °F) and 7% CO2 for 48 h. Colonies of each colony type were counted up to 350 CFU/10 µL; counts greater than 350 were reported simply as “greater than 350 CFU/10 µL”. The counts were then binned into 4 categories: None = 0; Low =1 to 49; Medium = 50 to 200 and High = greater than 200. Identification of the bacteria was conducted as previously reported.29
Surgical Technique.
All rats were maintained on a surgical plane of anesthesia as described above. For each anesthetic event, rats received subcutaneous fluids at 100 mL/kg/day after anesthesia to maintain hydration. The experimental procedures were performed in two equivalent cohorts. The first cohort received Buprenorphine-Sustained Release (SR) Laboratory (ZooPharm Ve terinary Compounding Pharmacy, Laramie, WY) subcutaneously at 1.0 mg/kg on Day 0. Rats also received Meloxicam-SR (ZooPharm Veterinary Compounding Pharmacy, Laramie, WY) subcutaneously at 4.0 mg/kg for 3 d alternated with acetaminophen (Children's Mapap Acetaminophen Liquid (160 mg/5 mL), Major Pharmaceuticals, Livonia, MI) in 2 oz gel cups (MediGel Sucralose, Clear H2O, Portland, ME) for 24 h administered as previously described.31
The surgical technique consisted of taking biopsies. The skin was aseptically prepped with BD ChloraPrep One-Step (2% s/v Chlorhexidine gluconate and 70% v/v Isopropyl alcohol, Becton, Dickinson and Company, El Paso, TX) according to product directions. The surgeon prepped aseptically, with hairnet, face mask, and sterile surgical gloves. A sterile drape was placed over the rat. On Day 0, 4 full-thickness skin punch biopsies, identified as sites A, B, C, and D, were taken from the hairless region of each rat (clipped or depilated) with an 8 mm biopsy punch (Robbin Instruments, Chatham, NJ). The punch biopsies were taken at the outside corners of the 1 inch × 1 in. area defined for the bacterial sampling. Hemostasis was managed with sterile gauze. The open wounds were closed using 5-0 Monocryl RB-1 (Ethicon, Guaynabo, PR) in a simple interrupted pattern. After the initial 4 biopsies on each rat, another biopsy was performed at the same sites (sites A, B, C or D on days 1, 3, 7, and 10, respectively) using a 10 mm biopsy punch (Robbin Instruments, Chatham, NJ). Prior to the biopsy, the target site was aseptically prepped with ChloraPrep Single Swabstick (2% s/v Chlorhexidine gluconate and 70% v/v Isopropyl alcohol, Becton, Dickinson and Company, El Paso, TX) according to product directions.
Histopathologic Evaluation.
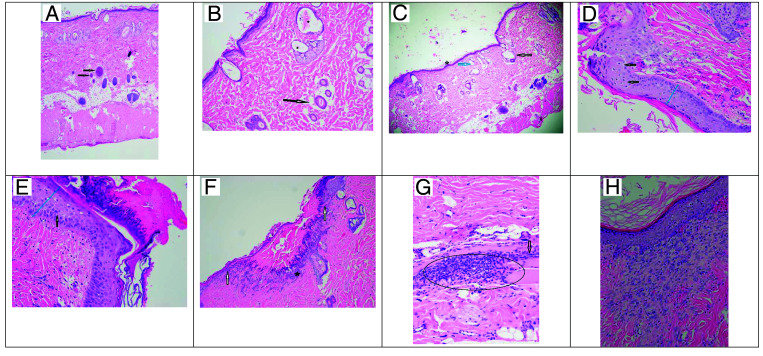
To microscopically examine the surgical sites for bacterial contamination and wound (biopsy) healing, the punch biopsies were collected into cassettes and placed in 10% neutral buffered formalin, routinely processed, paraffin-embedded, sectioned at 5µm, and stained with hematoxylin and eosin. The stained slides were evaluated by a veterinary pathologist who was blind to the hair removal method. Histopathology scoring was performed at both low-power (magnification, 100×) and high power (magnification, 200×) by using methods similar those previously published.26 The assessed criteria were dermal inflammation, follicular changes, fibroplasia, and epidermal hyperplasia. Each criterion was assigned a score ranging from 0 (absent) to 3 (robust). The scores were summed for each sample to obtain a cumulative histopathology lesion score, with a maximum possible score of 12. Images of stained slides were obtained at 200× magnification using a microscope (model BX41, Olympus) equipped with a digital camera (model DP22, Olympus) by using digital imaging software (cellSens Standard, Olympus Life Science Imaging Software). Representative images are presented in Figure 1.
