Key Points
Question
Can intravitreal aflibercept injection (IAI) be used as a prophylactic treatment against conversion to exudative age-related macular degeneration (eAMD) in high-risk eyes?
Findings
In a randomized, single-masked clinical trial of 128 patients at high risk for eAMD, there was no difference in the proportion of eyes converting to eAMD between the IAI (6 patients [9.5%]) and sham (7 patients [10.9%]) groups at month 24. Patients with eAMD for longer than 2 years in their fellow eye at baseline showed a lower conversion rate in the study eye compared with those with eAMD for no longer than 2 years in the fellow eye.
Meaning
Although these findings do not support IAI as a prophylactic against conversion to eAMD, such prophylaxis is needed.
This randomized clinical trial evaluates the use of intravitreal aflibercept as prophylaxis against conversion to exudative age-related macular degeneration (AMD) in patients with dry AMD and high risk for conversion.
Abstract
Importance
Anti–vascular endothelial growth factor (VEGF) agents may provide a prophylactic effect in high-risk eyes with intermediate dry age-related macular degeneration (AMD) against conversion to exudative AMD (eAMD), lowering the risk of vision loss.
Objective
To evaluate intravitreal aflibercept injection (IAI) as prophylaxis against the conversion to eAMD in high-risk eyes at 24 months.
Design, Setting, and Participants
This single-masked, sham-controlled, randomized clinical trial performed at 4 US clinical sites enrolled patients with intermediate AMD in 1 eye (study eye), defined as presence of more than 10 medium drusen (≥63 to <125 μm), at least 1 large druse (≥125 μm), and/or retinal pigmentary changes, and eAMD in the fellow eye. Patients were treated from June 23, 2015, to March 13, 2019.
Interventions
Intravitreal aflibercept injection (2 mg) or sham quarterly injection for 24 months (1:1 randomization).
Main Outcomes and Measures
The primary end point was the proportion of patients with conversion to eAMD at month 24 characterized by development of choroidal neovascularization, as assessed by leakage on fluorescein angiography and fluid on spectral-domain optical coherence tomography by an independent masked reading center.
Results
Of 128 patients enrolled, 127 (63 in the IAI group and 64 in the sham group) were included in the primary analysis (68 men [53.5%]; mean [SD] age, 76.5 [8.1] years). Baseline demographic and clinical characteristics were balanced between the groups. By month 24, 6 patients (9.5%) in the IAI group and 7 (10.9%) in the sham group developed eAMD (P = .98). Patients with a history of eAMD for longer than 2 years in their fellow eye at baseline showed a lower rate of conversion to eAMD in the study eye compared with those with a history of eAMD for 2 years or less in the fellow eye. Safety was consistent with previous studies involving intravitreal anti-VEGF injections.
Conclusions and Relevance
In this evaluation of quarterly anti-VEGF exposure as prophylaxis to reduce conversion of eyes with high-risk dry AMD to eAMD, the rates of conversion were not lower in the IAI group compared with the sham treatment group at month 24. Understanding the mechanism of conversion to eAMD and therapies that could prevent this event remains an important unmet need.
Trial Registration
ClinicalTrials.gov Identifier: NCT02462889
Introduction
Extensive research has evaluated risk factors and conversion rates for progression from intermediate dry age-related macular degeneration (AMD) to exudative AMD (eAMD), an event that can lead to precipitous vision loss. During a mean follow-up of 6.3 years, the Age-Related Eye Disease Study (AREDS) reported a 10% conversion rate in at least 1 eye of patients with bilateral drusen without advanced AMD. When considering only fellow eyes of patients with unilateral eAMD, the rate was 35% during the same period.1
Although there has been a tremendous effort to establish treatment prevention for this subset of patients, high-dose vitamins and antioxidants based on the AREDS have been the only proven treatments with a modest reduction in risk of 25% for 5 years.2 With the advent of anti–vascular endothelial growth factor (VEGF) agents for the treatment of eAMD, extensive data have been developed among patients with unilateral eAMD, including rate and time to conversion to eAMD in the second eye. Conversion rates of fellow eyes in these patients have been reported to range from 10% to 60% during 1 to 2 years of follow-up across multiple studies.3,4,5,6
With the overwhelming success of anti-VEGF agents in treating eAMD, it has been hypothesized that these agents may serve as prophylaxis against conversion to eAMD among high-risk eyes with dry AMD. Such a prophylactic effect could target the upregulation of VEGF and control the progression of the choroidal neovascularization (CNV), thereby lowering the risk of vision loss associated with eAMD. Ultimately, this effect would preserve vision-related quality of life, especially among patients who already have visual impairment in 1 eye due to eAMD.
