Abstract
BACKGROUND AND PURPOSE: Stent placement for intracranial atherosclerosis has become an alternative treatment technique; however, stent placement for middle cerebral artery (MCA) stenosis remains a technical and clinical challenge. Our purpose was to assess the feasibility, safety, and effectiveness of stent placement for MCA stenosis.
METHODS: Between May 1998 and August 2003, we performed stent placement for MCA stenosis (more than 50%) in 17 patients and retrospectively analyzed the technical success rate, complications, and outcomes over 10 months.
RESULTS: Stent placement was technically successful in 16 (94.1%) patients and failed in one patient (5.9%), because the stent did not reach the lesion due to carotid artery tortuousity. In 16 patients, postprocedural angiography showed restoration of the normal luminal diameter. Acute in-stent thromboses occurred in nine patients (56.3%) and was lysed with abciximab. The parent artery ruptured in two patients (12.5%): One died from massive subarachnoid hemorrhage, and the other patient received a second stent over the tear site. Stent jumping (distal migration at the time of balloon inflation) occurred in one patient (6.3%) and was solved by implanting a second stent. Periprocedural complications included subacute in-stent thrombosis (n = 1, 6.3%) and minor stroke (n = 1, 6.3%); these were relieved with heparin therapy or local thrombolysis. Fifteen patients experienced symptomatic relief or were stable at follow-up. Angiographic follow-up (n = 6) revealed no in-stent restenosis.
CONCLUSION: Stent placement for symptomatic MCA stenosis was technically feasible and effective in alleviating symptoms and improving cerebral blood flow.
Atherosclerosis of the intracranial vessels is a widespread vascular disease (1). It may develop selectively in intracranial vessels, particularly in blacks and Asians (2, 3). In patients with intracranial atherosclerotic stenosis, the increased risk of stroke, heart disease, and death has been observed consistently (4–6). In their study of 66 patients with stenosis of more than 50% in the intracranial internal carotid artery (ICA), Marzewski et al (4) reported that 12.1% had transient ischemic attacks (TIAs), 15.2% had a stroke, and 50% died during an average follow-up of 3.9 years. Although the natural history of middle cerebral artery (MCA) stenosis has not been studied as thoroughly as that of intracranial ICA stenosis, some investigators have reported that the prognosis of patients with this disease is not favorable (5, 6).
Traditionally, antiplatelet combination therapy has been used as the first treatment, but its stroke and death rates are notable (7). Revascularization by means of extracranial–intracranial bypass surgery in stenotic intracranial arteries may be another therapeutic option, but it does not have proved efficacy, as compared with medical therapy (8). In the report by the EC/IC Bypass Study Group (8), patients who received surgery for MCA stenosis fared substantially worse than those who received medical therapy.
In terms of preventing stroke, the benefits of revascularization of stenotic arteries have been unequivocally demonstrated in patients with cervical carotid artery disease (9). However, the optimal treatment of patients with intracranial atherosclerotic lesions is still controversial. Although percutaneous balloon angioplasty has been reported to be useful and safe for the treatment of stenotic intracranial arteries (10), percutaneous balloon angioplasty of the MCA still poses a risk of vascular dissection, elastic recoil, vasospasm, and high-grade residual stenosis because of the small caliber of the vessel (11–14).
In the coronary, peripheral, and extracranial cerebral circulations, stent placement increases the safety and efficacy of balloon angioplasty for the treatment of arterial lesions (15–17). More recently, elective stent placement of atherosclerotic intracranial artery stenosis has become possible as an alternative treatment technique because of the introduction of the newer, more flexible coronary stents (18–21). However, stent placement to treat MCA stenosis remains a technical and clinical challenge. The purpose of our study was to investigate the feasibility, complications, and effectiveness of stent placement for symptomatic MCA stenosis.
Methods
Patient Selection
Between May 1998 and August 2003, we retrospectively analyzed the medical records of all patients who underwent stent-assisted angioplasty of the MCA at three hospitals (Pusan National University Hospital, Pusan; Chosun University Hospital, Kwangju, Metrohospital, Anyang, Korea). We assessed the following parameters: patient’s age and sex, clinical presentation, degree of stenosis before and after stent placement, antiplatelet and anticoagulation regimen, procedure-related complications, and clinical and angiographic outcomes at follow-up.
