Abstract
Worldwide, patients with urothelial carcinoma characterized by a bone-exclusive metastatic spread usually present with poor performance status, have limited access to active therapy, and have a poor outcome. Consequently, treatments offered and outcomes should be improved in this rare subgroup.
Background:
Patients with exclusive bone metastatic spread from urothelial carcinoma (UC) throughout their disease course represent a rare subgroup with unique clinical features. These patients deserved special consideration in a retrospective multicenter study.
Patients and Methods:
Analyses were made from a pool of 1911 patients with a diagnosis of metastatic UC, from 23 centers. Baseline characteristics, access to treatment, and outcomes were analyzed according to metastatic spread. Univariable and multivariable Cox analyses were performed.
Results:
A total of 128 evaluable patients (6.7%), diagnosed between February 1997 and April 2013, were identified. Eastern Cooperative Oncology Group performance status (PS) was ≥ 2 in 33.3% versus 17.7% of the remaining patients. Seventy-three (57%) received first-line chemotherapy, that was platinum-based in 50 patients (69%). Twenty-eight (21.9%) received second-line chemotherapy (vs. 75.9% and 32.2%, respectively, of the remaining patients). In multivariable analyses, no clinical factor was significantly associated with overall survival (OS). Among platinum chemotherapy-treated patients (total evaluable n = 972), significantly different relapse-free survival (RFS) and OS were observed according to bone metastases status (no bone metastases vs. bone metastases only vs. bone and other sites, P < .001). In these groups, 2-year RFS was 37.4%, 28.8%, and 25.9%, respectively. Two-year OS was 35.5%, 15.8%, and 23%, respectively.
Conclusion:
Patients with metastatic UC and bone-only metastases are less likely to receive systemic therapy than those with other metastases, likely because of their lower PS. The prognostic effect of having exclusive bone metastases or additional sites seems to be equally poor. These patients deserve new effective and tolerable agents, and improvements in the knowledge of their disease.
Keywords: Bladder cancer, Bone metastases, Outcomes of bone-only metastatic urothelial carcinoma, Outcomes of chemotherapy, Prognosis
Introduction
The development of metastases from urothelial carcinoma (UC) represents a relatively rare but deadly event, except for patients who present with a regional lymph node involvement, who might be suitable for combined modality treatment.1 Overall survival (OS) outcomes with conventional, platinum-based, chemotherapy depend on the possibility of administering cisplatin versus carboplatin-based regimens, in addition to key baseline factors.2–4 For cisplatin-ineligible patients, the possibility of administering immune checkpoint inhibitors as an alternative to carboplatin-based chemotherapy might further improve prognosis.5,6 However, the median OS of patients with metastatic UC might vary from < 10 to > 15 months, according to the treatment. During the past 2 decades, we have learned that OS probability is closely dependent on baseline patient- and disease-related factors. Conventionally, the presence of bone metastases, with or without liver or pulmonary involvement (LLB), is defined as “visceral metastases” from UC, and it is recognized as a negative prognostic factor in a model developed at the Memorial Sloan Kettering Cancer Center, together with Karnofsky performance status (PS), in patients receiving cisplatin-based chemotherapy.7 Subsequently, these factors have been augmented with additional factors like albumin, leukocyte count, hemoglobin level, and the number of metastatic sites.8,9 In more recent years, the presence of LLB metastases was included in a nomogram for OS calculation that was developed by the authors of the current study on the basis of the data obtained from the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) database.10 Interestingly, none of the 3 factors was an independent prognostic indicator of OS, although hepatic involvement in patients receiving second-line chemotherapy was independently prognostic for OS.11 There is a small subgroup of patients with metastatic UC whose tumor shows an exquisite bone tropism and who develop predominant or exclusive skeletal metastases. Literature about these patients is limited to a few case reports, and discordant findings have been reported regarding the clinical course and prognosis of such patients.12,13 However, these patients should constitute a special population because of their unique clinical course, including the occurrence of bone metastases-related complications, that are likely to cause early deterioration of PS and low rates of access to chemotherapy. Also, because of the lack of measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) in most cases,14 the likelihood of enrollment of such patients in clinical trials is very low. Consequently, access to effective systemic therapy that might prolong survival is frequently prevented by multiple factors, and the outcome is generally poor. This limitation might be well perceived nowadays, because the shifting therapeutic landscape of metastatic UC prompted investigators to develop a huge number of clinical trials that are combining various novel agents, with or without immunotherapy.15 We aimed to retrospectively analyze the population of patients with bone-limited metastatic UC in a multicenter study.
Patients and Methods
Patient Population
We performed a retrospective study encompassing individual patient-level data from patients with muscle-invasive or advanced UC or nonurothelial histology who have received systemic therapy during the course of their disease. This contemporary database includes data gathered from hospitals in the United States, Europe, Israel, and Canada. The RISC study was approved by the ethics committee at each participating institution. In March 2017, data were extracted to select patients with the following characteristics: any primary tumor site, predominant UC histology, and diagnosis of metastatic disease. Data analysis was performed externally by a senior statistician (G.R.P.). The arbitrary definition we used to define patients with bone-exclusive disease was the following: evidence at conventional imaging of metastatic bone metastases, presence of either single or multiple lesions, and no other metastatic site occurrence until the last follow-up or death, or until the receipt of third-line chemotherapy for those who accessed systemic therapy. We had no information about histologic confirmation of bone lesions, because it was not required in this database.