Methods
Study Design
The Prophylaxis Against Conversion to Neovascular Age-Related Macular Degeneration was a prospective, single-masked randomized clinical trial comparing a 2-mg intravitreal aflibercept injection (IAI) vs sham injection as prophylaxis against conversion to eAMD in high-risk eyes. The trial protocol is in Supplement 1. Institutional review board approval from Advarra, Inc (formerly Chesapeake Research Review) was obtained before initiating the study. Written informed consent was obtained from all patients. All patients received a stipend per visit. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Across 4 centers, 128 patients were randomized 1:1 to receive either quarterly IAI or sham injection for 24 months, from June 23, 2015, to March 13, 2019. Enrollment was stratified to balance the 2 groups for patients who were diagnosed with eAMD in their fellow eye no longer than 2 years vs those diagnosed longer than 2 years before enrollment.
Confirmation of eligibility was conducted by the Boston Imaging Reading Center based on review of fluorescein angiography and spectral-domain optical coherence tomography (SD-OCT) images before initiation of study treatment. This eligibility determination was performed within 1 hour to allow for same-day enrollment and treatment of eligible patients. Conversion to eAMD had to be confirmed by the reading center within 1 hour of submission and before same-day treatment could be administered. Treatment with IAI after eAMD diagnosis was according to standard of care per the treating investigator (J.S.H., D.M.B., S.P.S., SD, C.C.W., J.L.P., and D.S.B.).
Study assessments were conducted every 3 months and included manifest refraction with Early Treatment Diabetic Retinopathy Study best-corrected visual acuity testing, slitlamp examination, dilated fundus examination, SD-OCT using the Avanti device (Optovue), OCT angiography using the AngioVue device (Optovue), and fluorescein angiography. Fundus photography was also performed at baseline as well as the 12- and 24-month visits. Patients, visual acuity examiners, and the reading center were masked to study treatment assignment, whereas the investigators and coordinators (J.S.H., D.M.B., S.P.S., SD, C.C.W., J.L.P., and D.S.B.) were not masked.
Study Population
The study eye had to have a diagnosis of nonexudative (dry) AMD characterized by the presence of multiple intermediate drusen, 1 or more large drusen, and/or hyperpigmentary changes. Presence of geographic atrophy was not an exclusionary criterion. The fellow (nonstudy) eye had to have active eAMD (ie, leakage on fluorescein angiography and/or subretinal, intraretinal, or subretinal pigment epithelial fluid on OCT findings) or history of eAMD as confirmed by past treatment and diagnostic imaging. Patients with a history or the presence of serous pigment epithelial detachments, macular hole, vitrectomy, diabetic retinopathy more severe than 10 microaneurysms, blot hemorrhages, or diabetic macular edema in the study eye were excluded.
Outcome Measures
The primary outcome measure of the study was the proportion of patients with conversion to eAMD at 24 months as confirmed by the independent, masked reading center. Secondary outcome measures included the time to conversion to eAMD; rate of conversion based on duration of exudative disease in the fellow eye (≤2 years vs >2 years at baseline); rate of conversion based on presence of nonexudative CNV, defined as presence of a neovascular complex on OCT angiography without signs of leakage on either fluorescein angiography or OCT; mean change in best-corrected visual acuity; and mean change in growth of geographic atrophy. Incidence and severity of ocular and systemic events were also evaluated.
Statistical Power Calculation and Analysis
A 35% conversion rate during 2 years was assumed to detect a 70% reduction in conversion to eAMD. An 8% loss of participants during the 2-year study period was estimated. Based on these assumptions, a sample size of 128 patients was determined. The proportion of patients with conversion to eAMD was examined by treatment group using χ2 analysis. We used the Kaplan-Meier method to report conversion rate, calculated as the number of days between first treatment and conversion. Statistical significance was defined as 2-sided P < .05. Primary statistical analyses were performed based on intention to treat. No adjustment was made to the P values for multiple analyses. Analyses used Python, version 3.6 (Python.org) and the SciPy Statistics library.
