Abstract
Summary: A case is presented in which a complex multicystic hemosiderin-containing lesion developed adjacent to a previously documented developmental venous anomaly (venous angioma). This lesion had the characteristic MR imaging appearance of a cavernous malformation. Follow-up MR imaging demonstrated a decrease in both the size and complexity of this lesion, which suggests at least a portion of the lesion was due to sequelae of hemorrhage. This case further supports the association of a de novo, hemosiderin-containing lesion in association with developmental venous anomaly. Implications of these findings are that the commonly seen “cavernous malformations” in association with developmental venous anomaly are acquired lesions, and not congenital in origin. A review of the literature discussing the etiology of cavernous malformations and their reported association with the developmental venous anomaly is provided.
There are four types of commonly described vascular malformations occurring in the brain, including venous angioma, cavernous angioma, capillary telangiectasia, and the arteriovenous malformation (1). More recent descriptions in the literature have used the term “developmental venous anomaly” (DVA) to encompass the entities of venous angioma, venous malformation, and medullary venous malformation (2). The term “cavernous malformation” has also been used to describe cavernous angioma, cavernous hemangioma, cavernoma, and angiographically occult vascular malformation (3).
The etiology of cavernous malformations remains largely unknown, with some believing these lesions to be congenital and others acquired (4, 5). Only a few documented cases of de novo development of cavernous malformations are reported in the literature (6–8). One case report has documented the appearance or development of cavernous angioma adjacent to a developmental venous anomaly (venous angioma) in a young boy 3 years after radiation therapy for posterior fossa medulloblastoma (6). Latchaw (7) reports a similar case of a development of a hemosiderin-containing lesion adjacent to a venous angioma, also following radiation of a patient’s brain tumor on a follow-up MR image obtained 7 years later. Cakirer (8) describes a case of de novo formation of a cavernous malformation adjacent to a DVA in an otherwise healthy man presenting with throbbing headache. De novo appearance of cavernous angiomas has also been described in familial cases: a case associated with a craniopharyngioma and a case of multiple cryptic malformations associated with an extensive developmental venous anomaly (6).
Case Report
A 40-year-old right-handed man presented for evaluation of recent and relatively acute onset of headache, dizziness, and left-sided paresthesias involving the left upper extremity and left side of the face, primarily his forehead and cheek, sparing the mandible. He described the paresthesias in terms of dullness, numbness, soreness, and ache. He had also been undergoing continued evaluation of a >10-year history of left-sided abdominal pain, which remained indeterminate in origin. Noncontrast and gadolinium-enhanced MR imaging of the brain performed before the current presentation demonstrated a typical venous angioma within the right cerebellum, which was believed to be asymptomatic at that time (Fig 1A–D).
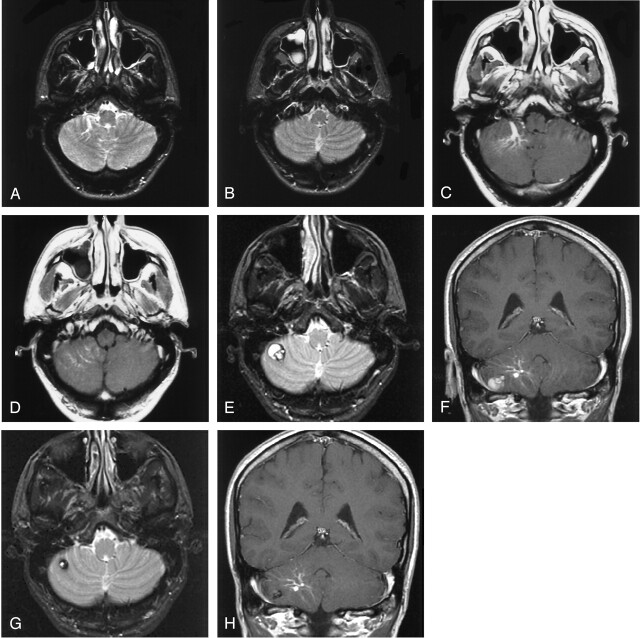
Fig 1.
De novo development of a lesion with the appearance of a cavernous malformation adjacent to an existing developmental venous anomaly.
A and B, T2-weighted axial MR imaging sections of the cerebellum obtained approximately 3 years before current presentation demonstrates a developmental venous anomaly within the right cerebellar hemisphere.
C and D, Postgadolinium-enhanced axial T1 images obtained at same levels of A and B demonstrate the characteristic enhancement and caput medusae distribution of the developmental venous anomaly, which drains anteriorly via an enlarged medullary vein.