Seventeen patients with symptomatic and significant MCA stenosis were included in the study. They included 12 men and five women with a mean age of 59 years (range, 41–74 years). All patients gave written informed consent for the procedure (as part of a protocol approved by the local institutional review board).
Significant stenosis was defined as stenosis of more than 50%, as estimated on the basis of digital subtraction angiography (DSA), according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (9). Symptomatic MCA stenosis was defined as the occurrence of one or more TIAs or non-disabling strokes in the MCA territory within 6 months.
In general, stent placement was recommended to decrease the risk of new or recurrent cerebral infarction in patients with significant atherosclerotic stenosis of the MCA in these situations: 1) TIA or infarction recurred or progressed despite optimal medical therapy, including anticoagulation or antiplatelet treatment. 2) Anticoagulation or antiplatelet treatment was contraindicated. 3) Patients had previous ischemic events or asymptomatic severe stenosis (more than 70%) with poor collateral cerebral circulation, or they had decreased cerebral perfusion on technetium-99m ethyl cysteinate dimer (ECD) single photon emission CT (SPECT) before and after the intravenous administration of acetazolamide 1g (Zoladin; Far-East Pharmaceuticals, Seoul, Korea). 4) Coronary artery bypass grafting was planned.
The exclusion criteria included the following: 1) stenosis distal to the M1 bifurcation; 2) severe neurologic deficits in the affected MCA territory; 3) life expectancy less than 5 years; 4) cardiac lesions likely to cause cardioembolism; 5) failure of the kidney, liver, or lung; and 6) chronic complete occlusion of the MCA.
Techniques of MCA Stent Placement
A neurologist (K.P.P.) or a neurosurgeon (C.H.C.) obtained a complete neurologic history and performed a complete examination in all patients. MR imaging with MR angiography was performed before cerebral angiography. Eight patients underwent 99mTc ECD basal SPECT and SPECT after acetazolamide administration. In all patients, aortic arch and cerebral angiography was performed to evaluate the extracranial and intracranial arteries. The rate of stenosis was calculated manually and also automatically on the basis of DSA (Multistar; Siemens, Erlangen, Germany) according to NASCET criteria.
All patients were premedicated with daily doses of aspirin 100 mg and clopidogrel 75 mg (Plavix; Sanofi-Synthelabo, Seoul, Korea) for at least 3 days before the procedure. In addition, low-molecular-weight nadroparin calcium (2850 IU/0.3 mL, Fraxiparine; Sanofi-Synthelabo) was subcutaneously injected two or three times a day during the same period.
Therapeutic procedures were performed during a second angiographic session. Patients were fully awake during the procedure, and their ECG, arterial oxygen saturation, and blood pressure were appropriately monitored. Percutaneous access was obtained via the right femoral artery, and a 6F or 7F sheath was inserted. Baseline activated clotting times (ACT) was obtained before the procedure. Then, patients received systemic heparinization and a bolus injection of heparin 3000–5000 IU just before the therapeutic procedure. An additional 1000-IU bolus of heparin was administered every hour to maintain an activated clotting time of longer than 250 seconds or twice the baseline value throughout the procedure.
A 6F or 7F guiding catheter (Envoy; Cordis Endovascular Corporation, Miami, FL) was positioned in the distal cervical ICA. After a preliminary angiogram was obtained, the catheter was connected to a continuous saline flush. Preprocedural angiograms were then obtained in orthogonal planes. The stenotic segment of the MCA was crossed with a 160-cm-long 0.014-in microwire (Transend 14; Target/Boston Scientific, Natick, MA) that was navigated into the insular portion of the MCA to ensure maximal support; this allowed tracking of the balloon-mounted stent catheter. Occasionally, predilation with a coronary balloon (Hayate; Terumo Medical Corporation, Tokyo, Japan) was required, especially when the diameter of the stenotic segment was less than the profile of the stent catheter. In general, a 1.5-mm balloon diameter was used to allow advancement of the stent catheter.