Reading Center Evaluation
Diagnosis of eAMD was based on multimodal imaging results, including color fundus photography, SD-OCT, and fluorescein angiography. This diagnosis included evaluation for the presence of CNV on fluorescein angiography and presence of subretinal and/or intraretinal fluid associated with CNV. Graders were masked to OCT angiography results at the time of diagnosis of exudation.
Optical coherence tomographic angiography grading was performed by separate graders who were masked to the results of standard imaging, including fluorescein angiography and additional structural SD-OCT (although of necessity, when graders evaluate OCT angiography, they are able to view the antecedent volume scans but not the high-resolution scans). For review of OCT angiography, graders reviewed the en face scans after manual correction of segmentation where needed. They also reviewed the structural B-scans with flow overlay in the entire volume and then made an assessment on the presence or the absence of CNV. Exudative vs nonexudative determination was based on the criteria defined above, including exudation on multimodal imaging.
Results
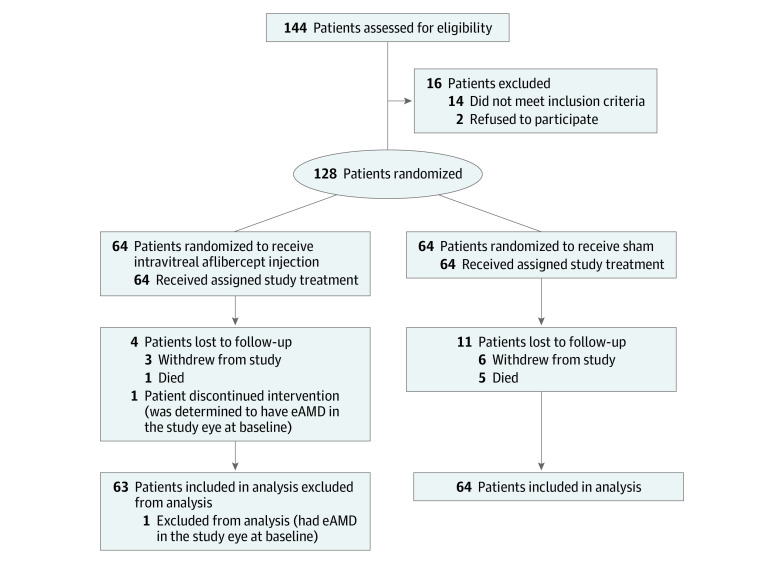
Of 128 patients enrolled, 127 (63 in the IAI and 64 in the sham groups) were included in the primary analysis (59 women [46.5%] and 68 men [53.5%]; mean [SD] age, 76.5 [8.1] years). One patient in the IAI group was found to have eAMD at enrollment subsequent to the first study treatment and was excluded from the primary analysis (Figure 1). Groups were balanced with respect to demographic and baseline characteristics (Table). Three patients in the IAI group and 6 in the sham group withdrew before the month 24 visit. One death occurred in the IAI group and 5 deaths in the sham group during the study period.
Figure 1. Study Flowchart.
eAMD indicates exudative age-related macular degeneration.
Table. Baseline Characteristics.
| Characteristic | Treatment group | |
|---|---|---|
| IAI (n = 63)a | Sham (n = 64) | |
| Age, mean (SD), y | 76.1 (7.4) | 76.9 (8.8) |
| Female, No. (%) | 27 (42.9) | 32 (50.0) |
| Duration of eAMD in fellow eye ≤2 y, No. (%) | 30 (47.6) | 31 (48.4) |
| Nonexudative choroidal neovascularization, No. (%) | 4 (6.3) | 6 (9.4) |
| BCVA, mean (SD), ETDRS letters | 78.7 (8.8) | 77.3 (10.1) |
| Proportion of patients with GA in study eye, No. (%) | 17 (27.0) | 8 (12.5) |
Abbreviations: BCVA, best-corrected visual acuity; eAMD, exudative age-related macular degeneration; ETDRS, Early Treatment Diabetic Retinopathy Study; GA, geographic atrophy; IAI, intravitreal aflibercept injection.