Figure 1.
Dermal pathology on Days 0, 3, and 10. (A) Normal full thickness intact skin with the black arrows indicating normal hair follicles (40× magnification). (B) Day 0 Depilatory method with the arrow indicating a normal hair follicle with central hair shaft. The asterisks (*) indicate hair follicles that are moderately dilated with hair shaft fragments, flattened follicular epithelium, and compressed sebaceous glands (10× magnification). (C) Day 0 Clipper method with the arrow indicating a markedly dilated hair follicle void of the hair shaft and a compressed sebaceous gland. The blue arrow indicates a moderately dilated hair follicle with no hair shaft (40× magnification). (D) Day 3 Clipper method shows mildly transmural thickened epidermis (blue line) and the arrow shows single cell necrosis (20× magnification). (E) Day 3 Depilatory method shows a moderate transmural thickened epidermis (blue line) with the arrow showing cellular bridging (edema). A thick mat of serocellular debris is seen covering the epidermis (20× magnification). (F) Day 3 Clipper method with the white arrows indicating a demarcation for a discontinuous epidermis; black astrick indicating inflammatory cellular infiltration covered by a mat of serocellular material (10× magnification). (G) Day 3 Depilatory method with a large aggregate of inflammatory cells within the deep dermis marked by the oval; the black arrow indicates fibrosis (20× magnification). Image H: Day 10 Clipper method showing an area of organized fibrosis with fibroblasts stacked in a linear fashion subjacent to the epidermis (20× magnification).
ASEPSIS Evaluation.
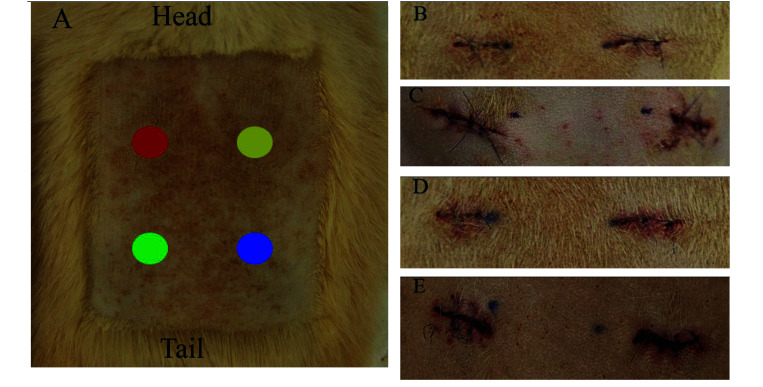
The initial 4 biopsy sites were photographed (Canon EOS Rebel T1i) on Day 0, Day 1 and Day 3. On Day 0, the sites were photographed after all 4 punch biopsy sites were closed. This photograph was used as a baseline of the appearance of the incision immediately after closure. It was not evaluated as healing, and infection would not be evident at that early time point. Day 1 and Day 3 photographs were edited to select only for sites C and D for scoring using the ASEPSIS evaluation. All photographs were evaluated by 5 assessors who were blind to the hair removal group. Assessors used a modified-ASEPSIS wound chart similar to that described previously.41 Scores were assigned for 4 criteria: serous exudate, erythema, purulent exudate and separation of deep tissue. The scores were summed to arrive at a total wound score with a maximal possible score of 22. Total scores of 0 to 3 indicate satisfactory healing; 4 to 6 indicated disturbed healing; 7 to 12 indicate mild to moderate infection; and scores greater than 13 indicated severe infection. The average ASEPSIS wound score for each treatment condition was calculated and used for the statistical analysis (Figure 2).
Figure 2.
