Key Points
Question
What are the effects of intermediate-dose compared with standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit (ICU)?
Findings
In this randomized clinical trial that included 562 patients with COVID-19 admitted to the ICU, the primary outcome (a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days) occurred in 45.7% of patients in the intermediate-dose prophylactic anticoagulation group and 44.1% of patients in the standard-dose prophylactic anticoagulation group, a difference that was not statistically significant (odds ratio, 1.06).
Meaning
The results do not support routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients with COVID-19 admitted to the ICU.
Abstract
Importance
Thrombotic events are commonly reported in critically ill patients with COVID-19. Limited data exist to guide the intensity of antithrombotic prophylaxis.
Objective
To evaluate the effects of intermediate-dose vs standard-dose prophylactic anticoagulation among patients with COVID-19 admitted to the intensive care unit (ICU).
Design, Setting, and Participants
Multicenter randomized trial with a 2 × 2 factorial design performed in 10 academic centers in Iran comparing intermediate-dose vs standard-dose prophylactic anticoagulation (first hypothesis) and statin therapy vs matching placebo (second hypothesis; not reported in this article) among adult patients admitted to the ICU with COVID-19. Patients were recruited between July 29, 2020, and November 19, 2020. The final follow-up date for the 30-day primary outcome was December 19, 2020.
Interventions
Intermediate-dose (enoxaparin, 1 mg/kg daily) (n = 276) vs standard prophylactic anticoagulation (enoxaparin, 40 mg daily) (n = 286), with modification according to body weight and creatinine clearance. The assigned treatments were planned to be continued until completion of 30-day follow-up.
Main Outcomes and Measures
The primary efficacy outcome was a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days, assessed in randomized patients who met the eligibility criteria and received at least 1 dose of the assigned treatment. Prespecified safety outcomes included major bleeding according to the Bleeding Academic Research Consortium (type 3 or 5 definition), powered for noninferiority (a noninferiority margin of 1.8 based on odds ratio), and severe thrombocytopenia (platelet count <20 ×103/µL). All outcomes were blindly adjudicated.
Results
Among 600 randomized patients, 562 (93.7%) were included in the primary analysis (median [interquartile range] age, 62 [50-71] years; 237 [42.2%] women). The primary efficacy outcome occurred in 126 patients (45.7%) in the intermediate-dose group and 126 patients (44.1%) in the standard-dose prophylaxis group (absolute risk difference, 1.5% [95% CI, −6.6% to 9.8%]; odds ratio, 1.06 [95% CI, 0.76-1.48]; P = .70). Major bleeding occurred in 7 patients (2.5%) in the intermediate-dose group and 4 patients (1.4%) in the standard-dose prophylaxis group (risk difference, 1.1% [1-sided 97.5% CI, −∞ to 3.4%]; odds ratio, 1.83 [1-sided 97.5% CI, 0.00-5.93]), not meeting the noninferiority criteria (P for noninferiority >.99). Severe thrombocytopenia occurred only in patients assigned to the intermediate-dose group (6 vs 0 patients; risk difference, 2.2% [95% CI, 0.4%-3.8%]; P = .01).
Conclusions and Relevance
Among patients admitted to the ICU with COVID-19, intermediate-dose prophylactic anticoagulation, compared with standard-dose prophylactic anticoagulation, did not result in a significant difference in the primary outcome of a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days. These results do not support the routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients admitted to the ICU with COVID-19.
Trial Registration
ClinicalTrials.gov Identifier: NCT04486508
This randomized trial compares the effect of intermediate-dose vs standard prophylactic enoxaparin on a composite outcome of acute venous thromboembolism (VTE), arterial thrombosis, need for extracorporeal membrane oxygenation (ECMO), and all-cause mortality within 30 days among patients with COVID-19 admitted to the ICU.
Introduction
In the context of endothelial injury1,2 and a prothrombic milieu,1,3 venous and arterial microthrombosis and macrothrombosis are common manifestations of COVID-19.4 Venous thromboembolism (VTE) is the most commonly reported thrombotic complication, with higher incidence rates among critically ill patients.5 A 2020 systematic review estimated that 28% of critically ill patients with COVID-19 had VTE.6
However, limited evidence exists to guide the prophylactic antithrombotic regimen.7 Some retrospective observational studies suggest that anticoagulation beyond standard prophylactic doses was associated with reduced mortality,8 but others did not confirm these findings and, rather, suggested an elevated risk of bleeding.9 A small randomized trial suggested improved oxygenation with therapeutic anticoagulation compared with standard prophylaxis.10 However, the small sample size and other drawbacks limit the strength of this evidence.11 The uncertainty in optimal prophylactic anticoagulant regimen has translated into variability in expert recommendations, hospital policies, and clinicians’ decisions to use a variety of types and intensities of antithrombotic regimens.4,12,13,14,15 The present multicenter randomized trial investigated the effects of intermediate-dose vs standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit (ICU).
Methods
Trial Oversight
The Intermediate vs Standard-Dose Prophylactic Anticoagulation in Critically-ill Patients With COVID-19: An Open Label Randomized Controlled Trial (INSPIRATION) and INSPIRATION-statin (INSPIRATION-S) studies, designed by an international committee, had a 2 × 2 factorial design and was conducted in Iran with 10 enrolling centers in Tehran and Tabriz. The Rajaie Cardiovascular Medical and Research Center and the Tehran Heart Center were the study coordinating centers. The study protocol was approved by the Rajaie Cardiovascular Medical and Research Center ethics committee and accepted by other participating sites. All patients or their health care proxies provided written informed consent. An independent data and safety monitoring committee monitored the study results.
Design
The trial design has been described previously16 and the study protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2. This study was a multicenter randomized trial with a 2 × 2 factorial design comparing intermediate-dose vs standard-dose prophylactic anticoagulation (first hypothesis) and statin therapy vs matching placebo (second hypothesis) among patients with COVID-19 admitted to the ICU. The current article summarizes the results from the first hypothesis, an open-label randomized clinical trial with blinded outcome adjudication. Patient recruitment for the second (statin) hypothesis is underway.
Trial Population
Patients admitted to the ICU with polymerase chain reaction testing–confirmed COVID-19 within 7 days of the index hospitalization were eligible for inclusion. Patients with life expectancy less than 24 hours, an established indication for therapeutic-dose anticoagulation, weight less than 40 kg, pregnancy, history of heparin-induced thrombocytopenia, platelet count less than 50 ×103/µL, or overt bleeding were excluded. The full list of eligibility criteria16 are available in the study protocol in Supplement 1.