One patient had eAMD in the study eye at baseline.
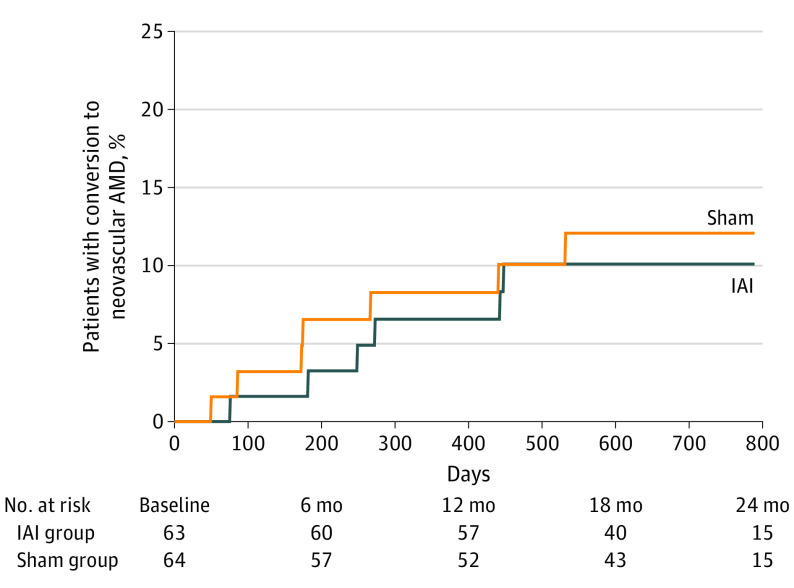
By month 24, 6 patients (9.5%) in the IAI group and 7 (10.9%) in the sham group developed eAMD (P = .98). Of these, 4 (66.7%) in the IAI group and 6 (85.7%) in the sham group experienced conversion during 12 months. Time to development of eAMD is shown in Figure 2. Of these 13 patients, 8 were determined to have conversion at a scheduled study visit, and 5 were diagnosed with eAMD at an unscheduled visit.
Figure 2. Time to Conversion to Exudative Age-Related Macular Degeneration (AMD) in the Overall Population.
IAI indicates intravitreal aflibercept injection.
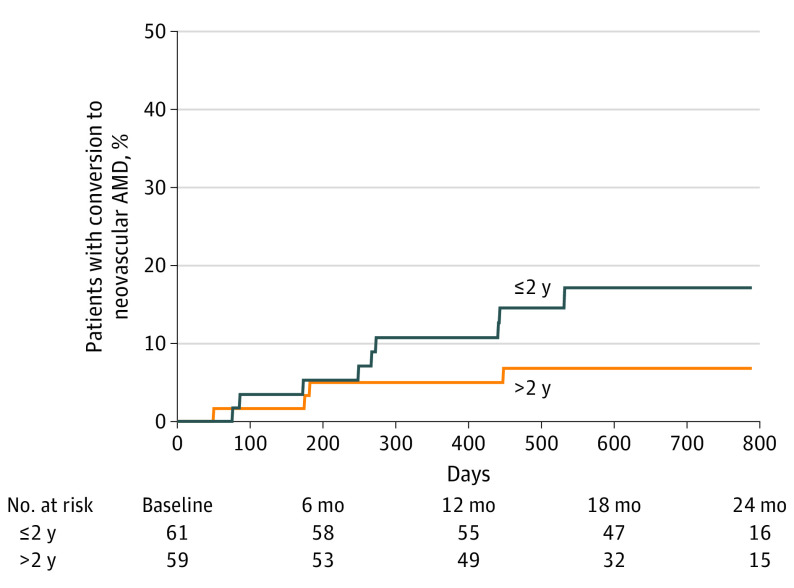
Patients with a history of eAMD in their fellow eye of longer than 2 years (IAI group: 2 [6.3%]; sham group: 2 [6.3%]) at baseline showed a lower rate of conversion to eAMD in the study eye compared with patients with a history of eAMD in the fellow eye of no longer than 2 years (IAI group: 4 [12.9%]; sham group: 5 [15.6%]). Time to development of eAMD based on duration of eAMD in the fellow eye is shown in Figure 3.