A S660 coronary stent (AVE, Medtronic, Minneapolis, MN) was used in nine patients, a Flexmaster coronary stent (JoMed GmbH, Rangendirgen, Germany) was used in six, and a Gfx coronary stent (AVE; Medtronic) was used in two. The coronary stent was advanced over the microwire and positioned across the stenosis by using the roadmap imaging and external stent markings. The correct position of stent was angiographically confirmed. We then began to deploy the stent using the roadmap image.
The balloon was slowly inflated by using a multistage technique to prevent vascular dissection or rupture by the dog-bone phenomenon of the balloon (Fig 1). In this technique, the balloon-inflation pressure was increased by 1 atm, step by step. At first, the pressure was slowly elevated to 4 atm, and angiography was performed after balloon deflation to identify the results and possible complications. If the gap between the distal end of the stent and the parent artery remained on angiography, balloon inflation was then performed with 5 atm. This procedure was repeated until the gap between the distal end of the stent and the parent artery disappeared. The distal tip of the balloon was then pulled back into the distal end of the stent. Using the same technique, we restarted inflating the balloon at the previous pressure and continued to the point where the gap disappeared. Balloon pressures did not exceed the burst pressure of the stent and balloon. When no gap was present between the stent and the parent artery, we ended stent deployment and waited for 1 hour to identify possible complications, such as acute in-stent thrombosis or previously undetected vascular rupture.
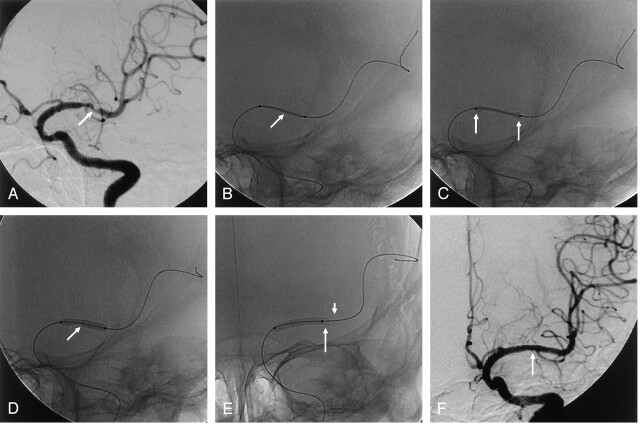
Fig 1.
A 71-year-old woman with recurrent TIA symptoms.
A, Anteroposterior oblique left ICA angiogram shows severe stenosis (about 70%, arrow) in the M1 portion of the left MCA.
B, Balloon-mounted coronary stent (arrow) is positioned at the lesion, and the microwire is anchored in the distal branch of the MCA.
C, At 4 atm, the balloon is initially expanded at the distal and proximal ends of the stent (dumbbell phenomenon, arrows).
D, At 6 atm, the balloon expands in the center section containing the stent (arrow).
E, When no gap is present between the distal end of the stent and the parent artery on angiography, the balloon tip (long arrow) is pulled back into the distal end of the stent (short arrow) to prevent dissection and rupture of the parent artery. Balloon inflation is restarted.
F, Immediate postprocedural anteroposterior left ICA angiogram shows excellent recanalization (arrow) of the diseased segment, with preservation of the lateral lenticulostriate arteries.
When poststenting angiography showed any filling defect, we considered it acute in-stent thrombosis and instantly attempted thrombolysis. Abciximab 2 mg was intra-arterially administered through a guiding catheter, and angiography was performed after 10–20 minutes to identify undissolved thrombus. If clot remained on angiography, an additional 2 mg of abciximab was administered. This procedure was repeated until complete thrombolysis was achieved and the dose of abciximab equaled the weight-adjusted intravenous dose. Complete dissolution of the acute in-stent thrombus occurred when no filling defect was identified on subsequent angiography. Although abciximab is injected intravenously after coronary interventions, this was not done here because of the risk of cerebral hemorrhage. If no complications were noted on final angiography, we completed the procedure after measuring the rate of residual stenosis on the angiogram. Technical success was defined as more than 50% revascularization of the stenotic lesion after stent deployment.