Randomization and Study Drugs
Randomization was done using an electronic web-based system with permuted blocks of 4 and allocation sequence concealment. Eligible patients were allocated in 1:1 ratio to receive intermediate-dose or standard-dose prophylactic anticoagulation. The primary anticoagulant agent in both groups was enoxaparin. Unfractionated heparin was used in the case of severe kidney insufficiency. For patients who weighed less than 120 kg and had a creatinine clearance greater than 30 mL/min, enoxaparin, 1 mg/kg daily, was assigned as intermediate-dose anticoagulation. Enoxaparin, 40 mg daily, was the control group standard-dose prophylactic anticoagulation regimen. In both groups, predefined modifications were advised according to body weight and creatinine clearance (eTables 1-3 in Supplement 3). The assigned treatments were planned to be continued until the 30-day follow-up, irrespective of hospital discharge status.
Study Outcomes
The primary efficacy outcome was a composite of adjudicated acute VTE, arterial thrombosis, treatment with extracorporeal membrane oxygenation (ECMO), or all-cause mortality within 30 days of enrollment. Secondary efficacy outcomes included all-cause mortality, adjudicated VTE, and ventilator-free days. Prespecified exploratory outcomes included objectively clinically diagnosed type I acute myocardial infarction, stroke, and acute peripheral arterial thrombosis; rate of discharge from the ICU; incident atrial fibrillation; new in-hospital kidney replacement therapy; and ICU length of stay. Diagnostic tests were performed based on clinical judgment of the treating clinicians; no systematic screening for thrombotic events was required by the study protocol.
The prespecified safety outcomes included major bleeding (Bleeding Academic Research Consortium type 3 or 517) and severe thrombocytopenia (platelet count <20 ×103/µL). Clinically relevant nonmajor bleeding was defined as clinically significant bleeding that warranted attention from medical personnel but did not fulfil criteria for major bleeding. Mild thrombocytopenia (platelet count <100 ×103/µL) and moderate thrombocytopenia (platelet count <50 ×103/µL) were also assessed as post hoc safety outcomes.
The full list of study outcomes and their definitions can be found in Supplement 3. For patients who did not die during hospitalization, regular follow-up was pursued by structured weekly phone interviews. A clinical events committee blinded to the treatment assignment adjudicated the primary, secondary, and exploratory outcomes.
Statistical Analysis
Power calculation was performed for 2-sided superiority testing for the primary efficacy outcome in patients who were randomized and were not excluded due to violation of the eligibility criteria, did not withdraw consent, and received at least 1 dose of the study drug (see Supplement 2 for terms used previously for describing the analytic populations).16 According to the estimates obtained from the enrolling centers, a 55% event rate for the primary outcome in the standard-dose prophylactic group was presumed. Considering a 2-sided α of .05 and using the Z approximation formula for comparing 2 proportions between independent groups, a sample size of 544 patients (272 per group) was estimated to provide 80% power to detect a 12% absolute risk reduction in the primary outcome with intermediate-dose compared with standard-dose prophylactic anticoagulation (eMethods in Supplement 3). Considering a 10% dropout rate during the study for withdrawal of consent or postrandomization exclusions, 600 patients were planned for enrollment. No interim efficacy analyses were planned to minimize the type I error rate.
In a prespecified secondary analysis, estimating bleeding event rates of 5.5% in the standard-dose group and 6.5% in the intermediate-dose anticoagulation group18,19 and a 1-sided α of .025, the same sample size provided 79.5% power to show noninferiority of intermediate-dose anticoagulation compared with standard-dose prophylactic anticoagulation with respect to major bleeding, with a noninferiority margin of 1.8 based on the odds ratio. The selection of a 12% difference in the primary outcome as the minimal clinically important difference to power the study, as well as the selection of an odds ratio of 1.8 as the basis for declaring noninferiority with regard to major bleeding events, were based on investigator consensus (eMethods in Supplement 3).
Because the study included a 2 × 2 factorial design, a Mantel-Haenszel χ2 test was performed to assess the interaction between anticoagulation intensity and statin use for the primary outcomes prior to the assessment of the primary efficacy and prespecified safety outcomes. Because the tests of interaction between the 2 interventions were nonsignificant (P = .97 for the primary efficacy outcome and P = .22 for major bleeding), the anticoagulation hypothesis is presented independently.
Given the short follow-up duration, logistic regression with odds ratio as the effect measure was prespecified for the primary analyses. Accounting for study sites as random effect was done post hoc in sensitivity analyses using mixed-effects logistic regression models for binary outcomes and linear mixed-effects models for interval outcomes.
Time to events for the primary outcomes were plotted with Kaplan-Meier curves. In sensitivity analyses, the proportionality assumption was met (based on Schoenfeld residuals) and results were repeated with unadjusted Cox proportional hazards models. Definitions of different cohorts used for sensitivity analyses can be found in Supplement 3.
There were no missing outcomes for participants in the final analysis. The rate of missing values for baseline characteristics was trivial (<5% in all cases) and did not warrant multiple imputations according to the prespecified statistical analysis plan. The only exception was baseline D-dimer, which was not available in 66.5% of the patients at baseline due to unprecedented national increase in use of D-dimer assays leading to temporary shortage. Because the rate of missing rate data was greater than 20%, according to the prespecified statistical analysis plan, multiple imputations were not performed.
The association between the assigned anticoagulation regimen and the primary outcome was assessed in the study subgroups. Prespecified subgroup analyses (based on age, sex, cigarette smoking, diabetes, hypertension, heart failure, obstructive airway disease, time from symptom onset to randomization, corticosteroid use, renin-angiotensin-aldosterone system inhibitor use, and baseline D-dimer level) as well as post hoc subgroup analyses (based on coronary artery disease, body mass index, time receiving the assigned treatment, and aspirin use) were performed. For evaluation of the homogeneity of odds ratios across subgroups, the Woolf test was applied. The interaction between the intervention and specific subgroups was assessed via the Cochran-Mantel-Haenszel χ2 test. All hypothesis tests, except for the test of noninferiority for major bleeding, were 2-sided. A P value <.05 was considered significant for the primary efficacy outcome. For noninferiority testing for major bleeding, a 1-sided P value <.025 was considered significant. No adjustment was performed for the P value thresholds with respect to multiplicity of comparisons. Because of the potential for type I error, findings for analyses of all other outcomes should be interpreted as exploratory. Statistical analyses were performed using R statistical software package, version 4.0.3 (R Core Team).