Figure 3. Time to Conversion by Duration of Exudative Age-Related Macular Degeneration (AMD) in the Fellow Eye.
Patients are stratified between those with a history of exudative AMD in the fellow eye of longer than 2 years vs no more than 2 years.
The number of study eyes with presence of nonexudative CNV increased from baseline during the study period from 4 (6.3%) in the IAI group and 6 (9.4%) in the sham group to 11 (17.5%) and 13 (20.3%), respectively, at any time before conversion to eAMD. Among these eyes, 3 of 11 (27.3%) in the IAI group and 4 of 13 (30.8%) in the sham group developed eAMD. This accounts for only conversions that occurred after determination of the presence of nonexudative CNV. Case examples of courses of patients with nonexudative CNV at baseline are shown in the eFigure in Supplement 2.
Mean change in best-corrected visual acuity remained relatively stable over time (IAI group: −2.8 [95% CI, 0.70 to 6.64] letters; sham group: 0.2 [95% CI, −4.39 to 2.52] letters; P = .10); eyes were censored at the time of eAMD development. Mean growth in geographic atrophy was 0.34 (95% CI, −0.07 to 0.36) mm2 in the IAI group and 0.43 (95% CI, 0.11 to 0.96) mm2 in the sham group (P = .67).
Safety
One patient in the IAI group had a loss of 31 letters from the month 9 to month 12 visits, resulting from progression of geographic atrophy into the fovea. The patient continued to receive study treatment through month 24.
Nonfatal cerebrovascular accidents occurred in 2 patients, 1 in each treatment group. In addition, in the sham group, 1 patient had a fatal cerebrovascular accident, 1 had a fatal cerebrovascular accident and heart attack, and 1 had a fatal congestive heart failure. One patient in each treatment group died because of pneumonia, and 1 in the sham group died of natural causes. None of these events were attributed to the study drug or the study procedure.
Discussion
This study evaluated prophylactic quarterly treatment with IAI to reduce conversion of high-risk eyes with dry AMD to eAMD. By month 24, conversion rates in the IAI group were not lower than in the sham group and overall were lower than originally estimated. One reason for lack of a prophylactic effect could be an inadequate dosing frequency, and more frequent dosing than a quarterly regimen could have a different effect. Quarterly dosing was selected owing to concern with the increased treatment burden that more frequent dosing would necessitate. The on-mechanism trigger that leads to the development of eAMD may be unrelated to upregulation of VEGF, and hence, there could be no role for prophylactic anti-VEGF to reduce the risk of developing CNV among eyes with the intermediate stage of AMD.7
In planning the present study, estimated conversion rates in second eyes when the first eye had previously developed eAMD were obtained from prior published studies.3,4,5,6 Patients were typically enrolled in these studies within a short time from their diagnosis of eAMD, often weeks to months. Conversion to eAMD in the fellow eyes of unilateral eAMD in these studies is limited to a 2-year study period. We suspect that because patients enrolled in these studies had been diagnosed with eAMD in their first eye within 1 to 2 years of conversion in the second eye, a potential overestimation of conversion rates for the second eye is plausible and led to an underpowering of the study.
In addition, more patients discontinued the study (withdrew or died) in the sham group (n = 11) than in the IAI group (n = 4). Specifically, for patients who withdrew (6 in the sham group and 3 in the IAI group), most did so within the first year, and it is unknown whether they had subsequent conversion to eAMD. Considering the small sample size, this imbalance could have had an effect on estimated conversion rates.
Because timing of eAMD diagnosis in the first eye could influence the rate of conversion to eAMD in the second eye, in the present trial, patients were stratified a priori based on this finding. The present study validates the observation that rate of conversion to eAMD in the second eye decreases over time after eAMD diagnosis in the first eye. In our study, the rate of conversion was higher in eyes whose fellow eyes had been diagnosed with eAMD within 2 years of baseline compared with those whose fellow eyes were diagnosed earlier. In addition, most of the conversions in the IAI and sham groups were within the first year.