Statistical Analyses
Patient, disease, and outcome characteristics were summarized using descriptive statistics, with frequencies and percentages used for categorical variables and medians and interquartile range used for continuous variables.
In this descriptive, retrospective analysis, assessments included the proportion of patients with bone-exclusive metastatic UC who received treatment and the outcomes of such patients. Additional analyses included the prognostic effect of clinical baseline factors, as well as the survival outcomes of those who received platinum-based chemotherapy according to the bone metastatic spread. OS was the primary end point, whereas relapse-free survival (RFS) and objective response (OR) were the secondary end points. OS as well as RFS were measured from the date of first diagnosis of metastatic disease, whereas the OR was assessed at each site by the local investigators. The Kaplane–Meier method was used to estimate time-to-event outcomes. Cox proportional hazards regression was used to investigate potential prognostic factors of OS and RFS. Complete case analysis was performed, and no multiple imputation was performed for missing data. Because clinical data were missing in many cases, because of the retrospective nature of this analysis, multivariate models were constructed on the basis of prespecified factors that were hypothesized to be clinically important, and univariable analyses were left as exploratory only. To evaluate the outcomes in the platinum-treated patients subgroup, we resorted the database of the 1020 patients that was used to construct the RISC nomogram,10 from which we stratified patients according to the metastatic sites as follows: bone metastases only versus bone with other metastatic sites versus other metastatic sites. Treatment center was used as a stratification factor throughout the analyses. All analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC).
Results
Patient, Disease, and Treatment Characteristics and Outcomes
The study flow chart, with patient selection and reasons for study exclusion, is presented in Figure 1. Among the 1911 patients diagnosed with metastatic UC, a total of 128 evaluable patients (6.7%; ie, the study group) were identified, diagnosed between February 1997 and April 2013, from 23 contributing centers. Table 1 presents the baseline characteristics of the study group, coupled with those of patients from the remaining RISC population (n = 1781; control group). There were no substantial differences between the 2 groups according to the clinical and laboratory parameters, except for more Eastern Cooperative Oncology Group (ECOG) PS ≥ 2 cases in the study group (33.3% vs. 17.7%). Most importantly, fewer patients had access to first-line chemotherapy in the study group compared with the control group (57.0% vs. 75.9%), and the same was also for second-line therapy (21.9% vs. 32.2%), and third-line therapy (5.5% vs. 13%). Among the 55 patients who did not receive any systemic therapy in the study group, 24 received palliative radiotherapy on metastatic bone lesions. No information was available regarding the administration of bisphosphonates in the study group.
Figure 1.
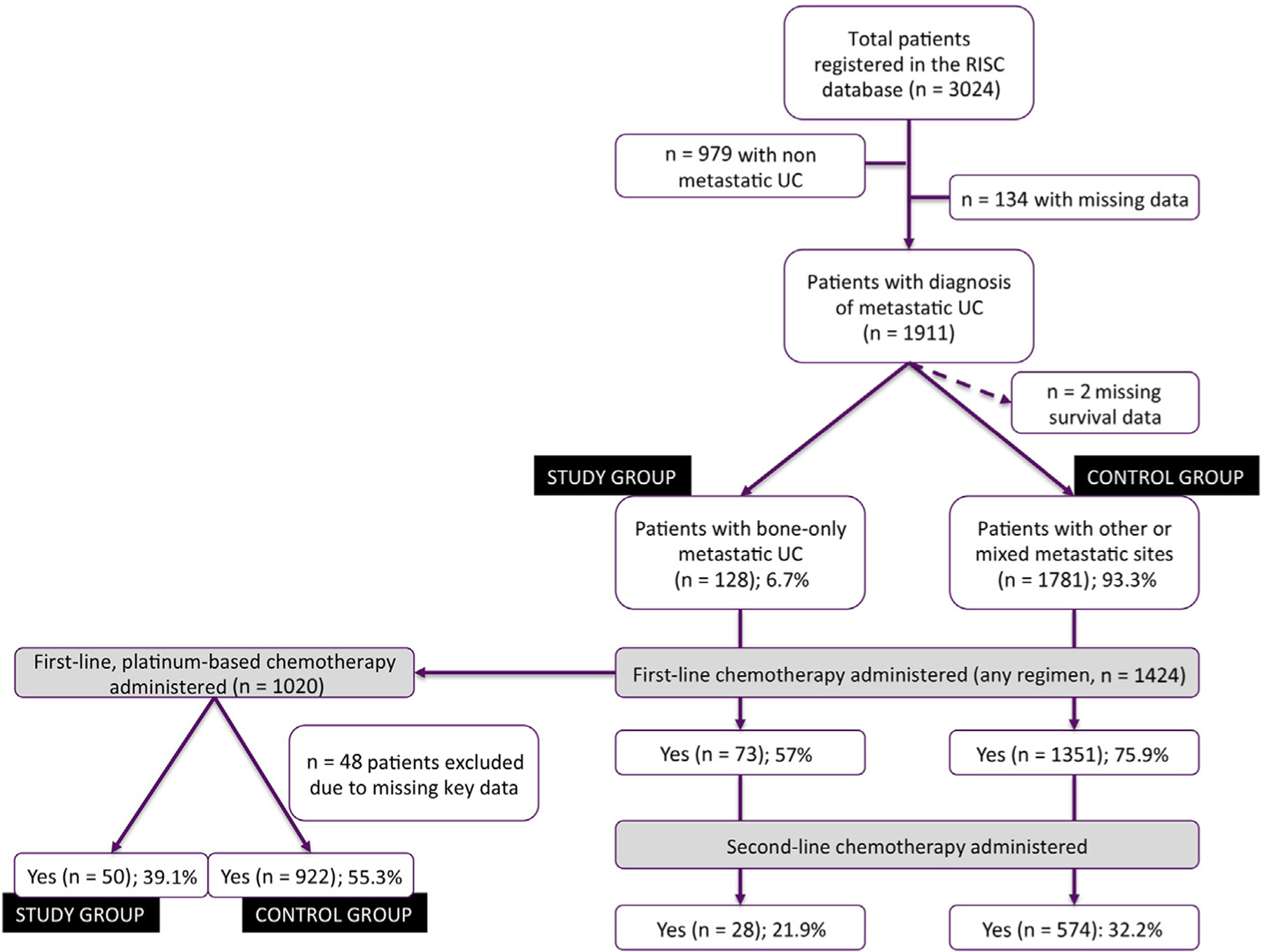
Study Flow Chart, With Counts and Reasons for Patient Selection
Abbreviations: RISC = Retrospective International Study of Invasive/Advanced Cancer of the Urothelium; UC = urothelial carcinoma
Table 1.