Results
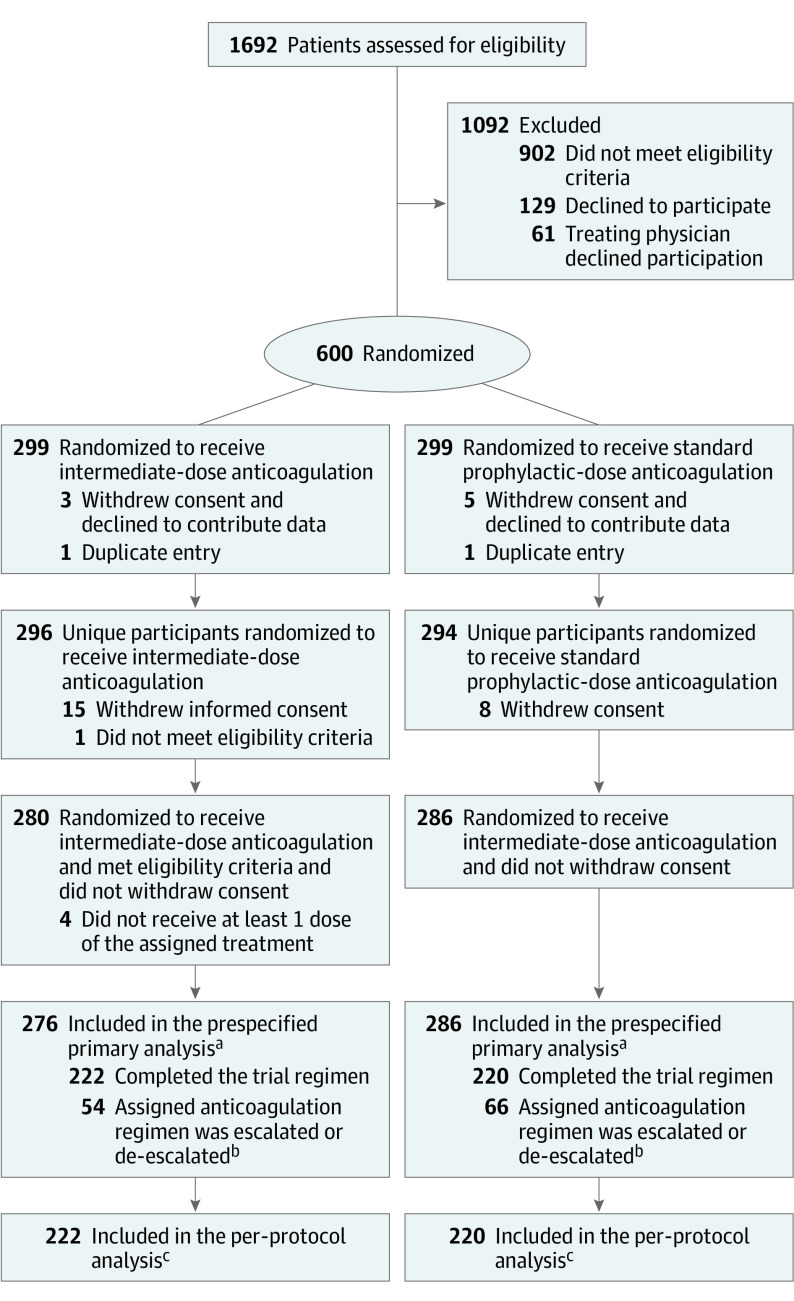
Between July 29, 2020, and November 19, 2020, a total of 1692 patients were screened for eligibility and 600 underwent randomization, of whom 4 died before receiving the first dose of the study drug, 2 were excluded due to duplicate entry, and 32 were excluded for other reasons (Figure 1). Ultimately, 562 patients (93.7%) were included in the prespecified primary analysis (Figure 1; eTable 4 in Supplement 3).
Figure 1. Flow of Participants in a Study of the Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation Among Patients With COVID-19 Admitted to the Intensive Care Unit .
aSome patients had more than 1 reason for exclusion from the primary analysis cohort.
bReasons for discontinuation of the trial regimen are summarized in eTable 4 in Supplement 3.
cPatients who were randomized and were not excluded due to violation of the eligibility criteria or withdrawal of informed consent and continued their assigned anticoagulation regimen until 30-day follow-up or the occurrence of the prespecified efficacy outcome. See eTable 13 in Supplement 3 for the per-protocol safety cohort.
As shown in Table 1, the study population had a median (interquartile range [IQR]) age of 62 (50-71) years, 237 patients (42.2%) were women, and the median (IQR) body mass index was 27 (24.6-29.4). The 2 study groups were balanced with respect to baseline characteristics, expect for history of cigarette smoking, which was more frequent in the intermediate-dose group (Table 1).
Table 1. Baseline Characteristics of the Prespecified Primary Analysis Population in a Study of the Effect of Intermediate- vs Standard-Dose Prophylactic Anticoagulation Among Patients With COVID-19 Admitted to the Intensive Care Unit.
| Characteristic | Median (IQR) | |
|---|---|---|
| Intermediate dose (n = 276) | Standard dose (n = 286) | |
| Age, y | 62 (51-70.7) | 61 (47-71) |
| Sex, No. (%) | ||
| Women | 114 (41.3) | 123 (43.0) |
| Men | 162 (58.7) | 163 (57.0) |
| Body mass index | 26.7 (24.4-29.1) | 27.2 (24.3-29.1) |
| Current smoker | 35 (12.7) | 21 (7.3) |
| Coexisting conditions, No. (%) | ||
| Hypertension | 131 (48.0) | 118 (41.2) |
| Diabetes | 82 (29.7) | 73 (25.6) |
| Hyperlipidemia | 75 (27.2) | 68 (23.8) |
| Coronary artery disease | 45 (16.3) | 33 (11.5) |
| Obstructive airway disease | 23 (8.3) | 16 (5.6) |
| Heart failure | 7 (2.5) | 6 (2.1) |
| Ischemic cerebrovascular accidents | 6 (2.2) | 11 (3.8) |
| Hemorrhagic stroke | 0 | 0 |
| Venous thromboembolism | 0 | 0 |
| Duration of symptoms prior to hospitalization, d | 7 (4-8) | 7 (5-10) |
| Duration of hospitalization before randomization, d | 4 (2-6) | 4 (3-6) |
| Baseline indicators of illness severity, No. (%) | ||
| Patients with systolic blood pressure <100 mm Hg at the time of randomization | 25 (9.0) | 33 (11.5) |
| Vasopressor agent support within 72 h of enrollment | 63 (22.8) | 64 (22.3) |
| Fraction of inspired oxygen >50% at the time of randomization | 112 (40.5) | 122 (42.6) |
| Acute Physiology and Chronic Health Evaluation II score at the time of randomizationa | 8 (5-11) | 8 (5-11) |
| Acute respiratory support, No. (%) | ||
| Nasal cannula | 10 (3.6) | 14 (4.9) |
| Face mask | 33 (12.0) | 27 (9.4) |
| Reservoir mask | 76 (27.5) | 96 (33.6) |
| High-flow nasal cannula | 9 (3.3) | 6 (2.1) |
| Noninvasive positive pressure ventilation | 93 (33.7) | 85 (29.7) |
| Invasive positive pressure ventilation (endotracheal intubation) | 55 (19.9) | 58 (20.3) |
| Medication history, No. (%)b | ||
| Baseline medication | ||
| Aspirin | 91 (33.0) | 81 (28.3) |
| Platelet ADP P2Y12 receptor inhibitors | 7 (2.5) | 6 (2.1) |
| Cotreatment | ||
| Antiviral therapy | 226 (81.9) | 217 (75.9) |
| Remdesivir | 168 (60.9) | 170 (59.4) |
| Favipiravir | 52 (18.8) | 43 (15.0) |
| Lopinavir/ritonavir | 3 (1.1) | 3 (1.0) |
| Atazanavir/ritonavir | 27 (9.8) | 19 (6.6) |
| Corticosteroid use | 262 (94.9) | 262 (91.6) |
| Renin-angiotensin-aldosterone system inhibitors | 78 (28.3) | 74 (25.9) |
| Tocilizumab | 34 (12.3) | 40 (14.0) |
| Laboratory values at baselinec | ||
| Creatinine, mg/dL | 1.1 (0.9-1.2) | 1.1 (0.9-1.3) |
| White blood cell count, /µL | 9800 (7300-13 400) | 10 000 (7525-12 500) |
| Hemoglobin level, g/dL | 13.1 (11.9-14.5) | 13.2 (11.9-14.5) |
| Platelet count, ×103/µL | 239 (183-309) | 230 (173-301) |
| D-dimer, ng/mL | 1037 (460-3121) (n = 97) | 910 (410-2380) (n = 91) |
| Prothrombin time, s | 13.6 (12.6-15.0) | 13.7 (12.6-15.0) |
| International normalized ratio | 1.0 (1.1-1.2) | 1.0 (1.1-1.2) |
| Activated partial thromboplastin time, s | 32 (28-38) | 31 (27.4-36) |
Abbreviation: IQR, interquartile range.