Photographs of surgical sites on Days 0, 1 and 3. (A) Representative of hair removal site (clipper method) with rat orientation noted. Each colored circle represents a punch biopsy site: A (red circle), B (yellow circle), C (green circle), D (blue circle). After Day 0, in which all 4 sites were initially created, the healing punch biopsy site was removed on Days 1, 3, 7 and 10 respectively. The following images are of only Sites C and D which were used for the ASEPSIS scoring. (B) Day 1 Clipper method ASEPSIS scoring photo. The wound total average score is a 1 (satisfactory healing). (C) Day 1 Depilatory method ASEPSIS scoring photo. The wound total average score is a 1 (satisfactory healing). Sensitivity reaction can be seen in the red circular lesions between the punch biopsy sites. (D): Day 3 Clipper method ASEPSIS scoring photo. The wound total average score is a 3 (satisfactory healing). (E) Day 3 Depilatory method ASEPSIS scoring photo sensitivity resolving sensitivity reaction can be seen between the incision sites. The wound total average score is a 2 (satisfactory healing).
Statistical analysis.
ASEPSIS wound scoring was evaluated among the 5 assessors using Kendall coefficient of concordance. Agreement was assessed for each day and each method. Using the median wound score from each animal, a linear mixed model for repeated measures assessed the main effects of day, hair removal method, and the interaction of the 2. For histopathology, a similar mixed model approach was used. Model means for both ASEPSIS and histopathology scores are reported as age-adjusted, given the significance of age in both models. Pairwise comparisons evaluating the interactions from both models were adjusted using the Sidak method. Bacterial load for each animal was categorized from 0 to 3, 0 representing no growth, and 3 representing heavy growth. Associations between hair removal method and bacterial load grouping were assessed on each day using a χ2 test. Unless otherwise stated, Type I error is controlled at 5%, with all tests 2-sided. Analysis was conducted in SPSS statistics software (version 25, IBM North America, NY, NY). All continuous results are presented as mean ± SEM.
Results
Interassessor agreement regarding ASEPSIS wound score.
The Kendall coefficient of concordance for the assessor's ASEPSIS wound scores were 0.74 for Day 1 and 0.66 for Day 3 for the clipping hair removal method, indicating moderate to substantial agreement. For the depilatory methods, concordance was 0.35 on Day 1 and 0.39 on Day 3, weak to moderate agreement. In addition, assessor scores showed significant concurrence (P < 0.001) for both clipping and the depilatory method Day 1 (P = 0.04) and Day 3 (P = 0.02).
ASEPSIS Score.
The ASEPSIS Day 1 mean score for the clipping method was 2.1 ± 0.4, which significantly (P = 0.03) higher than the mean of the depilatory method (0.48 ± 0.54). The Day 3 ASEPSIS mean scores between methods were not significantly different, although the Day 3 mean for the depilatory method (2.1 ± 0.5) was significantly (P = 0.01) higher than the Day 1 mean (0.48 ± 0.54). The clipping method had no significant change from Day 1 (2.1 ± 0.4) to Day 3 (2.5 ± 0.4) (Table 1).
Table 1.
Comparisons of ASEPSIS means between hair removal methods on each day.
| Method |
|||
| Day | Clippers | Depilatory | P value |
| 1 | 2.10 ± 0.45 | 0.48 ± 0.54 | 0.03 |
| 3 | 2.51 ± 0.45 | 2.07 ± 0.54 | 0.53 |
Results reported as age-adjusted means with standard error. P values are adjusted via the Sidak method.
Histopathology Evaluation.
The clipping method score increased at each time point from 1.5 ± 0.2 on Day 0 to reach the highest score of 8.7 ± 0.3 on Day 10. With the depilatory method, the highest score was on Day 3 (8.4 ± 0.2) and fell through Day 10, but none of the differences were significant. No significant difference existed between the 2 methods on any day. The mean scores for each method had a significant (P < 0.001) increase on Days 1, 3, 7, and 10 as compared with Day 0.
Bacterial Assessment.