Patient and Disease Characteristics at the Time of Diagnosis of Metastatic UC, RISC Database
| Characteristic | Statistic | Bone Only Data Set (n = 128) | Other (n = 1781) |
|---|---|---|---|
| Age at Diagnosis | Median (IQR), years | 69 (60–76) | 66 (60–75) |
| Gender | Male | 104 (81.3) | 1368 (77.1) |
| Female | 24 (18.7) | 413 (23.3) | |
| Missing | – | 8 | |
| Race | Asian | 3 (2.3) | 40 (2.2) |
| Black | 2 (1.6) | 73 (4.1) | |
| Hispanic | 4 (3.1) | 24 (1.3) | |
| White | 116 (90.6) | 1611 (90.5) | |
| Not stated | 3 (2.3) | 10 (0.6) | |
| Other | – | 23 (1.3) | |
| Ethnicity | Hispanic or Latino | 15 (12.1) | 142 (8.4) |
| Not Hispanic/Latino | 109 (87.9) | 1556 (91.6) | |
| Missing | 4 | 83 | |
| Smoking Status | Current smoker | 34 (26.6) | 348 (19.5) |
| Former smoker | 41 (32.0) | 689 (38.7) | |
| Never smoker | 31 (24.2) | 453 (25.4) | |
| Not stated | 22 (17.2) | 291 (16.4) | |
| BMI | Mean (SD) | 25.4 (3.7) | 26.6 (4.8) |
| Missing | 81 | 833 | |
| Charlson Comorbidity Index | Mean (SD) | 1.9 (2.3) | 1.9 (2.4) |
| Missing | 7 | 136 | |
| ECOG PS | 0 | 8 (14.0) | 354 (33.7) |
| 1 | 30 (52.7) | 510 (48.6) | |
| ≥2 | 19 (33.3) | 186 (17.7) | |
| Missing | 71 | 731 | |
| Hemoglobin, g/dL | Median (IQR) | 12 (10.6–13.3) | 12 (10.7–13.4) |
| Missing | 63 | 638 | |
| Platelet × 103/μL | Median (IQR) | 293 (222.7–381) | 292 (222–380) |
| Missing | 65 | 640 | |
| Calcium, mg/dL | Mean (SD) | 9.4 (1.0) | 9.3 (0.6) |
| Missing | 71 | 769 | |
| Albumin, g/dL | Mean (SD) | 3.6 (0.7) | 5.4 (0.6) |
| Missing | 78 | 849 | |
| Primary Tumor Location | Bladder | 113 (94.2) | 1473 (85.3) |
| Renal pelvis | 5 (4.2) | 163 (9.4) | |
| Ureter | 2 (1.6) | 77 (4.4) | |
| Urethra | – | 14 (0.9) | |
| Missing | 8 | 54 | |
| Histology | UC | 90 (70.3) | 1376 (77.3) |
| UC with variant histology | 15 (11.7) | 148 (9.4) | |
| Adenocarcinoma | 4 (3.1) | 45 (2.5) | |
| Squamous-cell carcinoma | 3 (0.8) | 37 (2.6) | |
| Micropapillary variant | 2 (0.8) | 15 (0.8) | |
| Other | 1 (0.8) | 160 (9.0) | |
| Previous Neoadjuvant Chemotherapy | Yes | 20 (15.6) | 277 (16.4) |
| Missing | – | 95 | |
| Previous Adjuvant Chemotherapy | Yes | 20 (15.6) | 277 (16.5) |
| Missing | – | 103 | |
| Surgical Removal of Primary Tumor | Yes | 64 (50.0) | 1049 (60.1) |
| Missing | – | 36 | |
| Time From Diagnosis to Metastatic UC, Months | Median (IQR) | 5.3 (0–10) | 5 (0–15) |
| Sites of Metastases | Bone only | 128 (100) | – |
| Bone and other | – | 362 (20.3) | |
| Lung or liver | – | 500 (28.1) | |
| Other | – | 919 (51.6) | |
| First-Line Chemotherapy Administered | Yes | 73 (57.0) | 1351 (75.9) |
| No | 55 (43.0) | 430 (24.1) | |
| Duration of First-Line Chemotherapy, Months | Median (IQR) | 2.8 (0–49.0) | 2.0 (1–4) |
| Number of Cycles | Median (IQR) | 4 (2–6) | 4 (3–6) |
| First-Line Chemotherapy Regimen | Gemcitabine with cisplatin | 23 (31.5) | 352 (26.0) |
| Gemcitabine with carboplatin | 21 (28.8) | 264 (19.5) | |
| MVAC or DD-MVAC | 5 (6.9) | 164 (12.1) | |
| Other or missing | 24 (32.9) | 571 (42.4) | |
| First-Line, Platinum-Based Chemotherapy | Yes | 50 (39.1) | 922 (55.3) |
| Missing | – | 114 | |
| Second-Line Chemotherapy Administered | Yes | 28 (21.9) | 574 (32.2) |
| Third-Line Chemotherapy Administered | Yes | 7 (5.5) | 232 (13.0) |
Data are presented as n (%) except where otherwise noted.