The Acute Physiology and Chronic Health Evaluation II score is an index for the severity of the disease that ranges from 0 to 71 and includes 3 components: Acute Physiology Score, age, and chronic health status. Higher scores indicate poorer outcome.
No patients received monoclonal antibodies or convalescent plasma.
Normal ranges of measured laboratory tests were defined as follows: 0.6-1.4 mg/dL for creatinine, 4500-11 000 /µL for white blood cell count, 13.5-17.5 g/dL for men and 12-15.6 g/dL for women for hemoglobin level, 150-450 ×103/µL for platelet count, <500 ng/mL for D-dimer level, 13.3-14.3 s for prothrombin time, and 33.9-35.3 s for activated partial thromboplastin time.
The median (IQR) duration of receiving the assigned treatment was similar between the 2 groups (20 [7-30] days in both groups). Overall, 442 patients (78.6%) received the assigned treatment for the full study duration or until reaching an efficacy outcome (eTables 4 and 5 in Supplement 3).
Efficacy Outcomes
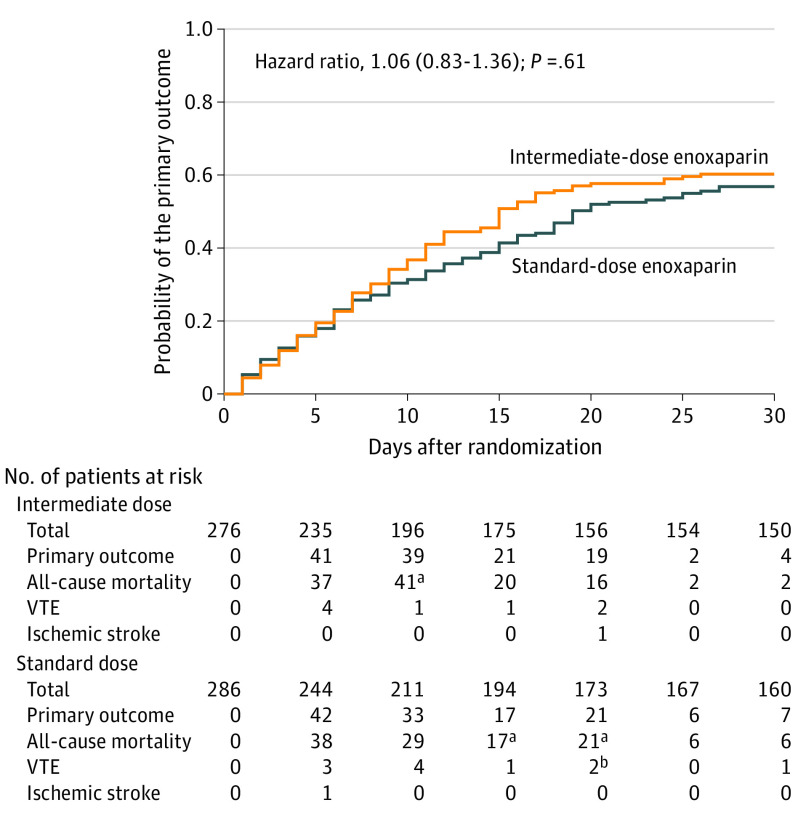
In the prespecified primary analysis cohort, the primary efficacy outcome occurred in 126 of 276 patients (45.7%) in the intermediate-dose group and 126 of 286 patients (44.1%) in the standard-dose prophylactic anticoagulation group (absolute risk difference, 1.5% [95% CI, −6.6% to 9.8%]; odds ratio, 1.06 [95% CI, 0.76-1.48]; P = .70) (Table 2). With respect to secondary efficacy outcomes, during 30-day follow-up, all-cause mortality occurred in 236 patients (42.0%) and was not significantly different in the intermediate-dose group compared with the standard-dose prophylaxis group (119 [43.1%] vs 117 [40.9%]; risk difference, 2.2% [95% CI, −5.9% to 10.3%]; odds ratio, 1.09 [95% CI, 0.78-1.53]; P = .50). VTE events occurred in 19 patients (3.4%), including 12 episodes of deep vein thrombosis and 7 pulmonary embolism events. The risk of VTE was not significantly different between the intermediate-dose and standard-dose groups (3.3% vs 3.5%; risk difference, −0.2% [95% CI, −3.2% to 2.7%]; odds ratio, 0.93 [95% CI, 0.37-2.32]; P = .94) (Table 2). The median (IQR) number of ventilator-free days was 30 (1-30) in the study population, with no significant difference between the intermediate-dose and standard-dose groups (30 [3-30] vs 30 [1-30] days; P = .50). The 30-day Kaplan-Meier curves for the primary composite outcome, VTE, and all-cause mortality are shown in Figure 2 and eFigures 1 and 2 in Supplement 3.
