Abstract
BACKGROUND AND PURPOSE: In the early stage of Multiple Sclerosis (MS), conventional MR imaging parameters such as T2 lesion load fail to explain the clinical status of patients. In the present work, we aimed to determine the ability of magnification transfer imaging to better reflect the relationship between local tissue damage and functional status of MS patients.
METHODS: We performed a comparative statistical mapping analysis on brain tissue magnetization transfer ratio (MTR) data measured in 18 patients with clinically isolated syndrome suggestive of MS (CISSMS) and 18 matched control subjects.
RESULTS: In the patients with CISSMS, a pattern of significant low MTR values was observed in the white matter, corpus callosum, bilateral occipitofrontal fascicles, right fornix, right parietal white matter, external capsule, right superior longitudinal fasciculus (SLF), right inferior longitudinal fasciculus, optica radiata, parietal white matter, right cingulum, gray matter, bilateral thalamus, bilateral caudate, right insula, and left Brodmann area (BA) 8. No correlation was found between local MTR decrease and Expanded Disability Status Scale score. Significant correlations between MTR and MS Functional Composite scores (Spearman rank test, P < .05) were observed in the left BA40, right SLF, right frontal white matter, splenium, and anterior corpus callosum. Local MTR values correlated with Paced Auditory Serial Addition Test scores in the left BA40, right BA4, right SLF, and splenium.
CONCLUSION: Statistical mapping analysis of brain MTR data provides valuable information on the relationship between the location of brain tissue damage and its functional impact in patients with MS, even in the earliest stage of the disease.
MR imaging has become a technique of choice in the diagnosis of multiple sclerosis (MS) because of its ability to depict demyelinating lesions on T2-weighted images and blood-brain barrier disruption on gadolinium-enhanced T1-weighted images, as well as to characterize lesion dissemination in time and space (1). However, the extent of tissue damage expressed as the T2 lesion load correlates weakly with the clinical status of patients with MS (2, 3). Causes of discrepancy are multifactorial. First, the T2 lesion load has been shown not to reflect the entire extent of tissue damage in patients with MS when parametric MR imaging techniques, like diffusion tensor imaging or magnetization transfer ratio (MTR) imaging, depict structural abnormalities in the normal-appearing brain tissue, as well as in white matter and gray matter. Second, compensatory cortical reorganization, as shown by functional MR imaging studies, plays a role in this mismatch between tissue injury and functional status of patients with MS (4, 5). This cortical reorganization is present in several functional systems (motor, visual, and cognitive) and even at the earliest stage of MS (6, 7). Noninvasive parametric MR imaging reflects, to some extent, the effect of tissue damage on functional status of patients with MS. Indeed, metrics derived from normal-appearing brain MTR or diffusion tensor imaging histograms were reported to correlate with the Expanded Disability Status Scale score (EDSS) (8, 9) in patients with MS at an advanced stage of the disease.
Spatial information on tissue injury is of particular interest. Location of MS lesions assessed with statistical mapping analysis performed on T2 lesions correlates with several functional impairments in patients with long-standing MS (10). Moreover, statistical mapping analysis performed on gray matter maps has recently shown that the mean thickness of cortical ribbon in the motor cortex was related to the EDSS scores in patients with long-standing MS (11). However, this approach was not sufficiently sensitive to evidence significant cortical thickening in patients with MS who had a short disease duration (< 3 years) (11).
In the present study, we combined MTR measurements to evidence subtle tissue damage, with spatial information given by statistical mapping analysis applied to brain tissue MTR data. The objective was to better characterize the effect of brain tissue damage on the functional status of patients in the earliest stage of MS, namely, in patients with clinically isolated syndrome suggestive of MS (CISSMS).
Methods
Subjects
We evaluated 18 patients with CISSMS who, at inclusion, fulfilled at least the dissemination-in-space criterion among the criteria of McDonald et al (1): dissemination in space demonstrated by MR imaging, or positive CSF plus two or more MR imaging-detected lesions consistent with MS. Fourteen patients had a diagnosis of MS, and four had a diagnosis of “possible MS” according to the McDonald’s criteria when comparing the first MR images obtained after the relapse with the conventional MR images obtained at the same time as MTR imaging (at least 3 months after the onset and steroid treatment). The functional status of patients was evaluated by using EDSS (12) and the MS Functional Composite (MSFC) score (13, 14). Perceptuomotor abilities were evaluated by using the Trail Making Test version A (TMTA), which consists of connecting ascending numbers from 1 to 20 dispersed randomly on a sheet of paper. The extent of depression and fatigue was evaluated by using the Montgomery-Asberg Depression Rating Scale (MADRS) and the Modified Fatigue Impact Scale (MFIS). Eighteen age- and sex-matched healthy control subjects were also included in this protocol. All subjects gave their informed consent to participate in this protocol, which was approved by the local committee on ethics.