Immediately after stent placement, the neurologist (K.P.P.) or neurosurgeon (C.H.C.) obtained a complete neurologic history and performed a complete examination. All patients underwent nonenhanced brain CT for an evaluation of possible hemorrhagic complications. MR diffusion and fluid-attenuated inversion recovery imaging was performed on the first postprocedural day. Between postprocedural days 7 and 15, 99mcTc-ECD SPECT was performed to assess cerebral blood flow. These images were then compared with the preprocedural SPECT scans. When periprocedural ischemic symptoms developed while the patients were still in the hospital, repeat angiography was performed.
After the procedure, patients received aspirin 100 mg and clopidogrel 75 mg daily. Fraxiparine 2850 IU was also subcutaneously administered two or three times a day for at least 3 days. Patients were discharged home from 3–15 days after the procedure when their neurologic condition was determined to be stable.
Follow-up Clinical and Angiographic Outcomes
The time between stent placement and last clinical follow-up ranged from 1 to 25 months (mean, 10 months). In six patients, follow-up angiography was performed 3–10 months after stent placement (mean, 5.7 months).
To assess the clinical outcome at last follow-up, we used a five-grade scale, as follows: I was excellent (neurologic improvement without neurologic complications); II, good (no neurologic improvement and no neurologic complications); III, fair (transient neurologic complications); IV, poor (neurologic deterioration and permanent neurologic complications); and V, (death associated with the procedure).
Angiographic in-stent restenosis was defined as stenosis of more than 50% on follow-up DSA.
Results
The patients’ characteristics, stents used, complications, and outcomes are presented in the Table. All patients presented with symptoms of recurrent TIA (n = 9), infarction (n = 5), or both (n = 3). All had stenosis of the M1 segment of the MCA, which was considered to be directly responsible for their symptoms. These patients were selected for stent-assisted angioplasty on the basis of a number of inclusion criteria, including failure of optimal medical treatment (n = 10), ischemic events with poor collateral cerebral circulation or decreased cerebral blood flow on acetazolamide SPECT (n = 7).
Patient characteristics, stents used, complications, and outcomes of stenting for symptomatic MCA stenosis
| Patient/Sex/Age (y) | Clinical Presentation | Stent (mm)* | Complication | Complication Management | Stenosis Degree (%) |
Neurologic Outcome | Follow-Up |
||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Poststenting | DSA | Ischemic | ||||||
| 1/M/48 | TIA | G 2.5 × 9 | None | NA | 65 | NA | Fail | NA | NA |
| 2/M/51 | TIA | G 3.0 × 9 | Rupture | NA | 75 | NA | Death | NA | NA |
| 3/M/71 | TIA | S 2.5 × 9 | None | NA | 70 | 0 | Excellent | NA | None, 25 mo |
| 4/M/67 | TIA | S 2.5 × 15 | AT | Abciximab 8 mg IA | 80 | 5 | Excellent | 6 mo, no RS | None, 17 mo |
| 5/F/74 | TIA | S 2.5 × 15 | None | NA | 70 | 0 | Excellent | NA | None, 17 mo |
| 6/F/58 | TIA, infarct | S 2.5 × 18, S 2.5 × 12 | Rupture, AT | Second stent overlap, abciximab 10 mg IA | 90 | 0 | Good | 10 mo RS | None, 15 mo |
| 7/M/66 | TIA, infarct | F 2.5 × 9 | None | NA | 60 | 0 | Excellent | NA | None, 11 mo |