Table 2. Primary, Secondary, and Exploratory Outcomes Within 30 Days of Enrollment in the Prespecified Primary Analysis in a Study of the Effect of Intermediate- vs Standard-Dose Prophylactic Anticoagulation Among Patients With COVID-19 Admitted to the Intensive Care Unit (ICU).
| Outcome | No. (%) | Absolute difference (95% CI), % | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Intermediate dose (n = 276) | Standard dose (n = 286) | ||||
| Primary outcome | |||||
| Composite of adjudicated acute venous thromboembolism, arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause mortalitya | 126 (45.7) | 126 (44.1) | 1.5 (−6.6 to 9.8) | 1.06 (0.76 to 1.48) | .70 |
| Secondary outcomes | |||||
| All-cause mortality | 119 (43.1) | 117 (40.9) | 2.2 (−5.9 to 10.3) | 1.09 (0.78 to 1.53) | .50 |
| Adjudicated venous thromboembolism | 9 (3.3) | 10 (3.5) | −0.2 (−3.2 to 2.7) | 0.93 (0.37 to 2.32) | .87 |
| Ventilator-free days, median (IQR)b | 30 (3 to 30) | 30 (1 to 30) | 0 (0 to 0) | NA | .50c |
| Exploratory outcomes | |||||
| Objectively clinically diagnosed type I acute myocardial infarctiond | 0 | 0 | |||
| Objectively clinically diagnosed stroke | 1 (0.4) | 1 (0.3) | 0.1 (−0.9 to 0.9) | 1.03 (0.06 to 16.65) | .97 |
| Objectively clinically diagnosed acute peripheral arterial thrombosis | 0 | 0 | |||
| ICU length of stay, median (IQR) | 5 (2 to 10) | 6 (3 to 11) | −1 (−4 to 3) | NA | .14c |
| Patients discharged from the ICU | 169 (61.2) | 174 (60.8) | 0.3 (−7.6 to 8.4) | 1.01 (0.72 to 1.42) | .72 |
| Incident atrial fibrillation | 2 (0.7) | 6 (2.1) | −1.3 (−3.3 to 0.5) | 0.34 (0.0 to 1.49) | .16 |
| New in-hospital kidney replacement therapy | 10 (3.6) | 7 (2.4) | 1.1 (−1.6 to 4.0) | 1.49 (0.58 to 3.86) | .41 |
| Safety outcomes | |||||
| Major bleedinge | 7 (2.5) | 4 (1.4) | 1.1 (−1.1 to 3.4) | 1.83 (0.53 to 5.93) | .33 |
| BARC classification | |||||
| Type 3a (hemoglobin drop of 3-5 g/dL or any transfusion) | 3 (1.1) | 4 (1.4) | −0.3 (−2.1 to 1.5) | 0.78 (0.17 to 3.49) | .73 |
| Type 3b (hemoglobin drop >5 g/dL) | 1 (0.4) | 0f | 0.3 (−0.3 to 1.0) | .30 | |
| Type 3c (intracranial hemorrhage) | 1 (0.4) | 0f | 0.3 (−0.3 to 1.0) | .30 | |
| Type 5 (fatal bleeding) | 2 (0.7) | 0f | 0.7 (−0.2 to 1.7) | .14 | |
| Clinically relevant nonmajor bleeding (BARC type 2)g | 12 (4.3) | 5 (1.7) | 2.5 (−0.2 to 5.4) | 2.55 (0.92 to 7.04) | .07 |
| Composite of major and non-major bleeding | 17 (6.2) | 9 (3.1) | 3.0 (−0.4 to 6.4) | 2.02 (0.89 to 4.61) | .08 |
| Thrombocytopenia | |||||
| Mild (<100 ×103/µL)h | 50 (18.2) | 57 (19.9) | −1.4 (−7.9 to 5.0) | 0.89 (0.58 to 1.36) | .62 |
| Moderate (<50 ×103/µL)h | 14 (5.1) | 20 (7.0) | −0.8 (−4.6 to 2.8) | 0.71 (0.35 to 1.44) | .61 |
| Severe (<20 ×103/µL) | 6 (2.2) | 0f | 2.2 (0.4 to 3.8) | .01 | |
Abbreviations: IQR, interquartile range; NA, not applicable.
All venous thromboembolism events were diagnosed by the online clinical event committee. Each event was confirmed only if guideline-recommended imaging tests were presented (see definitions of outcome events in Supplement 3). Acute arterial thrombosis was defined as type I acute myocardial infarction, ischemic stroke, and acute peripheral arterial thrombosis. No patients received extracorporeal membrane oxygenation.
Difference between the total number of days alive after enrollment and the total number of days receiving invasive mechanical ventilation.
Calculated using Mann-Whitney U test.
Type I myocardial infarction was defined as an increase and/or decrease in cardiac troponin values with at least 1 value above the 99th percentile upper reference limits with at least 1 of the following: symptoms of ischemia, new or presumed new ischemic electrocardiographic (ECG) change, development of pathologic Q waves on the ECG findings, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with ischemic etiology confirmed by coronary angiography, intravascular imaging, or autopsy. Myocardial injury was noted in 6 patients with a combination of cardiac biomarker rise and electrocardiographic changes. Coronary angiography was only pursued in 1 patient (with normal coronary vasculature), thus type I myocardial infarction was not adjudicated in any participants.
Major bleeding consisted of Bleeding Academic Research Consortium (BARC) type 3 and 5, which defines type 3a as overt bleeding plus hemoglobin drop of 3 to 5 g/dL or any transfusion with overt bleeding; type 3b as overt bleeding plus hemoglobin drop 5 g/dL, cardiac tamponade, or bleeding requiring surgical intervention for control; type 3c as intracranial hemorrhage; and type 5 as fatal bleeding.17
For events with zero incidence in 1 group, only absolute risk difference was reported.
Clinically significant bleeding that warranted attention from the medical personnel but did not fulfil criteria for major bleeding.
Post hoc outcomes.
Figure 2. Primary Outcome in the Prespecified Primary Cohort in a Study of the Effect of Intermediate-Dose vs Standard-Dose Prophylactic Among Patients With COVID-19 Admitted to the Intensive Care Unit.
The primary outcome was a composite of adjudicated acute arterial thrombosis, venous thromboembolism, extracorporeal membrane oxygenation, or all-cause mortality during 30 days from enrollment. The prespecified primary cohort consisted of patients who received at least 1 dose of the study drug, were not excluded, and did not withdraw consent. The median (interquartile range) follow-up time was 30 (9-30) days in the intermediate-dose group and 30 (10-30) days in the standard-dose prophylactic anticoagulation group.
aAll-cause mortality events were censored by precedent venous thromboembolism (VTE) events. In some cases, the thrombotic events occurred in the prior window (ie, in the first 5 days).
bOne of the 2 VTE events was censored by a precedent ischemic stroke event.
No statistically significant differences were detected in the exploratory outcomes. There were no cases of adjudicated type I myocardial infarction. The rate of ischemic stroke was 0.3% in the intermediate-dose group and 0.4% in the standard-dose group (risk difference, 0.1% [95% CI, −0.9% to 0.9%]; odds ratio, 1.03 [95% CI, 0.06-16.65]; P = .97). No other acute arterial thrombotic events were identified. No patients received ECMO during the study period.