MR Imaging Acquisition
All subjects were imaged with a 1.5-T commercially available unit (Magnetom Vision Plus; Siemens, Erlangen, Germany). The MR imaging protocol included localizer scout imaging, transverse fast spin-echo proton density-weighted and T2-weighted sequences (2600/15/85 [TR/TE1/TE2], 44 contiguous sections, 3-mm section thickness, 90° flip angle, 240-mm FOV, 256 x 256 matrix), and transverse proton density-weighted spoiled gradient-echo sequences (500/4.7 [TR/TE], 44 contiguous sections, 3-mm section thickness, 30° flip angle, 240-mm FOV, 256 x 256 matrix) performed without and with MT saturation (1.5-kHz off-water resonance, 500°). A T1-weighted spin-echo sequence (650/10 [TR/TE], 25 contiguous sections, 5-mm section thickness, 90° flip angle, 240-mm FOV, 256 x 256 matrix) was also performed 5 minutes after injection of a gadolinium-based contrast agent (0.1 mmol/L/kg Dotarem, Guerbet, Roissy, France).
MTR Image Processing
MTR maps were calculated on a voxel-by-voxel basis according to the following equation: MTR(%) = (M0 − Mmt)/M0, where M0 and Mmt are the images obtained without and with MT saturation pulse, respectively. MTR maps were then co-registered onto the corresponding T2-weighted images of each subject. T2 lesions were delineated by using a semiautomated method (interactive thresholding technique written on the IDL Interactive Data Language platform, version 5.2; Resarch System Inc., Boulder Co.). MTR maps were spatially normalized into the Montreal Neurologic Institute (MNI) space by using the T1 anatomic template provided in the SPM2 software (Wellcome Institute, London, England). The spatial normalization algorithm preserved voxel intensities (concentrations) even in regions where values had been stretched by warping (15, 16). The spatial transformation was also applied onto the T2 lesion mask. Then the normalized T2 lesion mask was applied onto the normalized MTR maps to obtain normalized MTR maps of T2 lesions. Differences between normalized brain MTR images and normalized T2 lesions MTR maps gave the normalized normal-appearing brain MTR map. After segmentation of the normalized normal-appearing brain MTR maps by using voxel intensities and prior knowledge procedures (SPM2), three maps representing fractions of gray matter, normal-appearing white matter, and CSF were obtained. Pixels with a percentage of gray matter plus white matter greater than 90% were used to mask the normalized normal-appearing brain MTR map. The normalized normal-appearing brain MTR map was finally added to the normalized T2 lesion MTR map to obtain a normalized brain tissue MTR map.
Statistical Mapping Analysis
Statistical mapping analysis offers the ability to compare quantitative parameters derived from various imaging modalities in all parts of the brain without prior hypothesis about effect location. This makes the technique sensitive to local variations that could be hindered when considering the whole data set, as is done when using histogram analysis.
This technique is used extensively to study brain activation observed by functional imaging (positron emission tomography or functional MR imaging) (17). It has been useful to evaluate the variations in morphologic parameters such as local percentages and local concentrations of gray matter reflecting the extent of local cortical atrophy referred to as voxel-based morphometry (18). Comparison of images from various modalities such as thickness of cortical ribbon in patients with MS (11) or variations in gray matter MTR in epileptic patients (19) can be assessed. Recent studies have reported the usefulness of statistical mapping analyses to define patterns of macroscopic lesion location measured on T2-weighted images (10) or to determine the possible relationship between local characteristics of tissue (i.e., T2 lesion or cortical thickness) and various functional scores (10, 11).
Before statistical comparison, normalized brain tissue MTR maps were smoothed by using a 6-mm Gaussian filter to minimize remaining spatial differences between subjects and to better meet the conditions of random field theory (17). Group analyses performed on normalized data allow definition of common patterns of tissue damage and correlations between location of tissue damage and functional scores. A two-sample t test was used to compare brain MTR maps of patients and control subjects on a voxel-by-voxel basis. Clusters were located on a Talairach atlas after transformation of MNI coordinates into Talairach coordinates (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). Statistical threshold of Zt = 4.17 (P = .0001, k = 10, uncorrected for multiple comparison) was chosen to detect MTR abnormalities. The resultant foci were characterized in terms of spatial extent k (17). This correction describes the probability that a region of the observed size could have occurred by chance over the entire volume analyzed. The corrected P value selected was P < .05.
Correlation between Local MTR Decrease and Functional Scores
Only functional scores shown to significantly differ between patients and control subjects were used for subsequent correlation studies with local MTR decrease in patients. Correlations were first assessed with the statistical mapping analysis by using a multiple regression model, taking into account the effect of age (P < .01, k = 5). Statistical correlation maps were masked by the regions of significant MTR decrease observed in patients compared with control subjects (P < .0001, k = 10, corrected for multiple comparisons). Raw data from the surviving clusters were extracted by using the MarsBar toolbox (SPM), and correlations with functional scores were confirmed by using Spearman rank correlation tests.
Results
Patient Characteristics
Clinical characteristics of the patients are summarized in Table 1. No differences in age, sex, and educational level were observed between patients and control subjects (Table 2). A mean ± SD T2 lesion load of 2.15 ±1.4 cm3 was measured in the patients. At the time of exploration, the mean duration of disease since the first clinical onset was 6.6 ± 4.9 months in this population that comprised 14 patients with MS and four with possible MS, according to McDonald’s criteria. Follow-up exploration performed 6 months and 1 year later showed conversion of the four possible MS cases to MS according to McDonald’s criteria (dissemination in time criteria additional to new T2 lesion load or new gadolinium-enhanced lesions).