| 8/M/60 | Infarct | F 2.5 × 9 | None | NA | 65 | 0 | Excellent | NA | None, 11 mo |
| 9/F/52 | Infarct | F 2.5 × 9 | None | NA | 70 | 0 | Good | 6 mo, no RS | None, 9 mo |
| 10/F/49 | TIA, infarct | F 2.5 × 9, F 2.5 × 12 | Stent jumping, AT, minor stroke | Second stent overlap, abciximab 10 mg IA, IV heparin | 50 | 0 | Fair | 3 mo, no RS | None, 9 mo |
| 11/M/60 | TIA | F 2.75 × 12 | AT, SAT | Abciximab 10 mg IA, IA thrombolysis | 70 | 0 | Fair | 3 mo, no RS | None, 9 mo |
| 12/M/67 | TIA | F 2.5 × 9 | AT | Abciximab 10 mg IA | 60 | 0 | Good | 6 mo, no RS | None, 8 mo |
| 13/M/60 | TIA | S 2.5 × 9 | AT | Abciximab 16 mg 1A | 70 | 0 | Excellent | NA | None, 7 mo |
| 14/M/63 | Infarct | S 2.5 × 9 | AT | Abciximab 16 mg IA | 65 | 0 | Excellent | NA | None, 4 mo |
| 15/M/41 | Infarct | S 2.5 × 12 | None | NA | 60 | 0 | Excellent | NA | None, 3 mo |
| 16/F/71 | TIA | S 2.5 × 15 | AT | Abciximab 10 mg IA | 60 | 0 | Excellent | NA | None, 2 mo |
| 17/M/53 | Infarct | S 2.5 × 12 | AT | Abciximab 10 mg IA | 70 | 0 | Excellent | NA | None, 1 mo |
Note.—Abbreviations: AT, acute in-stent thrombosis; F, Flexmaster (JoMed); G, Gfx (AVE; Medtronic); IA, intra-arterial; IV, intravenous; NA, not applicable; RS, in-stent restenosis; S, S660 (AVE; Medtronic); and SAT, subacute in-stent thrombosis.
Stent implantation was successful in all patients except patient 1.
We achieved technical success in 16 (94.1%) of 17 patients. The procedure failed in one patient (5.9%) because the stent catheter could not reach the stenosis due to the tortuousity of the carotid artery. Two stents each were used in two patients because of stent jumping distal to the stenotic lesion (n = 1) or arterial rupture at the time of balloon inflation (n = 1). The remaining patients were treated by using one stent each. Eighteen stents were implanted: 10 S660 stents (AVE, Medtronic), seven Flexmaster stents (JoMed), and one Gfx stent (AVE; Medtronic) were implanted in 16 patients.
Mean preprocedural stenosis was 67.6% (range, 50–90%), and mean stenosis immediately after stent placement was 0.3%. Poststenting stenosis was 0% in 15 patients and 5% in one patient.
Procedure-related complications included acute in-stent thrombosis (n = 9, 56.3%) (Figure 2), arterial rupture (n = 2, 12.5%), and stent jumping (n = 1, 6.3%). To dissolve acute in-stent thrombosis, abciximab 2 mg was repeatedly administered through the guiding catheter for at least 10 minutes until the thrombus disappeared completely. In all nine cases, final angiography showed total dissolution of the thrombosis within 40–80 minutes. The total dose infused was 8 mg in one case, 10 mg in six cases, and 16 mg in two cases. Immediate postprocedural nonenhanced brain CT showed no hemorrhagic complications related to abciximab. Stent jumping was resolved by implanting a second stent. The parent artery was ruptured during balloon inflation in two patients. One of these patients (in whom an oversized stent had been used because of a lack of experience) died from massive subarachnoid hemorrhage. The second had severe total M1 stenosis, which was controlled by using the balloon and the placement of a second stent over the tear site (Figure 3). Most of the procedure-related complications were thus resolved.
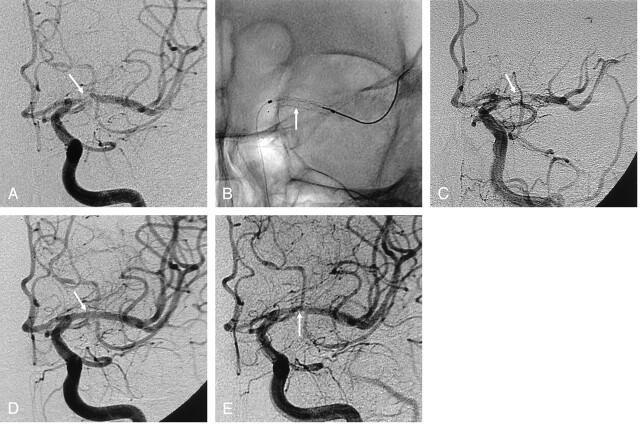
Fig 2.