The median (IQR) ICU length of stay was 6 (2-11) days (5 [2-10] days in the intermediate-dose group vs 6 [3-11] days in the standard-dose group; P = .14), and a total of 343 patients (61.0%) were discharged from the ICU, including 169 patients (61.2%) assigned to receive the intermediate-dose regimen and 174 patients (60.8%) assigned to receive the standard-dose prophylaxis regimen (risk difference, 0.3% [95% CI, −7.8% to 8.4%]; odds ratio, 1.01 [95% CI, 0.72-1.42]; P = .72). New in-hospital kidney replacement therapy was performed in 17 patients (3.0%) (3.6% in the intermediate-dose group vs 2.4% in the standard-dose group; risk difference, 1.1% [95% CI, −1.6% to 4.0%]; odds ratio, 1.49 [95% CI, 0.58-3.86]; P = .41) and new atrial fibrillation was detected in 8 patients (1.4%), without a significant difference between the intermediate-dose group and the standard-dose group (0.7% vs 2.1%; difference, −1.3% [95% CI, −3.3% to 0.5%]; odds ratio, 0.34 [95% CI, 0.00-1.49]; P = .16).
Safety Outcomes
There were 7 (2.5%) major bleeding events in the intermediate-dose group and 4 (1.4%) in the standard-dose prophylactic anticoagulation group (risk difference, 1.1% [1-sided 97.5% CI, −∞ to 3.4%]; odds ratio, 1.83 [1-sided 97.5% CI, 0.00-5.93]), which did not meet the noninferiority criteria (Pfor noninferiority >.99). There was 1 case of intracranial hemorrhage and 2 cases of fatal bleeding events in the intermediate-dose group. Clinically relevant nonmajor bleeding occurred in 12 patients (4.3%) in the intermediate-dose group and 5 patients (1.7%) in the standard-dose group (risk difference, 2.5% [95% CI, −0.2% to 5.4%]; odds ratio, 2.55 [95% CI, 0.92-7.04]; P = .07) (Table 2). Severe thrombocytopenia occurred only in patients assigned to the intermediate-dose group (6 vs 0; risk difference, 2.2% [95% CI, 0.4%-3.8%]) (Table 2). No significant differences were observed between the 2 study groups with respect to less severe forms of thrombocytopenia (Table 2).
Sensitivity Analysis
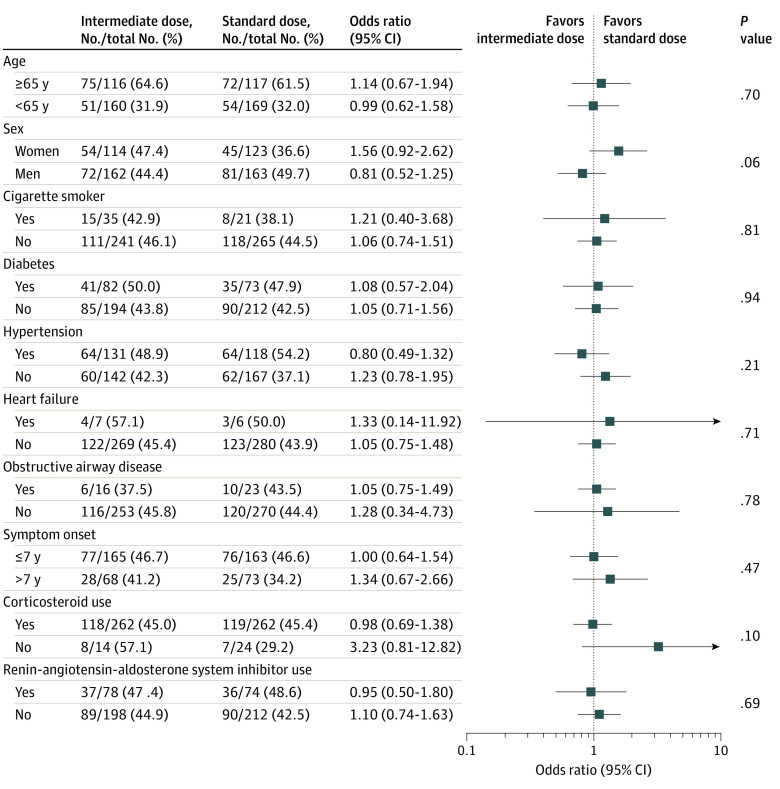
Findings from the per-protocol analyses and other sensitivity analyses were similar to those from the primary analyses (eTables 8-18 in Supplement 3). Findings were consistent in subgroup analyses. No particular subgroups were identified in which use of intermediate-dose prophylactic anticoagulation was associated with significant reduction in the primary outcome (Figure 3; eFigure 4 in Supplement 3).
Figure 3. Subgroup Analysis for the Primary Outcome in a Study of the Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation Among Patients With COVID-19 Admitted to the Intensive Care Unit.
P values indicate tests of interaction between the treatment group and each of the assessed variables for the primary composite outcome.
Discussion
In this multicenter randomized clinical trial of patients with COVID-19 admitted to the ICU, intermediate-dose compared with standard-dose prophylactic anticoagulation did not improve the primary composite efficacy outcome or its major components, including all-cause mortality and VTE. Results were consistent in sensitivity analyses and in key prespecified subgroups. Although bleeding events were rare, both major and clinically relevant nonmajor bleeding events were nonsignificantly more frequent with intermediate-dose anticoagulation, and noninferiority for major bleeding was not demonstrated. Furthermore, severe thrombocytopenia was observed only in patients assigned to receive intermediate-dose prophylactic anticoagulation.
Establishing the optimal antithrombotic prophylactic regimen in patients with COVID-19 is essential because of the reported excess rates of microvascular and macrovascular thrombosis.4,20,21,22 The observation of heightened VTE risk in patients receiving standard-dose prophylaxis23,24,25 encouraged some experts to advocate escalated-dose prophylaxis.26 Although the primary end point event rate was slightly lower than expected, there was no signal for benefit. Further, noninferiority for major bleeding was not confirmed. In line with these results, an interim analysis of critically ill patients enrolled in 3 pivotal trials testing therapeutic-dose vs standard prophylactic anticoagulation (ACTIV-4a, REMAP-CAP, and ATTACC) led the data and safety monitoring board to pause further enrollment because of futility for efficacy and potential excess of safety events, and additional clarifications are awaited.27
Several potential explanations exist for the observed lack of benefit with intermediate-dose prophylactic anticoagulation in this study. First, the intensity of intermediate-dose anticoagulation might have been insufficient to prevent thrombotic events compared with the standard-dose prophylactic regimen. Some studies completed before the COVID-19 pandemic indicated that intermediate-dose regimens may be effective for preventing thrombotic events.19,28 At the time of study design, some experts hypothesized that intermediate intensity of anticoagulation may be suitable for patients with COVID-19. Second, the timing of anticoagulation administration and its relation to the symptom onset might affect the effectiveness of anticoagulation.29 However, in this study, the results were consistent even among patients who received anticoagulation within the first 7 days from symptom onset.