Recently, with the availability of OCT angiography, eyes with nonexudative or silent CNV can be readily identified. These eyes harbor a neovascular complex, but there is no associated blood, retinal fluid, or thickening of the retina on OCT or evidence of leakage on fluorescein angiography. These eyes are generally observed without treatment unless evidence of exudation manifests. Multiple reports have indicated that presence of nonexudative CNV may increase the rate of conversion to exudative disease.8,9,10,11,12 Similarly, as observed in the present study, eyes with nonexudative CNV were more likely to undergo conversion to eAMD. In our study, no differences between the treatment groups were observed, and treatment with IAI did not affect the rate of conversion in these patients. However, the number of patients with nonexudative CNVs was small, and this study was not powered to detect a treatment effect if such an effect had occurred.
Strengths and Limitations
Strengths of this study include standardized protocol assessments and a full complement of imaging, including OCT angiography, at every visit in eyes with a high risk of conversion to eAMD. An independent reading center confirmed inclusion in the study as well as the conversion event within 1 hour, enabling same-day treatment. However, because of a lower than expected rate of conversion, our study could have been underpowered to detect a difference in conversion rate between the two treatment groups.
Conclusions
Careful clinical monitoring of fellow eyes of patients whose first eye has developed eAMD, especially in the first 1 to 2 years, remains critical. Understanding the trigger(s) driving conversion as well therapeutic options that may prevent this conversion remain important unmet needs. A larger study with an enriched population by eAMD duration in the first eye and the presence of nonexudative CNV in the study eye may demonstrate a value of quarterly anti-VEGF dosing not detected in the present study. On the other hand, a larger study may show the same results as noted in this study. Next-generation anti-VEGF agents, novel delivery methods allowing consistent and durable anti-VEGF intraocular residence time, and other therapeutic targets may have an effect on conversion of dry AMD to eAMD in a way that pulsatile quarterly dosing of IAI did not in this study. However, until such studies prove such effects, use of these agents for such prophylaxis is not supported by this study.
Trial Protocol
eFigure. Case Examples of Course of Patients With Nonexudative Choroidal Neovascularization at Baseline
Data Sharing Statement
References
- 1.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL III; Age-Related Eye Disease Study Research Group . Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report No. 19. Ophthalmology. 2005;112(4):533-539. doi: 10.1016/j.ophtha.2004.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119(10):1417-1436. doi: 10.1001/archopht.119.10.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lujan BJ ZL, Hopkins JJ. Comparison of spectral domain (SD-OCT) and fluorescein angiography (FA) conversion rates in fellow eyes of HARBOR patients. Presented at: 2012 Joint Meeting of the American Academy of Ophthalmology; November 10-13, 2012; Chicago, IL. [Google Scholar]
- 4.Maguire MG, Daniel E, Shah AR, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT Research Group) . Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(10):2035-2041. doi: 10.1016/j.ophtha.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol. 2010;149(6):939-946.e1. doi: 10.1016/j.ajo.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Parikh R, Avery RL, Saroj N, Thompson D, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients with age-related macular degeneration treated with intravitreal aflibercept or ranibizumab. JAMA Ophthalmol. 2019;137(8):914-920. doi: 10.1001/jamaophthalmol.2019.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438-451. doi: 10.1038/nri3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagi Y, Mohla A, Lee SY, et al. Incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration. JAMA Ophthalmol. 2018;136(8):905-911. doi: 10.1001/jamaophthalmol.2018.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-266. doi: 10.1016/j.ophtha.2017.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiferman MJ, Fawzi AA. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS One. 2019;14(6):e0217805. doi: 10.1371/journal.pone.0217805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey ST, Thaware O, Wang J, et al. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmol Retina. 2019;3(8):629-636. doi: 10.1016/j.oret.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Zhang Q, Motulsky EH, et al. Two-year risk of exudation in eyes with nonexudative age-related macular degeneration and subclinical neovascularization detected with swept source optical coherence tomography angiography. Am J Ophthalmol. 2019;208:1-11. doi: 10.1016/j.ajo.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Case Examples of Course of Patients With Nonexudative Choroidal Neovascularization at Baseline
Data Sharing Statement