A 67-year-old man with recurrent TIA symptoms.
A, Anteroposterior left ICA angiogram shows severe stenosis (about 90%, arrow) in the proximal M1 portion of the left MCA.
B, Balloon-mounted coronary stent (arrow) is successfully deployed.
C, Postprocedural ICA angiogram shows acute in-stent thrombosis (arrow).
D, Final ICA angiogram after thrombolysis with abciximab shows the MCA (arrow) with a smooth appearance, normalized luminal diameter, and preservation of the lenticulostriate arteries.
E, Angiogram at 10 months after the procedure shows asymptomatic, mild (20%) in-stent restenosis (arrow) due to intimal hyperplasia.
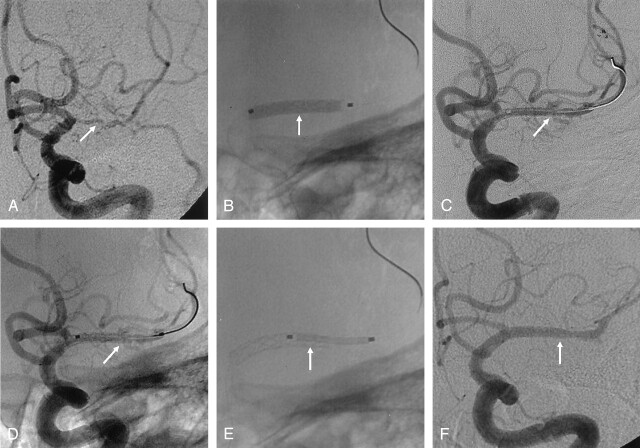
Fig 3.
A 58-year-old woman with recurrent TIA and infarction symptoms involving the left MCA territories.
A, Anteroposterior left ICA angiogram shows diffuse severe stenosis (about 90%, arrow) in the total M1 portion of the right MCA.
B, Anteroposterior radiograph shows the stent (arrow) during deployment in the M1 portion.
C and D, After stent deployment, left ICA angiogram shows extravasation of contrast agent in the stent (arrow) due to a vascular tear.
E, Second stent (arrow) is inserted over the tear site.
F, Postprocedural anteroposterior left ICA angiogram shows the near-normal diameter of the vessel, with no extravasation of contrast agent (arrow).
Periprocedural complications included subacute in-stent thrombosis (n = 1, 6.3%) and minor stroke (n = 1, 6.3%). One subacute in-stent thrombosis was dissolved by means of balloon angioplasty and intra-arterial thrombolysis with urokinase and abciximab. In one patient with minor stroke, angiography did not show in-stent thrombosis, and the symptoms were relieved with a continuous intravenous administration of heparin.
In 14 patients whose vessels were not ruptured during the procedure, postprocedural CT and MR imaging showed no hemorrhagic or distal embolic infarction.
Eight patients underwent preprocedural and postprocedural basal and acetazolamide SPECT. Preprocedural SPECT showed substantial flow reduction in the MCA territory of the lesion site. Postprocedural SPECT revealed augmentation of cerebral blood flow at the lesion site, as compared with that on preprocedural imaging.
Among 16 patients in whom stenting was successful, follow-up clinical outcomes were as follows: symptomatic relief in 10 (clinical grade I, 62.5%), neurologically stability in three (clinical grade II, 18.8%), stable but with transient neurologic complications in two (clinical grade III, 12.5%), and death in one (clinical grade V, 6.3%).
In six of the 15 patients who were neurologically stable on clinical follow-up, angiographic follow-up was performed and revealed no in-stent restenosis.