TABLE 1:
Clinical characteristics of the patients with CISSMS
| Patient No./Age (y) | Clinical Syndrome | McDonald’s Criteria | Months Since Onset | Test Scores |
T2 Lesion Load (cm3) | |||
|---|---|---|---|---|---|---|---|---|
| EDSS | PASAT | MSFC | TMTA | |||||
| 1/22 | Optic neuritis | MS | 7 | 1 | 30 | −1.758 | 32.66 | 1.78 |
| 2/35 | Hemispheric syndrome | MS possible | 7 | 0 | 37 | −1.108 | 34.00 | 2.36 |
| 3/36 | Optic neuritis | MS | 3 | 1 | 37 | −1.961 | 19.93 | 1.48 |
| 4/34 | Hemispheric syndrome | MS | 12 | 0 | 34 | −1.213 | 39.00 | 2.36 |
| 5/34 | Optic neuritis | MS | 4 | 0 | 30 | −1.106 | 14.78 | 1.78 |
| 6/37 | Spinal cord syndrome | MS possible | 5 | 2 | 21 | −2.093 | 29.00 | 1.32 |
| 7/27 | Optic neuritis | MS | 5 | 2 | 36 | −1.330 | 30.00 | 1.74 |
| 8/25 | Optic neuritis | MS | 4 | 0 | 32 | −0.631 | 32.47 | 2.03 |
| 9/25 | Brain-stem syndrome | MS | 6 | 0 | 34 | −0.377 | 19.00 | 1.19 |
| 10/36 | Hemispheric syndrome | MS | 6 | 1 | 45 | −1.082 | 37.62 | 5.94 |
| 11/22 | Spinal cord syndrome | MS | 3 | 1 | 54 | 0.354 | 27.19 | 0.58 |
| 12/40 | Hemispheric syndrome | MS possible | 4 | 0 | 51 | −0.532 | 49.00 | 3.38 |
| 13/38 | Optic neuritis | MS | 24 | 1 | 40 | −1.232 | 39.04 | 2.20 |
| 14/19 | Spinal cord syndrome | MS | 4 | 1 | 41 | −0.827 | 25.50 | 1.02 |
| 15/25 | Hemispheric syndrome | MS | 10 | 0 | 49 | 0.224 | 22.30 | 0.53 |
| 16/22 | Spinal cord syndrome | MS | 6 | 1 | 50 | 0.576 | 22.12 | 5.06 |
| 17/20 | Brain-stem syndrome | MS | 3 | 0 | 48 | 0.083 | 35.72 | 2.59 |
| 18/30 | Spinal cord syndrome | MS possible | 7 | 1 | 45 | 0.059 | 33.37 | 1.28 |
TABLE 2:
Demographics and clinical characteristics of the patients with CISSMS and control subjects
| Characteristic | Patients (n = 18) | Controls (n = 18) |
|---|---|---|
| Age (y), mean (SD) | 29.3 (7) | 25.27 (6.3) |
| Educational level (y), mean (SD) | 13.1 (2.4) | 14.7 (2.10) |
| Gender | ||
| Female | 16 | 16 |
| Male | 2 | 2 |
| Test Scores, mean (SD) | ||
| MSFC | −0.78 (0.8)* | −0.101 (0.60) |
| PASAT | 39.7 (8.9)† | 48.72 (6.78) |
| Nine-hole peg test (s−1) | 4.96 × 10−2 | 5.25 × 10−2 |
| (0.49 × 10−2) | (0.55 × 10−2) | |
| 25-foot walking test (s) | 4.75 (0.78) | 4.39 (0.76) |
| TMTA (s) | 30.2 (8.6)* | 22.6 (2.6) |
| Stroop (s) | 57.8 (9.8) | 56.8 (10.6) |
| MADRS | 8.9 (3.9) | 8.2 (4.2) |
| MFIS | 22.5 (13.4) | 25.5 (12.4) |
P < .05.
P < .005 (Mann Whitney U test).
Functional Status of Patients with CISSMS
The median EDSS score of the 18 patients was 1 (range, 0–2). No differences between patients and control subjects were observed in their performance of the nine-hole peg test and the 25-foot walking test (Table 2). Depression (MADRS) and fatigue (MFIS) scores were not different between patients and control subjects (Table 2). Significant differences in performances were observed during the TMTA. Low Paced Auditory Serial Addition Test (PASAT) scores in patients induced a significant difference in the MSFC score between patients and control subjects.
Statistical Mapping Analysis of Brain Tissue MTR Maps
Statistical t test comparison between brain MTR maps of patients and control subjects are reported in Figure 1 and Table 3. MTR abnormalities were observed in both white and gray matter.