Abbreviations: BMI = body mass index; DD-MVAC = dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; ECOG PS = Eastern Cooperative Oncology Group performance status; IQR = interquartile range; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; RISC = Retrospective International Study of Invasive/Advanced Cancer of the Urothelium; UC = urothelial carcinoma.
Relapse-Free Survival, OS Outcomes, and Results of the Cox Proportional Hazards Regression Analyses in the Bone-Only Metastatic Group
The median RFS and OS of patients in the study group were 6.4 months (95% confidence interval [CI], 5–7.7 months) and 10.8 months (95% CI, 6.7–14 months), respectively. In the study group, the more chemotherapy regimens the patients received throughout their treatment course the longer the OS, with a statistically significant trend (P < .001; Figure 2). Results of the univariable and multivariable analyses for OS are shown in Table 2. There were no baseline factors resulting as statistically significant associated with OS except for access to second- or third-line regimens; this factor was not included in the multivariable model. Regarding the RFS outcome, ECOG PS ≥ 2 (hazard ratio [HR], 1.99; 95% CI, 1.08–3.67; P = .027) and the presence of mixed histologies (HR, 3.06; 95% CI, 1.15–8.14; P = .008) were univariably significantly detrimental factors, but no multivariable model was constructed for RFS.
Figure 2.
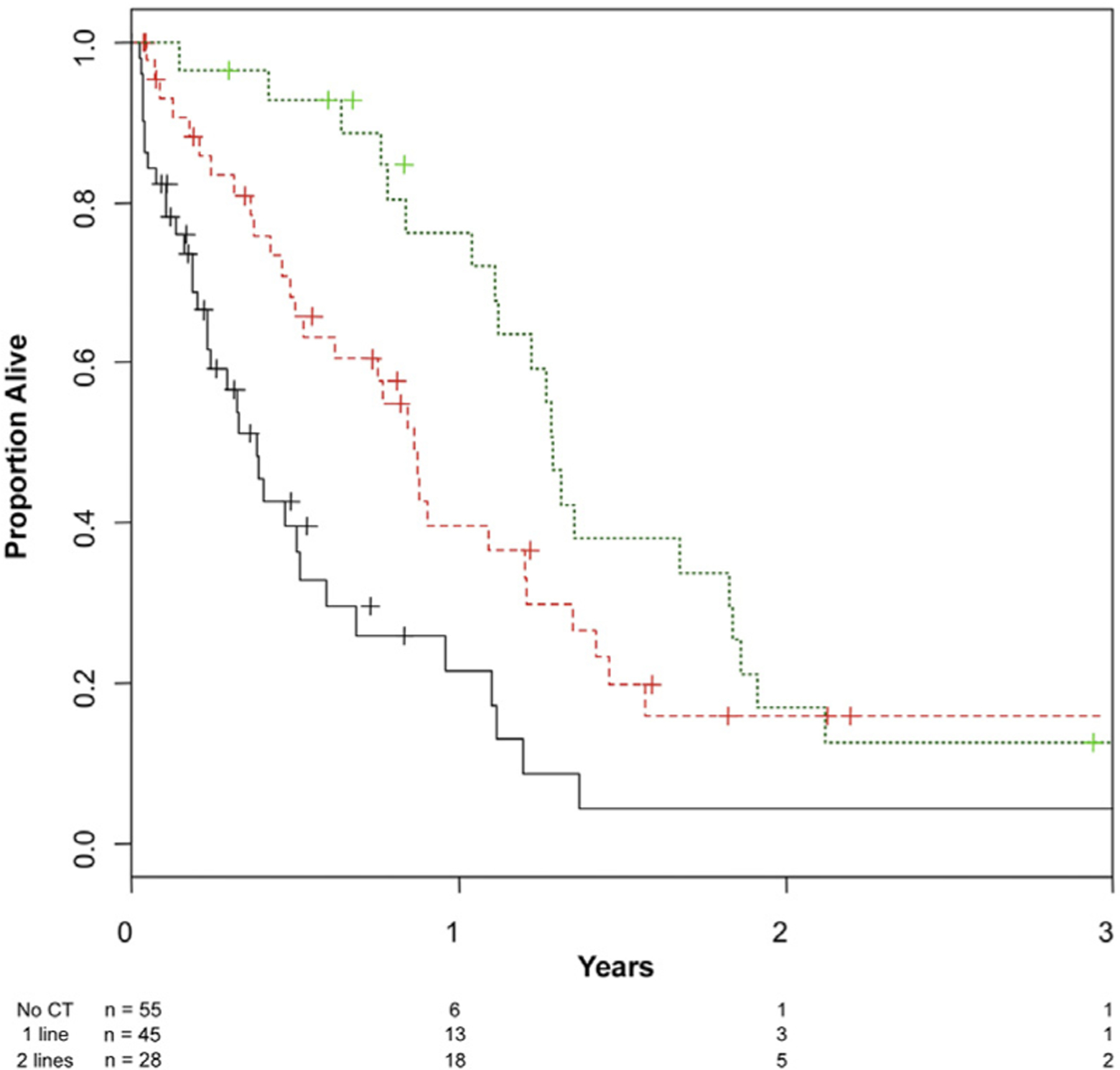
Kaplan–Meier Curves of Overall Survival (OS) in the Cohort of Patients With Bone-Only Metastases From UC, Stratified According to the Number of Treatment Regimens They Received. Green Line: OS of Patients Who Have Received 2 or More Chemotherapy (CT) Regimens for Metastatic UC; Red Line: OS of Patients Who Have Received First-Line CT Only; Black Line: OS of Patients Who Have Not Received Any CT
Abbreviation: UC = urothelial carcinoma
Table 2.
Results of the Univariable and Multivariable Cox Regression Analyses on OS
| Factor | Univariable Analyses | Multivariable Analyses | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (Continuous) | 1.01 | 1.00–1.03 | .15 | 1.02 | 0.99–1.05 | .18 |
| Time From Diagnosis to the Evidence of Metastatic UC | .31 | |||||
| ≥6 Months versus <6 months | 1.25 | 0.81–1.92 | ||||