The bacterial load across the 2 methods was statistically different only on Day 1, when the bacterial growth for the depilatory method was Low (67%, or 10 of 15 samples) compared with the clipping method, which was None (83%, or 15 of 18 samples) (P = 0.002). Both methods remained in either the None or Low growth category, except for the depilatory method on Day 3 in which 13%, or 2 of 15 samples had Medium growth (Table 2). The bacteria isolated prior to the aseptic preparation step represent 8 genera: Aerococcus spp., Bacillus spp., Corynebacterium spp., Enterobacter spp., Enteroccocus spp., Klebsiella spp., Proteus spp., and Staphylococcus spp. (Table 3). The most frequent organisms were the Staphylococcus spp. at 53% (30 out of 57) and Aerococcus spp. at 25% (14 out of 57). The other 6 genera ranged from 2% to 7% (1 to 4 out of 57) of the total bacteria found. After the antiseptic step, the only genera still present were the Staphylococcus spp. at 5% (3 of 57) and Aerococcus spp. at 2% (1 out of 57). All 4 isolates were found in the depilatory method.
Table 2.
Percent bacterial load of each hair removal method across Days 0, 1, and 3.
| Day 0 |
Day 1* |
Day 3 |
||||
| Bacterial Load | Clippers | Depilatory | Clippers | Depilatory | Clippers | Depilatory |
| None | 100 | 86.7 | 83.3 | 33.3 | 61.1 | 33.3 |
| Low | 0 | 13.3 | 11.1 | 66.7 | 33.3 | 53.3 |
| Medium | 0 | 0 | 5.6 | 0 | 5.6 | 13.3 |
| *P = 0.002 | ||||||
Data represents bacterial load from Clipper group (n = 18) and Depilatory (n = 15) across 3 d. Statistical significance on Day 1 in which majority of Clipper group is “None” while Depilatory is “Low”. High group is not listed as bacterial load never elevated above Medium. Note: Day 0 bacterial samples used in the table were collected after the aseptic preparation step.
Table 3.
Bacteria cultured from samples collected on Day 1 posthair removal and postaseptic preparation
| Bacteria | Total count (n = 57) | Post-hair removal proportion | Post-aseptic proportion |
| Aerococcus sp. | 14 | 0.25 | 0.02 |
| Bacillus cereus | 1 | 0.02 | 0 |
| Corynebacterium stationis | 3 | 0.05 | 0 |
| Enterobacter cloacae | 1 | 0.02 | 0 |
| Enterococcus casselitavus | 1 | 0.02 | 0 |
| Klebsiella sp. | 4 | 0.07 | 0 |
| Proteus mirabilis | 3 | 0.05 | 0 |
| Staphylococcus sp. | 30 | 0.53 | 0.05 |
Data represents the number of times the bacteria were cultured across the rat population. Proportions are relative to the original count.
Discussion
Guidance on rodent asepsis states that the hair should be removed, and the skin prepped with an antiseptic solution.6,30 Recently, research on skin preparations for surgical procedures in mice investigated aseptic preparations and their impact on SSI and healing.12,18,25 The impact of hair removal method has not been assessed in rats. The goal of this study was to determine whether the hair removal method affected wound healing and the development of SSI. The results of this study demonstrate that either method of hair removal is appropriate, with no differential effect on the development of SSI and satisfactory healing by Day 10.
In humans, recommended practice is to not remove the hair. However, in rodents, hair removal is necessary to ensure visualization of the surgical site and is considered a mandatory component of asepsis.10,35 The 3 common methods of removing hair are a razor blade, an electric clipper and a depilatory agent. This study originally planned on assessing all 3 methods. However, 2 different single-blade razors, BIC Sensitive Shaver disposable and Gallant Disposable Prep Razor (Process-Construction AB, Sweden), could not cut the rat hair such that a clear patch of skin was visible. We therefore only assessed clippers and depilatory agent.
Both clippers and the depilatory agent are effective methods of removing hair in rats. In contrast to a previous study17 in which the clipper caused widespread nicking and the depilatory agent appeared to cause no damage, our study found no apparent damage due to the clippers, while 10 of the 15 rats given the depilatory agent developed a mild to moderate sensitivity reaction (small nonerythemic bumps and petechiae of the skin). All sensitivity reactions were healed by Day 10 without requiring treatment. While 9 of the rats developed sensitivity reactions in less than 24 h, one rat did not develop a reaction until Day 3 after the original application. The total contact time did not exceed the product directions of 10 min, making the number of sensitivity reactions unexpected. The hair was not trimmed prior to the application of the depilatory agent, which may have contributed to the length of time the depilatory agent was in contact with the skin. Clipping the hair before using the depilatory agent may decrease contact time and decrease sensitivity reactions. Another possibility for the unexpected sensitivity reaction may be the way the depilatory agent was removed from the rat's skin. A standard 4 × 4 gauze cloth or tongue depressor was used to gently remove the chemically treated hair, and then the area was generously rinsed and wiped with a standard 4 × 4 gauze cloth. Despite the attention to gentleness, this may still have been too harsh a removal method given the skin's reaction to the depilatory agent. Using a soft gauze with a higher weave may protect the skin better.