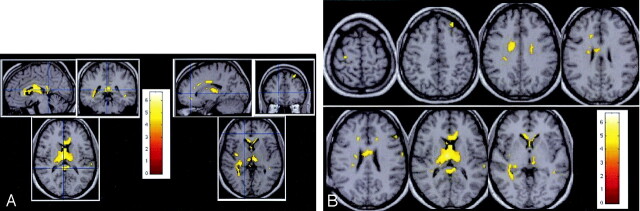
Fig 1.
Statistical mapping analysis. Patterns of significant MTR abnormalities are observed inside the corpus callosum, bilateral occipitofrontal fascicles, right fornix, right parietal white matter, external capsule, right SLF, right ILF, optica radiata, parietal white matter, right cingulum, bilateral thalamus, bilateral caudate, right insula, and the left BA8.
A, Multiplanar images of MTR decrease observed in patients with CISSMS compared with control subjects.
B, Transverse images of MTR decrease observed in patients with CISSMS compared with control subjects.
TABLE 3:
Location of MTR decrease in patients with CISSMS compared with control subjects
| Cluster No. | k Value | Corrected P Value | t Value | X | Y | Z | Location |
|---|---|---|---|---|---|---|---|
| 1 | 142 | .012 | 6.70 | 0 | −42 | 21 | CC splenium |
| 2 | 2112 | <.001 | 6.68 | −2 | −11 | 19 | CC genu |
| 6.41 | −6 | −27 | 7 | Left thalamus | |||
| 6.23 | 6 | −17 | 16 | Right fornix | |||
| 5.93 | 0 | −6 | 24 | CC body | |||
| 5.89 | 8 | 18 | 3 | Right caudate | |||
| 5.66 | −8 | 16 | 10 | Left caudate | |||
| 5.31 | 22 | −16 | 32 | Right OF | |||
| 5.30 | 28 | −30 | 24 | Right parietal WM | |||
| 5.21 | 8 | −27 | 11 | Right thalamus | |||
| 5.17 | 14 | −10 | 34 | Right cingulum | |||
| 3 | 50 | .040 | 6.22 | 36 | −17 | 8 | Right insula |
| 4 | 39 | .035 | 5.86 | 36 | 17 | −3 | Right insula |
| 4.30 | 38 | 6 | −4 | External capsule | |||
| 5 | 55 | .009 | 5.69 | −24 | 37 | 46 | Left BA8 |
| 6 | 236 | <.001 | 5.12 | 28 | −37 | 6 | Right optica radiata |
| 5.08 | 38 | −37 | 6 | Right ILF | |||
| 4.64 | 34 | −44 | 19 | Right SLF | |||
| 7 | 34 | .049 | 4.89 | −16 | −14 | 36 | Left cingulum |
Note.—CC indicates corpus callosum; OF, occipitofrontal fascicles; WM, white matter.
In the white matter, low MTR values were observed in the corpus callosum, right occipitofrontal fascicles, right fornix, right parietal white matter, external capsule, right superior longitudinal fasciculus (SLF), right inferior longitudinal fasciculus (ILF), right optica radiata, and right cingulum.
In the gray matter, MTR abnormalities in the patients were located in the bilateral thalamus, bilateral caudate, right insula, and left Brodmann area (BA) 8. Abnormalities observed in the left BA40, left BA4, bilateral BA21, and right BA6 did not survive to spatial extent correction.
Correlation between Brain MTR Decrease and EDSS Score
No correlations were found between local MTR values and EDSS scores.
Correlation Between Brain MTR and TMTA Score
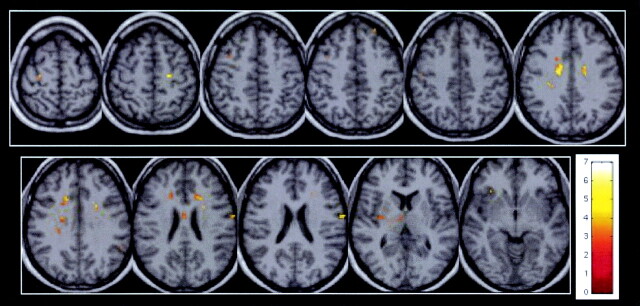
Significant correlations between MTR and TMTA scores (Spearman rank test, P < .05) were observed in the gray matter of patients inside the bilateral BA4, bilateral BA40, bilateral BA3, left BA8, right lateral sulcus, right BA6, and right claustrum (Fig 2 and Table 4). Correlations were also found in the white matter involving the body of corpus callosum, bilateral cingulum, left frontal white matter, and left occipitofrontal fascicles.
Fig 2.
In patients with CISSMS, significant correlations between MTR and TMTA scores (Spearman rank test, P < .05) are observed inside the bilateral BA4, bilateral BA3, right BA6, left BA8, left frontal white matter, left occipitofrontal fascicles (top row, left to right) and in the left frontal white matter, left occipitofrontal fascicles, body of corpus callosum, bilateral cingulum, bilateral BA40, right lateral sulcus, and right claustrum (bottom row, left to right).