Third, the study recruited a broad population of patients admitted to the ICU rather than targeting metrics such as D-dimer or specific metrics of illness severity. This was a pragmatic choice. A subgroup analysis showed that patients with baseline D-dimer elevation had outcomes consistent with the primary analysis. Fourth, the number of patients receiving mechanical ventilation at the time of enrollment in the present study was lower than some other cohorts.30,31 The study population correlated with the eligibility criteria of this trial, which did not allow the enrollment of the patients with extremely severe disease with estimated survival less than 24 hours. Nevertheless, many of the study participants were very ill, as indicated by the requirements for cardiopulmonary support and evidenced by the 30-day mortality rates. The possibility of a potential effect among patients who were admitted to the ICU and had more severe illness cannot be excluded or, alternatively, that heparin-based regimens might be effective in hospitalized patients not admitted to the ICU with an earlier stage of disease. In addition, it is possible that heparin-based regimens are not beneficial in critically ill patients with COVID-19,32 but that other agents may confer benefit.
Although the present study was unable to demonstrate noninferiority for the prespecified bleeding end point, major bleeding was infrequent in both study groups. Also, severe thrombocytopenia was noted in 6 patients who received intermediate-dose prophylactic anticoagulation, compared with zero patients who received standard-dose prophylactic anticoagulation, although no significant differences were noted in other platelet count measures. Because this outcome was not powered for hypothesis testing and its results were not adjusted for multiplicity, this finding should be considered exploratory. Numerous additional randomized trials with heparin-based and nonheparin agents are ongoing across the spectrum of COVID-19 illness severity and could identify potentially effective therapies in various subgroups of illness severity with COVID-19.33
Limitations
This study has several limitations First, this trial, by design, required the estimated survival of greater than 24 hours at the determination of site physicians and exclusion of patients receiving ECMO at the time of randomization (because ECMO needs escalated-dose anticoagulation). This meant that the most severely ill patients (eg, those with unstable maximized ventilatory support or those receiving multiple vasopressor agents at the time of screening) were not included in the trial. This issue should be considered for the external validity of the findings. Second, anticoagulation assignment was open-label. Using a double-dummy design during the COVID-19 pandemic was not considered feasible. However, the allocation sequence was concealed and outcomes were blindly adjudicated and analyzed. Third, the VTE event rate in the present study was lower than that reported in some other studies.6,25,34 This may in part correlate with lack of systematic routine screening, similar to results from other multicenter studies that did not use systematic screening and reported lower rates of VTE.9,35 Other factors that may have contributed include lower acuity of care in patients admitted to the ICU in this study compared with some other studies36,37 or the possible effect of anti-inflammatory therapies (including steroids38) on mitigating microthrombosis or macrothrombosis.7 Some recent studies suggest that the majority of thrombotic events in critically ill patients with COVID-19 include catheter-associated thrombosis, isolated distal deep vein thrombosis, or subsegmental pulmonary embolism, all of which are less severe forms of VTE and less likely to affect mortality.6,34 Fourth, although all-cause mortality rates in the present study are in line with other reports of critically ill patients,30,31,34 the CI for the primary outcome was relatively wide. Consequently, the possibility of a small benefit or a small and important harm cannot be excluded. Fifth, due to resource limitations, the study focused only on clinical events that were assumed to directly affect hard clinical end points. In this setting, the case report form did not collect information related to radial arterial line exchange (due to nonfunctioning) or nonfunctioning dialysis lines. Sixth, only 4 study participants weighed more than 120 kg, limiting the generalizability of the results to patients with higher body weight or obesity.
Conclusions
Among patients with COVID-19 admitted to the ICU, intermediate-dose prophylactic anticoagulation, compared with standard-dose prophylactic anticoagulation, did not result in a significant difference in the primary outcome of a composite of venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days. These results do not support the routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients with COVID-19 admitted to the ICU.
Trial protocol
Statistical analysis plan
Investigators and Committees
Eligibility criteria
Definitions of Outcome Events
eMethods
Study outcomes in hospitalized versus post-discharged patients
Prespecified subgroup analysis of primary outcome in patients with or without D-dimer >1000 ng/mL
Post hoc subgroup analysis of major bleeding among aspirin and non-aspirin users
eFigure 1. Kaplan-Meier curve for all-cause mortality in the prespecified primary cohort
eFigure 2. Kaplan-Meier curve for the venous thromboembolism in the prespecified primary cohort.
eFigure 3. Kaplan-Meier curve for the landmark analysis showing the primary composite outcome in the first ten days and from 11 to 30 days of enrollment in the prespecified primary cohort
eFigure 4. Post-hoc subgroup analysis
eTable 1. Intervention and comparator dosing for the anticoagulation hypothesis
eTable 2. Dosing strategies for intermediate dose applied in study sites
eTable 3. Dosing strategies for standard dose anticoagulation applied in study sites
eTable 4. Reasons for post-randomization changes in the assigned treatment of patients in the prespecified primary cohort
eTable 5. Treatment adherence in the two study groups in the prespecified primary cohort
eTable 6. Demographic and clinical characteristics at baseline according to the assigned anticoagulation therapy in the total study population
eTable 7. Demographic and clinical characteristics at baseline according to the assigned anticoagulation therapy in patients who were randomized and were not excluded due to violation of the eligibility criteria nor withdrawal of informed consent
Table 8. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis incorporating the center random effect
eTable 9. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the total study population who allowed their data to contribute to the final results
eTable 10. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the total study population who allowed their data to contribute to the final results incorporating the center random effect
eTable 11. Primary, Secondary and exploratory outcomes within 30 days from enrollment in randomized patients who were not excluded due to violation of the eligibility criteria or withdrawal of informed consent.