| Charlson Score | .35 | .12 | ||||
| ≥2 versus <2 | 1.23 | 0.80–1.88 | 1.50 | 0.90–2.51 | ||
| BMI (Continuous) | 0.93 | 0.84–1.02 | .13 | |||
| Serum Calcium Level (Continuous) | 1.20 | 0.80–1.81 | .38 | |||
| Serum Albumin (Continuous) | 0.69 | 0.45–1.06 | .089 | |||
| Gender | .90 | |||||
| Female versus male | 0.97 | 0.56–1.67 | ||||
| Race | .20 | |||||
| White versus other | 1.72 | 0.75–3.94 | ||||
| Ethnicity | .55 | |||||
| Hispanic or Latino versus other | 0.82 | 0.43–1.57 | ||||
| Smoking History | .16 | .14 | ||||
| Current smoker | 0.67 | 0.36–1.25 | 1.08 | 0.52–2.22 | ||
| Former smoker | 1.33 | 0.26–0.93 | 0.61 | 0.31–1.23 | ||
| Never smoker | 0.77 | 0.28–1.10 | 0.53 | 0.24–1.18 | ||
| Not stated | Reference | Reference | ||||
| ECOG PS | .001 | |||||
| ≥2 versus <2 | 2.86 | 1.50–5.45 | ||||
| Primary Tumor | .56 | .30 | ||||
| Bladder versus other | 1.22 | 0.63–2.38 | 0.64 | 0.28–1.49 | ||
| Histology | .67 | .26 | ||||
| TCC | 0.91 | 0.39–2.12 | 0.85 | 0.34–2.12 | ||
| TCC and variant histologies | 1.33 | 0.49–3.61 | 1.72 | 0.55–5.40 | ||
| Other histologies | 0.77 | 0.21–2.73 | 0.75 | 0.19–2.96 | ||
| Not stated | Reference | Reference | ||||
| Neoadjuvant Chemotherapy | .092 | |||||
| No versus yes | 0.62 | 0.35–1.08 | ||||
| Adjuvant Chemotherapy | .19 | |||||
| No versus yes | 1.48 | 0.83–2.64 | ||||
| Neoadjuvant or Adjuvant Chemotherapy: Yes versus No | 1.00 | .73 | ||||
| 1.00 | 0.64–1.57 | 0.90 | 0.48–1.68 | |||
| Surgical Resection of the Primary Tumor | .95 | |||||
| No versus yes | 1.01 | 0.66–1.55 | ||||
| Number of Administered Chemotherapy Regimens for Metastatic Disease | < .001 | |||||
| No chemotherapy | 1.87–5.76 | |||||
| 1 Regimen | 3.28 | 0.89–2.69 | ||||
| ≥2 Regimens | 1.55 | Reference | ||||
| Type of First-Line Chemotherapy Regimen | .17 | |||||
| Gemcitabine with cisplatin | 0.92 | 0.45–1.88 | 0.28 | 0.13–0.59 | ||
| Gemcitabine with carboplatin | 1.87 | 0.92–3.79 | 0.51 | 0.27–0.99 | ||
| MVAC or DD-MVAC | 1.65 | 0.54–5.00 | 0.28 | 0.14–0.53 | ||
| Other regimen | Reference | Reference | ||||
Abbreviations: BMI = body mass index; DD-MVAC = dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; OS = overall survival; TCC = transitional-cell carcinoma; UC = urothelial carcinoma.