E and F, MR images obtained following patient’s presentation with acute onset of dizziness, headache, and left sided paresthesias demonstrate development of a complex lobulated cystic hemosiderin-containing lesion measuring approximately 1.5 cm × 1.2 cm in size in the lower right cerebellum. The lesion is located within parenchyma that is in the drainage pattern of the developmental venous anomaly.
G and H, Follow-up MR images obtained 2 years later demonstrate a decrease in the size of the hemosiderin-containing abnormality, which now measures approximately 1 cm in diameter. The lesion has also become less complex in appearance.
Because of a new onset of neurologic symptoms, repeat MR imaging of the brain was performed. This demonstrated a new 1.5 cm × 1.2 cm finding adjacent to the previously noted venous angioma, characterized as having a well-circumscribed, slightly lobulated appearance and heterogeneous signal intensity with hemosiderin deposition along the margins of the lesion (Fig 1E and F). The patient’s neurologic symptoms and headaches resolved over a period of 3 months following onset. It was thought that the dizziness and headache may have been related to development of the left cerebellar abnormality. The patient’s left-sided paresthesias were not well explained by the cerebellar location and were thought to be unrelated to the cerebellar process. The paresthesias did, however, resolve concurrently with improvement of headache and dizziness. The patient continued to work as a truck driver throughout this evaluation and did not seek disability status.
Follow-up MR imaging obtained approximately 2 years after the appearance of the presumed cavernous angioma demonstrated an interval decrease in the size of the hemosiderin-containing lesion, which now measured less than 1 cm at its greatest diameter (Fig 1G and H). The lesion also had a less complex appearance on the follow-up study, with a less lobulated and less cystic appearance. The cerebellar abnormalities remain asymptomatic nearly 2 years after most recent MR imaging. The patient and treating physicians have elected to continue to monitor this abnormality with imaging, and surgical resection continues to be deferred.
Discussion
The issues and controversies concerning developmental venous anomalies, cavernous malformations, hemorrhage and their association with each other are well summarized by Latchaw (7). There is no doubt that there is an association between DVA and a hemosiderin-containing entity that resembles either a cavernous malformation or an old hemorrhage. The association of cavernous malformations and developmental venous anomaly was not well recognized until the advent of CT and MR imaging of the brain. Review of the literature reveals that the first report, in 1984, of an association between venous angioma and cavernous angioma was based on imaging findings (9). This association has also been reported by many other authors (10–12). It is interesting that there is no mention of the association of these two entities on autopsy series from older literature.
The MR imaging appearance of the de novo hemosiderin-containing lesion within the right cerebellum presented in this report would be consistent with the “classic” appearance of a cavernous malformation (13); however, the abrupt onset on patient’s symptoms would suggest a more acute process, such as a hemorrhagic event. Careful scrutiny of the MR images before appearance of the hemosiderin-containing lesion does show an abnormal nidus or small cavernous malformation. Although it has been previously reported that the imaging appearance of a cavernous malformation may be indistinguishable from the sequelae of hemorrhage (6), it has become common practice to diagnose as cavernous malformations lesions having the “classic” MR imaging appearance of multiseptate hemosiderin-containing lesions, with most of these lesions never undergoing pathologic confirmation. These lesions may simply represent previous hemorrhage (7). In fact, Rigamonti et al’s (13) original MR imaging description of a cavernous malformation clearly included the imaging differential of a hemorrhage, as being indistinguishable from cavernous malformation. The exact nature of the cavernoma-like hemosiderin-containing entity associated with DVA remains indeterminate, because most reported cases have not been pathologically confirmed (7).
The patient in this case, as well as the patient described by Cakirer (8) presented symptomatically, concomitantly with the de novo appearance of the cavernoma-like lesion, which suggests that an acute event such as development of a hemorrhage could be responsible for the symptoms. Unlike the de novo cavernous malformation reported by Maeder et al (6), the lesion in this case became smaller and less complex in appearance, suggesting that at least part of the changes were related to hemorrhage. Maeder et al’s report (6) showed the opposite with initial description of the abnormality as having the appearance of a chronic hematoma, with follow-up imaging demonstrating continued increase in the size of the lesion, and subsequent development of complex multilobulated features more typical of a cavernous malformation. At least three of four patients in Rigamonti and Spetzler’s series (10) had acute-subacute presentations. Furthermore, in Wilms et al’s series (12), nine of 15 patients with coexisting cavernous malformation and DVA had acute presentation.