One reason for hair removal is to reduce bacterial load.30 Both hair removal methods had no or low bacterial growth after the antiseptic step. Because the depilatory agent is a chemical, it potentially could have antimicrobial properties. In one study,25 mice treated with a depilatory agent had less bacterial growth as compared with clipping. However, in our study, the depilatory-treated rats had a bacterial load of 2 [Low] of 15, while the clipper-treated rats had a load of 0 [None] of 18; this difference was not significant. As time progressed and bacteria began to recolonize the skin, the bacterial load of the depilatory-treated rats remained higher than the clipper treated rats. Because all wounds healed well, with no signs of infection, the method of hair removal is likely not clinically relevant. However, our data indicate that a depilatory agent should not be considered to reduce bacterial load.
Staphylococcus spp. was the most commonly isolated bacteria. This is not an unexpected finding, as Staphylococcus spp. is ubiquitous in the environment and is known as a common, commensal bacteria in both humans and mice.25,27,37 Characterizing the microflora of the rat skin was not a goal of this study; samples were taken prior to the aseptic step only to ensure that known pathogens were not present. A potential limitation of our bacterial characterization is the possibility of not capturing all bacteria and the potential of one species to outgrow another while plated. We may have missed bacteria by using a gentle rolling technique rather than a swabbing technique. Dilution of the transport medium may also have limited growth, although IDEXX has found their current standard balances the number of plates with too many colonies to count with those displaying no or limited growth. More fastidious organisms could be outcompeted by less fastidious organisms, resulting in not identifying some bacteria that may be present on the skin. As we found no published articles describing the microflora of rat skin, our work may provide an initial examination of rat skin microflora. We used a single step aseptic preparation, which is not a standard preparation method for our facility, as compared with the standard triplicate method. The bacterial counts after the aseptic step were reduced by 93%, and only 4 isolates were found. Because a standard is not available for the level of reduction that should be achieved with the antiseptic step, we considered this percentage of reduction to be effective as an aseptic process.
In the human health care setting, providers make a determination of a SSI based on gross evaluation, and they may have formal guidance on what constitutes an SSI.8 In laboratory animal medicines, such formal guidance is not available. However, several animal models of wound infection do exist.11 In rat and guinea pig models, surgical wounds were considered infected based on the presence of pus or an abscess.13,23,44 In human medicine, more than 80 methods and 6 grading systems have been described for assessing surgical wounds, with the ASEPSIS grading system being the most frequently used.7 We used the ASEPSIS grading system for this study. To support the ASEPSIS determination, we collected samples for histology. The rationale is that the histology would show indications of delayed wound healing or infection, and it is often the ’gold standard‘ for identifying infected wounds and for describing delayed wound healing.25,32,33
The mean ASEPSIS scores of both hair removal methods were low, indicating satisfactory healing. The Day 1 score for the depilatory method was significantly lower than the clipper method. However, by Day 3 their scores were statistically equivalent, and both scores were within the satisfactory healing category. The histopathology assessment revealed normal healing with no indication of deep dermal bacterial infection for either method. Mean scores in the Dermal Inflammation and Epidermal Hyperplasia categories were highest for both methods on Day 3, which indicates the incisions were in the acute healing stage. Scores then fell through Day 10. By Day 10, the highest scores were in the Fibroplasia category, indicating that the incisions had started forming scars, as characterized by organizing and remodeling fibroblasts. In the Follicular Change category on Day 0, we expected that the score would be zero, as others had previously reported no follicular change for their depilatory method group on Day 0.25 In our study, however, both hair removal methods had scores in the Follicular Change category. For the clipping method, the scores ranged from 1 to 3 (highest possible score of 3 possible) in 15 of 18 rats, whereas in the depilatory method group, scores ranged from 1 to 3 in 14 of 15 rats. Depilatory agents act by dissolving the hair, while clippers only cut the hair.36 Chemical components of the depilatory agent could potentially seep into the intradermal hair follicular lumen, damaging the hair shafts. However, clippers should have no influence on the intradermal follicular changes noted in the histopathology evaluation. Because the mean age of the rats on this study is 10 mo, this could be a nonspecific, age-related change. However, this is speculative, as no studies report skin changes in aging rats.