TABLE 4:
Spearman rank correlation test between local MTR and functional scores in patients with CISSMS
| Correlations | Location | r Value | P Value |
|---|---|---|---|
| MTR = f(TMTA) | Left BA 4 | −0.789 | .0011 |
| Right BA 4 | −0.643 | .0080 | |
| Right BA 40 | −0.699 | .0040 | |
| Left BA 39/40 | −0.552 | .0228 | |
| Right thalamus | −0.492 | .0424 | |
| Right BA 3/4 | −0.643 | .0080 | |
| Left BA 3/4 | −0.746 | .0021 | |
| Left BA 8 | −0.692 | .0043 | |
| Right lateral sulcul | −0.610 | .0119 | |
| Right BA 6 | −0.507 | .0367 | |
| Body of corpus callosum | −0.700 | .0038 | |
| Left cingulum | −0.674 | .0055 | |
| Left frontal WM | −0.814 | .0008 | |
| Left OF | −0.674 | .0055 | |
| Right claustrum | −0.668 | .0059 | |
| Right cingulum | −0.837 | .0006 | |
| MTR = f(PASAT) | Right BA 4 | 0.590 | .0149 |
| Left BA 40 | 0.698 | .0040 | |
| Splenium | 0.599 | .0136 | |
| Right SLF | 0.631 | .0093 | |
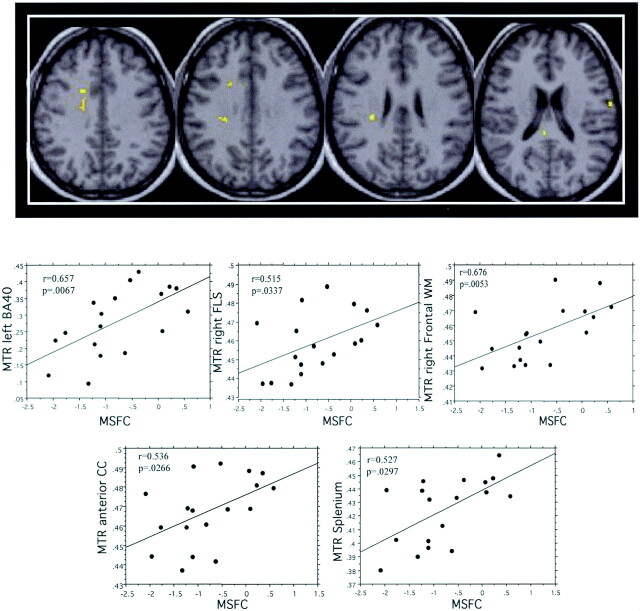
| MTR = f(MSFC) | Left BA 40 | 0.657 | .0067 |
| Splenium | 0.527 | .0297 | |
| Right SLF | 0.515 | .0337 | |
| Right frontal WM | 0.676 | .0053 | |
| Anterior corpus callosum | 0.536 | .0266 |
Note.—OF indicates occipitofrontal fascicles; WM, white matter.
Correlation Between Brain MTR Decrease and MSFC Score
Significant correlations between MTR and MSFC scores (Spearman rank test, P < .05) were observed in patients in the left BA40, right SLF, right frontal white matter, splenium, and projection of the anterior corpus callosum (Fig 3 and Table 4).
Fig 3.
In patients with CISSMS, significant correlations between MTR and MSFC scores (Spearman rank test, P < .05) are observed inside right SLF (FLS), right frontal white matter (WM), projections of the anterior corpus callosum (CC), splenium, and left BA40 (left to right).
Correlation Between Brain MTR Decrease and PASAT Score
Significant correlations between MTR and PASAT scores (Spearman rank test, P < .05) were observed in patients in the left BA40, right BA4, right SLF, and splenium (Fig 4 and Table 4).
Fig 4.
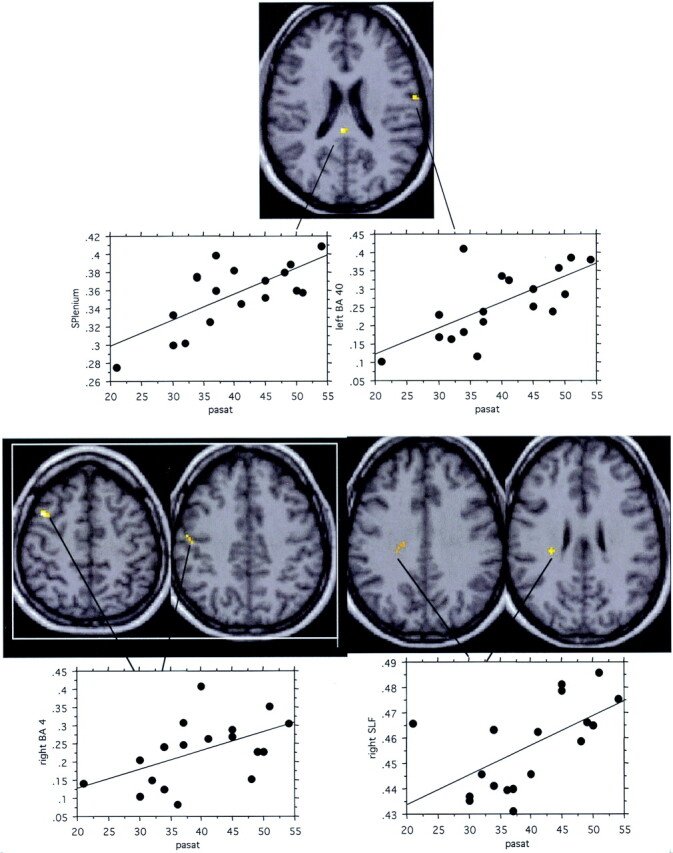
In patients with CISSMS, significant correlations between MTR and PASAT scores (Spearman rank test, P < .05) are observed in the splenium, left BA40, right BA 4, and right SLF.