eTable 12. Primary, Secondary and exploratory outcomes within 30 days from enrollment in randomized patients who were not excluded due to violation of the eligibility criteria or withdrawal of informed consent incorporating center random effect
eTable 13. Primary, Secondary and exploratory outcomes within 30 days from enrollment per-protocol cohort
eTable 14. Primary, Secondary and exploratory outcomes within 30 days from enrollment per-protocol cohort incorporating center random effect
eTable 15. Primary, Secondary and exploratory efficacy outcomes within 30 days from enrollment in patients with baseline D-dimer >1000 ng/ml in the prespecified primary analysis
eTable 16. Primary, Secondary and exploratory efficacy outcomes within 30 days from enrollment in patients with baseline D-dimer >1000 ng/ml in the prespecified primary analysis incorporating center random effect
eTable 17. In-hospital Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis
eTable 18. In-hospital Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis incorporating center random effect
eTable 19. Summary of venous thromboembolism diagnostic tests performed by enrolling centers
eTable 20. Study outcomes and laboratory tests in patients with severe thrombocytopenia
eTable 21. Selected baseline characteristics and assigned anticoagulation regimen in patients with major bleeding
eTable 22. Anatomical characteristics of adjudicated venous thromboembolic events
eReferences
Nonauthor collaborators. INSPIRATION Investigators
Data sharing statement
References
- 1.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268-277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038-3044. doi: 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: jacc state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799-801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiménez D, García-Sanchez A, Rali P, et al. Incidence of venous thromboembolism and bleeding among hospitalized patients with COVID-19: a systematic review and meta-analysis. Chest. 2021;159(3):1182-1196. doi: 10.1016/j.chest.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B, Madhavan MV, Gupta A, et al. ; Global COVID-19 Thrombosis Collaborative Group . Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(7):1004-1024. doi: 10.1055/s-0040-1713152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122-124. doi: 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Samkari H, Gupta S, Karp Leaf R, et al. Thrombosis, bleeding, and the effect of anticoagulation on survival in critically ill patients with COVID-19 in the United States. Res Pract Thromb Haemost. 2020;4. [Google Scholar]
- 10.Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359-366. doi: 10.1016/j.thromres.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B. Anticoagulation in COVID-19: randomized trials should set the balance between excitement and evidence. Thromb Res. 2020;196:638-640. doi: 10.1016/j.thromres.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. doi: 10.1007/s11239-020-02138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143-1163. doi: 10.1016/j.chest.2020.05.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramacciotti E, Macedo AS, Biagioni RB, et al. Evidence-based practical guidance for the antithrombotic management in patients with coronavirus disease (COVID-19) in 2020. Clin Appl Thromb Hemost. 2020;26:1076029620936350. doi: 10.1177/1076029620936350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872-888. doi: 10.1182/bloodadvances.2020003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B, Talasaz AH, Rashidi F, et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb Res. 2020;196:382-394. doi: 10.1016/j.thromres.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 18.Cook D, Meade M, Guyatt G, et al. ; PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305-1314. doi: 10.1056/NEJMoa1014475 [DOI] [PubMed] [Google Scholar]
- 19.Eck RJ, Bult W, Wetterslev J, et al. Intermediate dose low-molecular-weight heparin for thrombosis prophylaxis: systematic review with meta-analysis and trial sequential analysis. Semin Thromb Hemost. 2019;45(8):810-824. doi: 10.1055/s-0039-1696965 [DOI] [PubMed] [Google Scholar]
- 20.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi: 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438-e440. doi: 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. 2020;324(24):2548-2549. doi: 10.1001/jama.2020.23422 [DOI] [PubMed] [Google Scholar]
- 23.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743-1746. doi: 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spyropoulos AC. The management of venous thromboembolism in hospitalized patients with COVID-19. Blood Adv. 2020;4(16):4028. doi: 10.1182/bloodadvances.2020002496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NIH ACTIV trial of blood thinners pauses enrollment of critically ill COVID-19 patients. News release. National Institutes of Health . December 22, 2020. Accessed January 15, 2021. https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients
- 28.Pannucci CJ, Fleming KI, Agarwal J, Rockwell WB, Prazak AM, Momeni A. The impact of once- versus twice-daily enoxaparin prophylaxis on risk for venous thromboembolism and clinically relevant bleeding. Plast Reconstr Surg. 2018;142(1):239-249. doi: 10.1097/PRS.0000000000004517 [DOI] [PubMed] [Google Scholar]
- 29.Yamakawa K, Umemura Y, Murao S, Hayakawa M, Fujimi S. Optimal timing and early intervention with anticoagulant therapy for sepsis-induced disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2019;25:1076029619835055. doi: 10.1177/1076029619835055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287-291. doi: 10.1007/s11239-020-02145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talasaz AH, Sadeghipour P, Kakavand H, et al. Antithrombotic therapy in COVID-19: systematic summary of ongoing or completed randomized trials. J Am Coll Cardiol. Published online March 16, 2021. doi: 10.1016/j.jacc.2021.02.035 [DOI] [Google Scholar]
- 34.Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060-2072. doi: 10.1016/j.jacc.2020.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(2):135-144. doi: 10.1161/CIRCULATIONAHA.120.051936 [DOI] [PubMed] [Google Scholar]
- 36.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomazini BM, Maia IS, Cavalcanti AB, et al. ; COALITION COVID-19 Brazil III Investigators . Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307-1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
Investigators and Committees
Eligibility criteria
Definitions of Outcome Events
eMethods
Study outcomes in hospitalized versus post-discharged patients
Prespecified subgroup analysis of primary outcome in patients with or without D-dimer >1000 ng/mL
Post hoc subgroup analysis of major bleeding among aspirin and non-aspirin users
eFigure 1. Kaplan-Meier curve for all-cause mortality in the prespecified primary cohort
eFigure 2. Kaplan-Meier curve for the venous thromboembolism in the prespecified primary cohort.
eFigure 3. Kaplan-Meier curve for the landmark analysis showing the primary composite outcome in the first ten days and from 11 to 30 days of enrollment in the prespecified primary cohort
eFigure 4. Post-hoc subgroup analysis
eTable 1. Intervention and comparator dosing for the anticoagulation hypothesis
eTable 2. Dosing strategies for intermediate dose applied in study sites
eTable 3. Dosing strategies for standard dose anticoagulation applied in study sites
eTable 4. Reasons for post-randomization changes in the assigned treatment of patients in the prespecified primary cohort
eTable 5. Treatment adherence in the two study groups in the prespecified primary cohort
eTable 6. Demographic and clinical characteristics at baseline according to the assigned anticoagulation therapy in the total study population
eTable 7. Demographic and clinical characteristics at baseline according to the assigned anticoagulation therapy in patients who were randomized and were not excluded due to violation of the eligibility criteria nor withdrawal of informed consent
Table 8. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis incorporating the center random effect
eTable 9. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the total study population who allowed their data to contribute to the final results
eTable 10. Primary, Secondary and exploratory outcomes within 30 days from enrollment in the total study population who allowed their data to contribute to the final results incorporating the center random effect
eTable 11. Primary, Secondary and exploratory outcomes within 30 days from enrollment in randomized patients who were not excluded due to violation of the eligibility criteria or withdrawal of informed consent.
eTable 12. Primary, Secondary and exploratory outcomes within 30 days from enrollment in randomized patients who were not excluded due to violation of the eligibility criteria or withdrawal of informed consent incorporating center random effect
eTable 13. Primary, Secondary and exploratory outcomes within 30 days from enrollment per-protocol cohort
eTable 14. Primary, Secondary and exploratory outcomes within 30 days from enrollment per-protocol cohort incorporating center random effect
eTable 15. Primary, Secondary and exploratory efficacy outcomes within 30 days from enrollment in patients with baseline D-dimer >1000 ng/ml in the prespecified primary analysis
eTable 16. Primary, Secondary and exploratory efficacy outcomes within 30 days from enrollment in patients with baseline D-dimer >1000 ng/ml in the prespecified primary analysis incorporating center random effect
eTable 17. In-hospital Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis
eTable 18. In-hospital Primary, Secondary and exploratory outcomes within 30 days from enrollment in the prespecified primary analysis incorporating center random effect
eTable 19. Summary of venous thromboembolism diagnostic tests performed by enrolling centers
eTable 20. Study outcomes and laboratory tests in patients with severe thrombocytopenia
eTable 21. Selected baseline characteristics and assigned anticoagulation regimen in patients with major bleeding
eTable 22. Anatomical characteristics of adjudicated venous thromboembolic events
eReferences
Nonauthor collaborators. INSPIRATION Investigators
Data sharing statement