Several unexpected events occurred during the study. The initial event was the response of the first cohort to buprenorphine-SR. Pica behavior, compulsively bringing bedding into the mouth, was seen in 11 of 17 rats. Once anesthetized and examined, 2 of 11 rats had mouths full of bedding. Buprenorphine is an accepted analgesic in rats, with the buprenorphine-SR version commonly used to ensure dosing compliance and reduce stress due to handling.9,14,15 Pica is a known potential side effect of buprenorphine in rats.38 Some evidence indicates that pica development may be strain-dependent, with Sprague–Dawley rats being susceptible.16,38 In our facility, Sprague–Dawley is the most commonly used strain in surgical models, and buprenorphine-SR the preferred analgesic. While pica has only occasionally been seen, the rate of pica-like behaviors experienced in this study is unprecedented in our facility. While strain has been implicated as a potential factor, the possible influence of age has not. The most common age of rats in research ranges from 2 to 3 mo.21 However, the mean age of the rats in this study was 10 mo, and ranged from 4.5 to up to 23 mo. The advanced age of the rats in this group may have predisposed them to the behavioral side effects of buprenorphine-SR that were not seen in studies using younger rats.
The other unexpected event was 3 deaths, which we attributed to the age of the rats. One rat died within 24 h of the initial biopsy, and the other 2 rats required euthanasia based on veterinary guidance, one on Day 3 and the other on Day 7 after the initial biopsy. All 3 rats were the oldest animals used, with 2 of them 23 mo of age and the other 13 mo of age. Gross necropsy and histologic evaluations did not indicate an obvious cause of death, and no reasons for the deteriorating condition were found in the 2 euthanized rats. A possible contributor was that 2 injections of anesthetics at 24 h apart, in addition to the injection of buprenorphine-SR, was too stressful for the aged rats. Bradycardia and hypothermia are common problems in rats given injectable anesthetics like ketamine and xylazine, while the inhaled anesthetic, isoflurane, has less cardiorespiratory influence.40 Although an external heat source was provided, and subcutaneous fluids were administered to support the rats, the stress on their cardiovascular system may have exceeded their tolerance. While research is available on the effects of anesthetics in neonates, research on the effects of anesthesia in aged rats is lacking.39
In conclusion, both hair removal methods used in this study resulted in satisfactory healing of a biopsy site without dermal surgical site infections. We believe both methods are safe and effective hair removal methods. As sensitivity reactions occurred with the depilatory agent, a prudent strategy might be to shorten the hair length prior to application, thus shortening the contact time needed to achieve appropriate hair removal. Future research on the effects of using both clippers and a depilatory agent over the same area and its joint effects on dermal changes or SSI would be beneficial.
Acknowledgments
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Uniformed Services University of the Health Sciences, the Department of Defense, or the United States Federal Government. The authors want to especially thank Alli Oliver, Justin Brown, Andreanna Atkins, Kasie Carriveau, Constance Nichols, Amory Koch, Jennifer LeFors, Anna Mullins, Kerrie Farrar, Philip Bowling, Michael Bencivenga, and Warren W McNeil for their technical assistance in completing this study.
References
- 1.American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. 2009. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am 91Suppl 6:2–9. 10.2106/JBJS.I.00549. [DOI] [PubMed] [Google Scholar]
- 2.American College of Laboratory Animal Medicine. 2016. ACLAM position statement on rodent surgery. J Am Assoc Lab Anim Sci 55:822–823. [PMC free article] [PubMed] [Google Scholar]
- 3.Angel MF, Jorysz M, Schieren G, Knight KR, O'Brien BM. 1992. Hair removal by a depilatory does not affect survival in rodent experimental flaps. Ann Plast Surg 29:297–298. 10.1097/00000637-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Animal Welfare Regulations. 2013. 9 CFR § 3.129.