Discussion
White Matter Damage in CISSMS
Previous parametric MR or histologic studies have reported the presence of diffuse white matter injuries in patients with clinically definite MS (CDMS), inside and outside T2 lesions (20–24). MTR has been shown to be a sensitive method to evidence damage even in normal-appearing brain at the earliest stage of MS (25, 26). In the present study, patients with CISSMS showed white matter tissue damage affecting mostly long connecting fibers. Patterns of decreased MTR were observed inside the corpus callosum, the key structure linking the two hemispheres. Involvement of the corpus callosum at the earliest stage of MS has already been reported in a study based on a multimodal region-of-interest approach centered on this structure, showing a probable tissue defect related to a myelin abnormality rather than to axonal loss (27). In the group of patients with CISSMS, MTR decrease was observed in other connecting fibers: 1) the SLF, a large bundle comprising numerous connections between the frontal pole, the occipital lobe, and the posterior part of the temporal pole; 2) the occipitofrontal fascicles, more deeply situated than the SLF, running from the frontal to the occipitotemporal region; 3) the optica radiata fibers, arising from the lateral geniculate body to end in the cortex of the calcarine fissure (BA17), and 4) the external capsule (28). We also observed tissue damage inside the fibers linking mesial structures to the other cortical regions such as the fornix (the main output fiber system of the hippocampus terminating mainly in the mammilary body and the preoptic area after encircling the thalamus) and the cingulum (thin association fascicle concentric to the cingulate gyrus and to the parahippocampal gyrus) (28). More generally, one possible explanation for the involvement of long connecting fibers in MS could be that the probability for a long fiber to be affected by demyelination is higher because of its size. Moreover, white matter damage secondary to Wallerian degeneration may be particularly important in such a structure because of the high density of fibers in the same direction that could be affected by the same distant demyelinating lesion.
Gray Matter Damage in CISSMS
Recent histopathologic studies established that gray matter involvement is substantial in patients with CDMS (29–31). Although no histopathologic data are available in patients at an early stage of the disease, a few MR studies suggest the existence of gray matter damage as soon as the early phase of the disease. One study performed in patients with CISSMS reported abnormalities in gray matter MTR histogram parameters (25). Gray matter atrophy, assessed by MR imaging has also been demonstrated, suggesting the potential presence of early neuronal loss (23, 24, 32, 33). MR spectroscopy studies using a nonlocalized method aimed at evaluating the whole-brain N-acetylaspartate (NAA) reported a significant decrease of this global index reflecting neuronal dysfunction or axonal loss in patients with CISSMS (34). Axonal dysfunction related to indirect effect of inflammation involving macroglia activation macrophages also has been reported in patients with early MS (35).
Although it is now well admitted that gray matter damage can occur early in the course of the disease, it is not well understood whether gray matter changes are distributed diffusely or have predominant locations. A recent study focusing exclusively on the cortex by using an inflated brain method and conducted at a more advanced stage of the disease, showed that thickness of cortical ribbon was locally reduced in patients with long-standing MS (11), especially in the frontal cortex, in the temporal cortex, and additionally in the motor cortex in patients with severe disability. However, no significant differences in cortical thickness were observed in patients at the early stage of the disease (<3 years). In this population of patients with CISSMS with high risk of developing MS, statistical mapping analysis applied at the earliest stage of the disease on brain tissue MTR data has shown that this technique allows detection of patterns of gray matter damage.
Gray matter abnormality has been reported in thalamus (atrophy and NAA reduction) at the early stage of relapsing-remitting MS and appears to evolve into substantial neuronal loss (30–35% reduction) in patients with long-standing disease (mean disease duration, 23.5 years) (32, 36). Detection of structural abnormalities in basal ganglia could be related to the fact that it concentrates 4–7.3% of the whole-brain lesions, which represents, according to the small size of these structures, a high density of lesions (37, 38). Degenerative changes in axons within the white matter lesions and secondary degeneration of fiber tracts have been documented in patients with CDMS (39–41). Basal ganglia circuits, consisting of numerous afferent connections, efferent connections, and loop (feedback) connections, are organized in several basal ganglia–thalamocortical circuits (motor, oculomotor, prefrontal, and limbic) (42). The MTR decrease observed in the optica radiata, fornix, their projections on the thalamus, and the occipital lobe could be related to tissue damage involving a large visual network. Sailer et al (11) hypothesized that focal damage in the white matter (predominantly located around the horns of the ventricles within fiber connections to the frontal, temporal, and motor areas of the cortex) in CDMS could be responsible for remote retrograde cortical atrophy. Thus, diffuse white matter damage in patients with CISSMS (25) affecting connecting fiber tracts could be responsible for remote retrograde changes in highly connected gray matter. Cortical changes found inside left BA8 and right insula could also be explained by the importance of the output and input connections of those areas.