Outcomes of Platinum-Based Chemotherapy According to Bone Metastases Status
Activity and efficacy outcomes of platinum-based chemotherapy are shown in Table 3, coupled with the type of platinum chemotherapy administered. The proportion of patients who received cisplatin-based chemotherapy was similar between the “bone-only” and “no bone metastases” groups (62% and 66.2%, respectively), and lower for the remaining patients (55.4%; P = .014). OR were less frequent in the “bone-only” group (14%) compared with the other groups (38.3% and 37.7%; P = .003), and significantly lower RFS and OS outcomes were observed in the 2 groups of patient with bone metastases, as is shown in Figure 3 (overall P = .003 for RFS and P < .001 for OS). The median OS of bone-only metastatic patients was 14.4 months (95% CI, 10.8–17.4 months) versus 16.7 months (95% CI, 15.2–18.5) for those without bone metastases.
Table 3.
Platinum Chemotherapy Type, Response and Outcome of Patient Cohorts According to the Metastatic Sites, Including Patients Who Had Received Platinum-Based Chemotherapy Only
| Characteristic | Variable | No Bone Metastases | Bone Only | Bone and Other Metastases | P |
|---|---|---|---|---|---|
| n | 718 | 50 | 204 | ||
| Type of Platinum, n (%) | Cisplatin | 475 (66.2) | 31 (62.0) | 113 (55.4) | .014 |
| Carboplatin | 243 (33.8) | 14 (28.0) | 91 (44.6) | ||
| Platinum unknown | – | 5 (10.0) | – | ||
| Objective Response, n (%) | CR | 69 (9.6) | 2 (4.0) | 9 (4.4) | .003 |
| PR | 206 (28.7) | 5 (10.0) | 68 (33.3) | ||
| SD | 146 (20.3) | 17 (34.0) | 41 (20.1) | ||
| PD | 152 (21.2) | 17 (34.0) | 53 (26.0) | ||
| Other | 145 (20.2) | 9 (18.0) | 33 (16.2) | ||
| RFS | Events, n (%), | 569 (79.3) | 43 (86.0) | 182 (89.2) | .003 |
| Median (95% CI), months | 9.0 (8.2–9.9) | 7.0 (5.4–9.7) | 7.8 (7.0–9.2) | ||
| 3-Month (95% CI) | 89.2 (86.6–91.2) | 81.3 (67.2–89.8) | 85.3 (79.6–89.4) | ||
| 6-Month (95% CI) | 68.4 (64.5–71.5) | 62.2 (46.9–74.3) | 61.9 (54.8–68.2) | ||
| 1 Year (95% CI) | 37.4 (33.7–41.1) | 28.8 (16.5–42.3) | 25.9 (19.9–32.2) | ||
| OS | Deaths, n (%) | 446 (62.1) | 40 (80.0) | 148 (72.5) | < .001 |
| Median (95% CI), months | 16.7 (15.2–18.5) | 14.4 (10.8–17.4) | 12.0 (11.0–14.1) | ||
| 3-Month (95% CI) | 96.5 (94.8–97.6) | 91.7 (79.5–96.8) | 92.6 (88.1–95.5) | ||
| 6-Month (95% CI) | 87.4 (84.7–89.7) | 81.1 (66.8–89.7) | 82.0 (75.9–86.7) | ||
| 1 Year (95% CI) | 66.4 (62.6–69.9) | 55.5 (39.7–68.7) | 50.3 (42.9–57.3) | ||
| 2 Years (95% CI) | 35.5 (31.5–39.5) | 15.8 (6.6–28.6) | 23.0 (16.8–29.8) |
Abbreviations: OS = overall survival; RFS = relapse-free survival.
Figure 3.