- 5.Association of Surgical Technologist. [Internet]. 2008. AST standards of practice for skin prep of the surgical patient. [Cited 17 April 2020]. Available at: http://www.ast.org/uploadedfiles/main_site/content/about_us/standard_skin_prep.pdf
- 6.Bernal J, Baldwin M, Gleason T, Kuhlman S, Moore G, Talcott M. 2009. Guidelines for rodent survival surgery. J Invest Surg 22:445–451. 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- 7.Bruce J, Russell EM, Mollison J, Krukowski ZH. 2001. The measurement and monitoring of surgical adverse events. Health Technol Assess 5:1–194. 10.3310/hta5220. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Internet]. 2020. National Healthcare Safety Network (NHSN). Surgical Site Infections (SSI) Events. [Cited 17 April 2020]. Available at: https://www.cdc.gov/nhsn/faqs/faq-ssi.html.
- 9.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DM, Mciver R, Bianco R. 2000. The thin blue line: a review and discussion of aseptic Technique and postprocedural infections in rodents. Contemp Top Lab Anim Sci 39:27–32. Erratum.2001. Contemp Top Lab Anim Sci 40: 49. [PubMed] [Google Scholar]
- 11.Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. 2011. Animal models of external traumatic wound infections. Virulence 2:296–315. 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Valle JM, Fisk EA, Noland EL, Pak D, Zhang J, Crim MJ, Lawrence FR, Hankenson FC. 2018. Comparison of aqueous and alcohol-based agents for presurgical skin preparation methods in mice. J Am Assoc Lab Anim Sci 57:401–414. 10.30802/AALAS-JAALAS-17-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallon MT, Shafer W, Jacob E. 1996. Use of cefazoline microspheres to treat localized methicillin-resistant staphylococcus aureus infections in rats. J Surg Res 86:97–102. 10.1006/jsre.1999.5686. [DOI] [PubMed] [Google Scholar]
- 14.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. [Internet]. 2013. Freedom of Information Summary: BUPRELAB-RAT (Buprenorphine Extended-release Injection). [Cited 17 April 2020]. Available at: https://www.fda.gov/media/87135/download
- 16.Guillory AN, Clayton RP, Prasai A, Jay JW, Wetzel M, El Ayadi A, Herndon DN, Finnerty CC. 2018. Buprenorphine-sustained release alters hemodynamic parameters in a rat burn model. J Surg Res 232:154–159. 10.1016/j.jss.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton HW, Hamilton KR, Lone FJ. 1977. Preoperative hair removal. Can J Surg 20:269–274. [PubMed] [Google Scholar]
- 18.Huss MK, Casey KM, Hu J, Moorhead RC, Chum HH. 2020. Evaluation of 3 alcohol-based agents for presurgical skin preparation in mice. J Am Assoc Lab Anim Sci 59:67–73. 10.30802/AALAS-JAALAS-19-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IBM Corporation. 2017. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk (NY): IBM Corps. [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC: ): National Academies Press. [Google Scholar]
- 21.Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, Holmes A, Latcham J, Petrie A, Potter P, Rice A, Ritchie A, Stewart M, Strepka C, Yeoman M, Chapman K. 2016. Does age matter? The impact of rodent age on study outcomes. Lab Anim 51:160–169. 10.1177/0023677216653984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jose B, Dignon A. 2013. Is there a relationship between preoperative shaving (hair removal) and surgical site infection? J Perioper Pract 23:22–25. 10.1177/1750458913023001-203. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser AB, Kernodle DS, Parker RA. 1992. Low-inoculum model of surgical wound infection. J Infect Dis 166:393–399. 10.1093/infdis/166.2.393. [DOI] [PubMed] [Google Scholar]
- 24.Karegoudar JS, Prabhakar PJ, Vijayanath V, Anitha MR, Surpur RR, Patil VM. 2011. Shaving versus depilation cream for pre-operative skin preparation. Indian J Surg 74:294–297. 10.1007/s12262-011-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kick BL, Gumber S, Wang H, Moore RH, Taylor DK. 2019. Evaluation of 4 presurgical skin preparation methods in mice. J Am Assoc Lab Anim Sci 58:71–77. 10.30802/AALAS-JAALAS-18-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klopfleisch R. 2013. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology—a systematic review. BMC Vet Res 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, Kawano J. 2002. Isolation and species distribution of Staphylococci from animal and human skin. J Vet Med Sci 64:245–250. 10.1292/jvms.64.245. [DOI] [PubMed] [Google Scholar]