Functional Impairment in CISSMS
The patients with CISSMS exhibited only subtle functional impairment with low EDSS scores and normal performance on the nine-hole peg test and the 25-foot walking test. Depression (MADRS) and fatigue (MFIS) scores were not different between patients and control subjects. In contrast, abnormal performances were related to abnormal TMTA performances and to low PASAT scores, inducing a significant difference in the MSFC scores between patients and control subjects.
Correlation Between Tissue Injury and EDSS
In this population of patients at the earliest stage of MS, no correlation was observed between local tissue defect measured with MTR and the EDSS scores. These results are in accordance with those observed by Sailer et al (11) looking at correlations between EDSS scores and local cortical thickness in a population of patients with MS of short disease duration (<3 years). In addition, the EDSS has a nonlinear scale reflecting mainly motor deficit. In these patients with CISSMS, evaluated at least 3 months after the onset of their clinical symptoms, the motor deficit is absent or too subtle to be related to a given local tissue injury.
Correlation Between Tissue Injury and TMTA
TMTA performance has been reported to be a function of perceptuomotor abilities and the capacity to process verbal stimuli (43). Correlation with local MTR values in the BA40, right lateral sulcus, and BA6 known to play a role in the working memory loop could be in accordance with this statement. The TMTA has also been found to load heavily on a factor representing motor problem solving (44) and on a factor measuring visuomotor speed and coordination (45). Motor problem solving implied recruitment of primary motor and sensory motor areas like BA3, BA4, and BA6, whereas BA8 is implied in the occulomotor processes. Correlation between TMTA scores and tissue damage inside the corpus callosum could be related to defects in coordination processes, driven by rapid interhemispheric information exchange (10).
Correlation Between Tissue Injury and PASAT
Abnormalities of the MSFC score observed in this population of patients were only related to differences in the PASAT performances. Significant correlations (Spearman rank correlation P < .05) between local MTR and the PASAT (Table 4, Fig 4) were observed in the long anteroposterior connecting fibers, splenium, right BA4, and left BA40. PASAT is a complex information-processing task that mobilizes numerous systems like attention, verbal working memory, inhibition of a prepotent response, and mental calculation. Recent cognitive theories have emphasized the involvement of the long corticocortical projections in highly controlled information processing (46). One hypothesis may be that damage of long corticocortical connections between associative areas, such as corpus callosum or anteroposterior connecting fibers, could alter the performance of a high-level cognitive task involving several distant brain areas that have to interact rapidly.
Finally, local MTR abnormalities assessed with statistical mapping analysis help to explain 50–70% of the effect induced by tissue damage onto the brain functions tested with MSFC and TMTA scores. Unexplained effect could be related to the presence of cortical reorganization, known to be present at this stage of MS. One-shot MR imaging protocol including functional MR imaging and MTR maps would better reflect relations between brain damage and clinical functional status in patients with MS.
Conclusion
Statistical mapping analysis of brain MTR data shows the presence of a relationship between local brain tissue damage and the functional status of patients with MS. This method could be particularly relevant to explore the brain substrate of cognitive dysfunction, present in nearly half of patients with MS at the earliest stage of the disease.
Footnotes
Funded by Association pour la Recherche sur la Sclérose en Plaques, Centre National de la Recherche Scientifique, Institut Universitaire de France, and Biogen Idec.