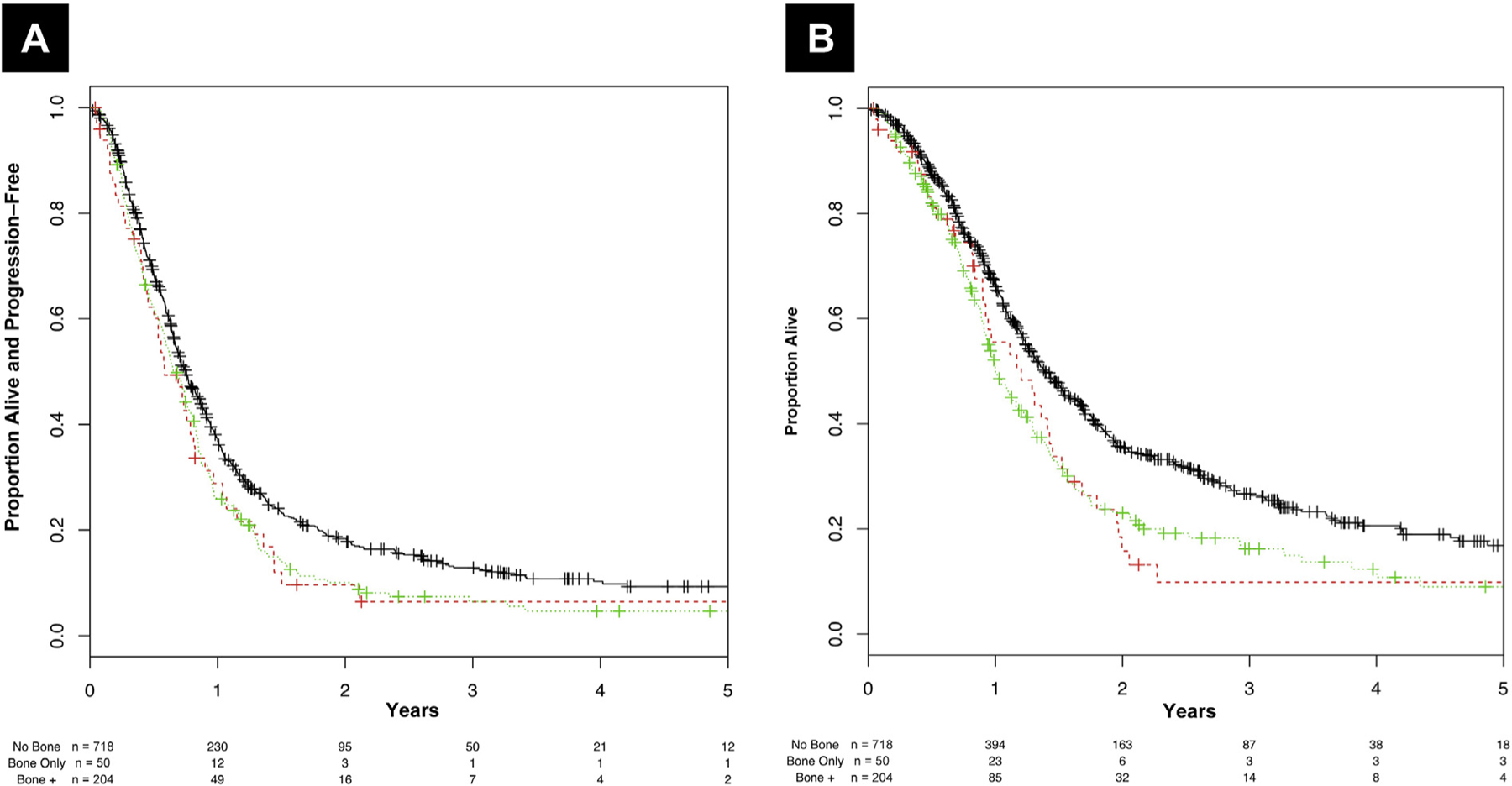
Kaplan–Meier Curves of (A) Relapse-Free Survival and (B) Overall Survival in the Cohort of Patients Who Received First-Line Platinum-Based Chemotherapy, Stratified According to the Sites of Metastatic Spread. Black Line: Patients Without Bone Metastases; Red Line: Patients With Bone Metastases Only; Green Line: Patients With Bone Metastases in Addition to Other Metastatic Sites (Bone +)
Discussion
In this report we present, for the first time to our knowledge, an analysis of patients with metastatic UC whose disease spread was limited to the bones throughout their clinical course. Such patients constitute an intriguing and rare subgroup who might deserve special focus for several reasons, which we attempted to analyze. Despite that skeletal involvement from UC is a frequent event, occurring in the range of 25% to 47% of patients with an advanced UC diagnosis,16–18 the development of exclusive bone metastatic spread occurred in < 7% of cases in our large population. In general, limited information is available in the literature regarding the incidence, treatment patterns, and clinical outcomes of such patients. An important finding of our study is that patients with bone-only metastases were less likely to receive chemotherapy than the rest of metastatic patients. A not negligible proportion of patients in the study group received supportive care alone or palliative radiotherapy on metastatic bone lesions, and this observation is likely to depend on patients’ poor PS, perhaps limited because of pain, at the time of diagnosis. Of note, the number of untreated patients is likely to be underestimated because of inherent biases of patient selection. In fact, only patients who received chemotherapy at any clinical stage (since neoadjuvant chemotherapy administration and onward) were eligible for the RISC project, but there might be additional patients who never received chemotherapy, developed bone metastatic disease, and were left out of the database. Critical barriers that limited treatment across lines of therapy include the ineligibility of many patients to receive platinum chemotherapy (cisplatin as well as carboplatin), mainly as a result of their poor PS of 2 at diagnosis. Furthermore, approximately 43% of patients with bone-only metastases did not receive any chemotherapy, and this limitation might be attributable to many reasons like the following: the need to delay systemic therapy in favor of palliative radiotherapy, or rapid decline in PS because of the onset of severe pain syndrome or bone metastases-related complications like hypercalcemia or skeletal-related events. All of these events would have led to systemic worsening of disease and prevent patients from receiving chemotherapy. Unfortunately, the occurrence of such events was not captured in the RISC database, but at least we knew that the recorded baseline laboratory levels were not significantly different compared with the total number of patients with metastatic UC. Among the patients who received platinum-based chemotherapy, there were some interesting findings. First, the major limitation in bone-only metastatic patients seems to be the ineligibility for chemotherapy rather than cisplatin. The small proportion of patients who could receive first-line chemotherapy were mainly administered cisplatin-based regimens, similar to those without bone metastases. Second, despite the administration of cisplatin-containing chemotherapy, bone metastases portended poor prognosis irrespective of the presence of additional sites, and the few patients who could receive additional therapies had the longest survival. An important limitation of our study lies in the methods used to identify bone metastases and to assess their response to treatment. In fact, skeletal involvement in metastatic UC patients can be under-recognized, depending on the staging procedures that are used as routine clinical practice at each center. Most importantly, the assessment of response to treatment in bony lesions might be subjected to huge discrepancies between investigators, especially in the context of conventional treatments administered outside of clinical trials, as in our case. No standard criteria are routinely used outside of clinical trials to identify and subsequently evaluate response in bone lesions. Furthermore, the adoption of non-RECIST criteria (like the Positron Emission Tomography Response Criteria in Solid Tumors criteria)19 in UC is still anecdotal and not widely used. Such a limitation also applies to our study, because the methods of response assessment were not recorded in the RISC database, and OR attribution (secondary end point in our study) was left to the investigators’ judgement. Not surprisingly, we observed less OR in the study group compared with the remaining groups, for which the ORs were similar despite small discrepancies in the rate of complete and partial responses. Of course, there might be also a chemoresistant underlying biology in the tumors that presented with distinct bone tropism, but unfortunately