The proximity of the de novo cavernoma-like lesions to the developmental venous anomaly in this case and other reported cases, strongly raises the possibility of a mechanism in which an underlying pathologic process related to the venous angioma leads to development of a cavernous malformation. Dillon et al (11, 14) have proposed that elevated venous pressure within the territory of a DVA may lead to a cascade of events leading to development of a cavernous malformation. Dillon (11) observed that these cavernoma-like lesions appear to arise at the distal radicles of venous malformations. This holds true for this case, as well as for the other reported de novo cavernoma cases when reviewing published images. In fact, the close association of the cavernous malformation and DVA was explicitly described in one report by Scamoni et al (15), in which at surgery a dark reddish mass embedded in xanthochromic subcortical white matter was found. The lesion was partially adherent to a dilated vein. Dillon et al have reported measurement of elevated pressure within DVAs (11, 14), suggesting this may be a key factor in subsequent development of a cavernoma-like lesion.
Consequently, this case and other reported cases support the hypothesis that cavernoma-like abnormalities seen in association with DVAs are most likely acquired rather than true congenital vascular malformations. It is also noted, reviewing articles on this topic, that the coexistence of cavernous malformations and DVAs is more common in the adult than child, which also supports an acquired etiology. Articles examining the natural history of cavernous malformations (16) have usually followed patients who have identified lesions meeting the MR imaging criteria of cavernous angioma. Few studies, however, comment on the etiology or de novo development of cavernous malformations. Cavernous malformations have been shown to increase in size, principally because of repeated self-limited intracavernous hemorrhagic events, and not because of tumoral cellular proliferation (17).
Conclusion
This case report supports the association of a de novo hemosiderin-containing lesion with developmental venous anomaly. The imaging findings and patient presentation in this case suggest that the origin of the cavernoma-like lesion is acquired, and at least partly on the basis of a hemorrhagic event.
References
- 1.Russel DS, Rubinstein LJ. Pathology of the Nervous System. 2nd ed. New York: Williams & Wilkins;1963. :345
- 2.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev 9:233–242 [DOI] [PubMed] [Google Scholar]
- 3.Abe T, Singer RJ, Marks MP, et al. Coexistence of occult vascular malformations and developmental venous anomalies in the central nervous system: MR evaluation. AJNR Am J Neuroradiol 1998;19:51–57 [PMC free article] [PubMed] [Google Scholar]
- 4.Garner TB, Del Curling O, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg 1991;75:715–722 [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg 1995;83:820–824 [DOI] [PubMed] [Google Scholar]
- 6.Maeder P, Gudinchet F, Meuli R, de Tribolet N. Development of a cavernous malformation of the brain. AJNR Am J Neuroradiol 1998;19:1141–1145 [PMC free article] [PubMed] [Google Scholar]
- 7.Latchaw RE, Truwit CL, Heros RC. Commentary: venous angioma, cavernous angioma, and hemorrhage. AJNR Am J Neuroradiol 1994;15:1255–1257 [Google Scholar]
- 8.Cakirer S. De novo formation of a cavernous malformation of the brain in the presence of a developmental venous anomaly. Clin Radiol 2003;58:251–256 [DOI] [PubMed] [Google Scholar]
- 9.Takamiya Y, Takayama H, Kobayashi K, et al. Familial occurrence of multiple vascular malformations of the brain. Neurol Med Chir (Tokyo) 1984;24:271–277 [DOI] [PubMed] [Google Scholar]
- 10.Rigamonti D, Spetzler RF. The association of venous and cavernous malformations: report of four cases and discussion of the pathophysiological, diagnostic, and therapeutic implications. Acta Neurochir 1988;92:100–105 [DOI] [PubMed] [Google Scholar]
- 11.Dillon WP. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol 1997;18:1839–1846 [PMC free article] [PubMed] [Google Scholar]
- 12.Wilms G, Bleus E, Demaeral P, et al. Simultaneous occurrence of developmental venous anomalies and cavernous angiomas. AJNR Am J Neuroradiol 1994;15:1247–1254 [PMC free article] [PubMed] [Google Scholar]
- 13.Rigamonti D, Drayer BP, Johnson PC, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg 1987;67:518–524 [DOI] [PubMed] [Google Scholar]
- 14.Dillon WP, Wilson CB, Hieshima GB, Rosenau W. Hemorrhagic venous malformations: the role of venous restriction. Paper presented at the 30th annual meeting of the American Society of Neuroradiology, St. Louis, MO, May 31–June 5,2002
- 15.Scamoni C, Dario A, Basile L. The association of cavernous and venous angioma: case report and review of the literature. Br J Neurosurg 1997;11:346–349 [DOI] [PubMed] [Google Scholar]
- 16.Del Gurling O, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg 1991;75:702–708 [DOI] [PubMed] [Google Scholar]
- 17.Maeder, Pozzati E, Giulani G, et al. The growth of cerebral cavernous malformations. Neurosurgery 1989;25:92–97 [DOI] [PubMed] [Google Scholar]