References
- 1.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 2.Miki Y, Grossman RI, Udupa JK, et al. Relapsing-remitting multiple sclerosis: longitudinal analysis of MR images–lack of correlation between changes in T2 lesion volume and clinical findings. Radiology 1999;213:395–399 [DOI] [PubMed] [Google Scholar]
- 3.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis: correlation between MRI parameters, clinical subtypes and symptoms. Brain 1998;121:687–697 [DOI] [PubMed] [Google Scholar]
- 4.Reddy H, Narayanan S, Arnoutelis R, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain 2000;123:2314–2320 [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Rocca MA, Falini A, et al. Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage 2002;15:537–546 [DOI] [PubMed] [Google Scholar]
- 6.Rocca MA, Mezzapesa DM, Falini A, et al. Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 2003;18:847–855 [DOI] [PubMed] [Google Scholar]
- 7.Aun B, Ibarrola D, Ranjeva J, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp 2003;20:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovaris M, Agosta F, Sormani MP, et al. Conventional and magnetization transfer MRI predictors of clinical multiple sclerosis evolution: a medium-term follow-up study. Brain 2003;126:2323–2332 [DOI] [PubMed] [Google Scholar]
- 9.Kalkers NF, Hintzen RQ, van Waesberghe JH, et al. Magnetization transfer histogram parameters reflect all dimensions of MS pathology, including atrophy. J Neurol Sci 2001;184:155–162 [DOI] [PubMed] [Google Scholar]
- 10.Charil A, Zijdenbos AP, Taylor J, et al. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. Neuroimage 2003;19:532–544 [DOI] [PubMed] [Google Scholar]
- 11.Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 2003;126:1734–1744 [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 13.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999;122:871–882 [DOI] [PubMed] [Google Scholar]
- 14.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999;5:244–250 [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston K. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of aging in 465 normal adult human brains. Neuroimage 2001;14:21–36 [DOI] [PubMed] [Google Scholar]
- 17.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45–53 [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Holmes AP, Price CJ, et al. Multisubject fMRI studies and conjunction analyses. Neuroimage 1999;10:385–396 [DOI] [PubMed] [Google Scholar]
- 19.Rugg-Gunn FJ, Eriksson SH, Boulby PA, et al. Magnetization transfer imaging in focal epilepsy. Neurology 2003;60:1638–1645 [DOI] [PubMed] [Google Scholar]
- 20.Tortorella C, Viti B, Bozzali M, et al. A magnetization transfer histogram study of normal-appearing brain tissue in MS. Neurology 2000;54:186–193 [DOI] [PubMed] [Google Scholar]
- 21.Evangelou N, Esiri MM, Smith S, et al. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 2000;47:391–395 [PubMed] [Google Scholar]
- 22.Allen IV, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 1979;41:81–91 [DOI] [PubMed] [Google Scholar]
- 23.De Stefano N, Matthews P, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 2003;60:1157–1162 [DOI] [PubMed] [Google Scholar]
- 24.Chard DT, Griffin CM, Barker GJ, et al. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 2002;125:327–337 [DOI] [PubMed] [Google Scholar]
- 25.Traboulsee A, Dehmeshki J, Brex PA, et al. Normal-appearing brain tissue MTR histograms in clinically isolated syndromes suggestive of MS. Neurology 2002;59:126–128 [DOI] [PubMed] [Google Scholar]
- 26.Iannucci G, Tortorella C, Rovaris M, et al. Prognostic value of MR and magnetization transfer imaging findings in patients with clinically isolated syndromes suggestive of multiple sclerosis at presentation. AJNR Am J Neuroradiol 2000;21:1034–1038 [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjeva JP, Pelletier J, Confort-Gouny S, et al. MRI/MRS of corpus callosum in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 2003;9:554–565 [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc.,1988
- 29.Kidd D, Barkhof F, McConnell R, et al. Cortical lesions in multiple sclerosis. Brain 1999;122:17–26 [DOI] [PubMed] [Google Scholar]
- 30.Peterson J, Bo L, Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50:389–400 [DOI] [PubMed] [Google Scholar]
- 31.Bo L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003;62:723–732 [DOI] [PubMed] [Google Scholar]
- 32.Cifelli A, Arridge M, Jezzard P, et al. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 2002;52:650–653 [DOI] [PubMed] [Google Scholar]
- 33.Quarantelli M, Ciarmiello A, Morra VB, et al. Brain tissue volume changes in relapsing-remitting multiple sclerosis: correlation with lesion load. Neuroimage 2003;18:360–366 [DOI] [PubMed] [Google Scholar]
- 34.Filippi M, Bozzali M, Rovaris M, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain 2003;126:433–437 [DOI] [PubMed] [Google Scholar]
- 35.Neumann H. Molecular mechanisms of axonal damage in inflammatory central nervous system diseases. Curr Opin Neurol 2003;16:267–273 [DOI] [PubMed] [Google Scholar]
- 36.Wylezinska M, Cifelli A, Jezzard P, et al. Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology 2003;60:1949–1954 [DOI] [PubMed] [Google Scholar]
- 37.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962;25:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lumsden C. The Neuropathology of Multiple Sclerosis. Amsterdam: North-Holland,1970
- 39.Matthews P, Pioro E, Narayanan S, et al. Assessment of lesion pathology in multiple sclerosis using quantitative MRI morphometry and magnetic resonance spectroscopy. Brain 1996;119:715–722 [DOI] [PubMed] [Google Scholar]
- 40.De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis: results of a longitudinal magnetic resonance spectroscopy study. Brain 1998;121:1469–1477 [DOI] [PubMed] [Google Scholar]
- 41.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285 [DOI] [PubMed] [Google Scholar]
- 42.Alexander G, Crutcher M. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13:166–171 [DOI] [PubMed] [Google Scholar]
- 43.Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol 1987;43:402–409 [DOI] [PubMed] [Google Scholar]
- 44.Goldstein G, Shelly CH. Statistical and normative studies of the Halstead Neuropsychological Test Battery relevant to a neuropsychiatric hospital setting. Percept Mot Skills 1972;34:603–620 [DOI] [PubMed] [Google Scholar]
- 45.Swiercinsky DP. Factorial pattern description and comparison of functional abilities in neuropsychological assessment. Percept Mot Skills 1979;48:231–241 [DOI] [PubMed] [Google Scholar]
- 46.Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci 2002;6:47–52 [DOI] [PubMed] [Google Scholar]