getting access to bone biopsy samples is difficult, and thus limits the possibility of performing translational studies. The few translational data on the activity of new drugs on bone metastases refer to the use of metabolic imaging as a tool to evaluate response to treatment. An example is represented by cabozantinib, a receptor tyrosine kinase inhibitor primarily targeting MET and vascular endothelial growth factor receptor, that showed distinct responses in bone lesions of chemotherapy-treated patients.20 In general, the lack of measurable disease according to RECIST criteria often prevents the possibility of including patients with exclusive bone metastases in clinical trials. The availability of multiple clinical trials with variously effective and well tolerated drugs would represent an important opportunity for these patients, allowing for an improvement of the critically low proportion of those who can currently receive systemic therapy.
Conclusion
To our knowledge, this study represents the largest clinical assessment of the characteristics, treatments, and outcomes of patients with bone-exclusive metastatic UC. Results showed that access rates for chemotherapy in this subgroup are lower than for the general population of patients with metastatic UC. Among those who received first-line, platinum-based chemotherapy, the outcomes were poor despite the administration of cisplatin chemotherapy, and notwithstanding the presence of additional metastatic sites. Bone-only metastatic patients deserve additional studies to better understand the biology underlying their disease, and clinically meaningful benefit might be obtained by allowing them access to safe and effective new agents.
Clinical Practice Points.
In a contemporary, international, retrospective study of patients with bone-only metastatic UC we found that the patterns of administered chemotherapy, PS, and outcomes are poor.
Patients with bone-exclusive, metastatic UC urgently deserve new active and well tolerated agents.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
These data were presented, in part, as a poster at the 2017 annual meeting of the European Society for Medical Oncology, Madrid, Spain, September 8–12, 2017
References
- 1.Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016; 388: 2796–810. [DOI] [PubMed] [Google Scholar]
- 2.Abida W, Bajorin DF, Rosenberg JE. First-line treatment and prognostic factors of metastatic bladder cancer for platinum-eligible patients. Hematol Oncol Clin North Am 2015; 29:319–28. [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012; 23:406–10. [DOI] [PubMed] [Google Scholar]
- 4.Sonpavde G, Galsky MD, Vogelzang NJ. First-line systemic therapy trials for advanced transitional-cell carcinoma of the urothelium: should we stop separating cisplatin-eligible and -ineligible patients? J Clin Oncol 2010; 28:e441–2. [DOI] [PubMed] [Google Scholar]
- 5.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balar A, Bellmunt J, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017. 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 7.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999; 17:3173–81. [DOI] [PubMed] [Google Scholar]
- 8.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013; 105:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galsky MD, Moshier E, Krege S, et al. Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin-based chemotherapy. Cancer 2013; 11:3012–9. [DOI] [PubMed] [Google Scholar]
- 10.Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Eur Urol 2017; 71:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28:1850–5. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JD, Cheng HH, Yu EY. Long-term survival in bone-predominant metastatic urothelial carcinoma. Clin Genitourin Cancer 2014; 12:e241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joudi FN, Dahmousch L, Spector DM, et al. Complete response of bony metastatic bladder urothelial cancer to neoadjuvant chemotherapy and cystectomy. Urol Oncol 2006; 24:403–6. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Smith K, Stenzl A, Bedke J. Immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol 2017; 72:477–81. [DOI] [PubMed] [Google Scholar]
- 16.Shinagare AB, Ramaiya NH, Jagannathan JP, et al. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol 2011; 196:117–22. [DOI] [PubMed] [Google Scholar]
- 17.Sengelov L, Kamby C, von der Maase H. Pattern of metastases in relation to characteristics of primary tumor and treatment in patients with disseminated urothelial carcinoma. J Urol 1996; 155:111–4. [PubMed] [Google Scholar]
- 18.Bianchi M, Roghmann F, Becker A, et al. Age-stratified distribution of metastatic sites in bladder cancer: a population-based analysis. Can Urol Assoc J 2014; 8:E148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 2006; 47:1059–66. [PubMed] [Google Scholar]
- 20.Apolo AB, Parnes HL, Madan RA, et al. A phase II study of cabozantinib in patients (pts) with relapsed or refractory metastatic urothelial carcinoma (mUC) (abstract 307). J Clin Oncol 2014; 32(suppl 4). [Google Scholar]