- 28.Orsi GB, Ferraro F, Franchi C. 2005. [The preoperative trichotomy: role in the prevention of surgical site infections]. [Article in Italian]. Ann Ig 17:401–412.16353677 [Google Scholar]
- 29.Philips BH, Crim MJ, Hankenson FC, Steffen EK, Klein PS, Brice AK, Carty AJ. 2015. Evaluation of presurgical skin preparation agents in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 54:788–798. [PMC free article] [PubMed] [Google Scholar]
- 30.Pritchett-Corning KR, Mulder GB, Luo Y, White WJ. 2011. Principles of rodent surgery for the new surgeon. J Vis Exp (47). 1–4. 10.3791/2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle LE, Raiciulescu S, Mullins AB, Foster CD. 2019. Evaluation of medicated gel as a supplement to providing acetaminophen in the drinking water of Sprague Dawley rats after surgery. The Internet Journal of Veterinary Medicine 15:1–8. DOI: 10.5580/IJVM.53539. [Google Scholar]
- 32.Schaudinn C, Dittmann C, Jurisch J, Laue M, Gunday-Tureli N, Blume-Peytavi U, Vogt A, Rancan F. 2017. Development, standardization and testing of a bacterial wound infection model based on ex vivo human skin. PLoS One 12:1–13. 10.1371/journal.pone.0186946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, Stoodley P, Swanson T, Tachi M, Wolcott RD, Global Wound Biofilm Expert P. 2017. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 25:744–757. 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 34.Smith MA, Dahlen NR. 2013. Clinical practice guideline surgical site infection prevention. Orthop Nurs 32:242–248, quiz 249–250. 10.1097/NOR.0b013e3182a39c6b. [DOI] [PubMed] [Google Scholar]
- 35.Spruce L. 2016. Back to basics: surgical skin antisepsis. AORN J 103:96–100, quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 36.Tanner J, Moncaster K, Woodings D. 2007. Preoperative hair removal: a systematic review. J Perioper Pract 17:118–121. 10.1177/175045890701700304. [DOI] [PubMed] [Google Scholar]
- 37.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, James GA, Olerud JE, Fleckman P. 2010. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci 49:588–591. [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AC, Kristal MB, Sallaj A, Acheson A, Martin LBE, Martin T. 2004. Analgesic efficacy of orally administered buprenorphine in rats: methodologic considerations. Comp Med 54:293–300. [PubMed] [Google Scholar]
- 39.Tsukamoto A, Konishi Y, Kawakami T, Koibuchi C, Sato R, Kanai E, Inomata T. 2017. Pharmacological properties of various anesthetics protocols in 10-day-old neonatal rats. Exp Anim 66:397–404. 10.1538/expanim.17-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsukamoto A, Niino N, Sakamoto M, Ohtani R, Inomata T. 2018. Validity of anesthetic protocols for the surgical procedure of castration in rats. Exp Anim 67:329–336. 10.1538/expanim.18-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson AP, Sturridge MF, Treasure T, Grüneberg RN. 1986. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 327:311–312. 10.1016/S0140-6736(86)90838-X. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. [Internet]. 2016. Global guidelines on the prevention of surgical site infection. Appendix 8: Summary of the systematic review on surgical site preparation. [Cited 18 April 2020]. Available at: https://www.who.int/gpsc/appendix8.pdf?ua=1.
- 43.World Health Organization. [Internet]. 2016. WHO surgical site infection prevention guidelines. Appendix 7: Summary of a systematic review on the effectiveness and optimal method of hair removal. [Cited 18 April 2020]. Available at: https://www.who.int/gpsc/appendix7.pdf.
- 44.Yarboro SR, Baum EJ, Dahners LE. 2007. Locally administered antibiotics for prophylaxis against surgical wound infection. J Bone Joint Surg Am 89:929–933. 10.2106/00004623-200705000-00002. [DOI] [PubMed] [Google Scholar]