Discussion
Endovascular treatment modalities for symptomatic MCA stenosis are balloon angioplasty and stent-assisted angioplasty for preventing recurrent stroke. The results of balloon angioplasty are tempered by the increased possibility of distal embolism, vascular dissection, elastic recoil, vasospasm, and immediate high-grade residual stenosis (11–14).
Compared with balloon angioplasty, stent-assisted angioplasty offers advantages including exclusion of the plaque and regions of dissection from the lumen of the vessel, prevention of vascular recoil and spasm, and improvement of the immediate and long-term patency of the treated lesion (19–22). In the past, the use of stent placement for intracranial arterial stenosis was limited by the inability of the stent catheter to track into the stenotic portion. More recently, however, marked advancements in stent technology have made stent placement for intracranial stenosis possible. The technical feasibility and clinically favorable short- and long-term outcomes of stent placement have been reported in patients with symptomatic intracranial ICA and vertebrobasilar artery stenosis (22, 23). Nevertheless, stent-assisted angioplasty of MCA remains technically difficult, and its durability and long-term effectiveness in the prevention of stroke are unknown. In this report, we describe our experience, which has taught us fundamental principles to successfully complete this procedure.
There are several important concerns in stent placement for symptomatic MCA stenosis. These include the selection of the stent, the technique of balloon inflation, the prevention and control of procedure-related complications, the preservation of the lateral lenticulostriate arteries arising in the M1 portion of the MCA, and the long-term clinical and angiographic results.
The first concern of MCA stent placement is stent selection. Because intracranial arteries are more delicate, thin-walled vessels with a greater risk for rupture, careful sizing of stents is important. Oversizing of a stent may result in vascular rupture or intimal dissection. In one of our patients, the vessel ruptured because we used an oversized stent. This was our first case, so we were somewhat lacking in experience. The choice of stent is influenced by its design, thickness, tractability, deliverability, radiopacity, and radial force, as well as its flexibility and compatibility after deployment. Our experience suggests that stent tractability in particular might be the most important factor in the stent placement of MCA stenosis because of the tortuousity of the carotid artery.
The second concern in MCA stent placement is the technique of balloon inflation in stent deployment. A rapid rate of inflation poses the serious risk of vascular rupture and dissection (24). Blankenship et al (25) reported an inverse relationship between the rate of inflation and the risk of dissection. Most coronary stents are premounted on the delivery balloon catheter. During inflation, the balloon is designed to initially expand at the distal and proximal ends (resembling a dumbbell or dog bone, 24) and then in the center section containing the stent. The rapid rate of inflation might transiently overexpand the proximal and distal ends of the delivery balloon before stent expansion. The dumbbell-shaped expansion of the delivery balloon may therefore contribute to vascular dissection or rupture. The shear stress applied to an atherosclerotic vessel wall is proportional to the rate of deformation (26). Hence, with reference to the risk of rupturing the vessel wall, the maximal pressure in the balloon may not be as important as the rate of inflation. To prevent vascular dissection or rupture, we slowly inflated the balloon by using a multistage technique and pulled its distal tip back into the distal end of the stent after the gap between the distal end of the stent and the parent artery disappeared. We thought that inflating the balloon after pullback prevents dissection or rupture of the parent artery distal to the stent because the diameter of parent artery there may be smaller than that of the inflated balloon.
The third concern is the prevention and control of procedure-related complications, the most common of which are thromboembolic events including acute in-stent thrombosis. Premedication with the antiplatelet combination of aspirin and clopidogrel has demonstrable benefit in preventing acute and subacute in-stent thrombosis after stent placement (27). Presently, abciximab is administered primarily by an intravenous route before coronary interventions as prophylaxis to reduce potential thromboembolic complications (28). However, we did not administer abciximab prophylactically because it is expensive and increases the risk of intracerebral hemorrhage (29). The prophylactic use of abciximab can also cause massive subarachnoid hemorrhage if a vascular tear occurs during a procedure. Instead, when acute in-stent thromboses developed, we injected abciximab intra-arterially through the guiding catheter. In all nine study patients, acute in-stent thromboses were completely lysed with the intra-arterial administration of abciximab.
Compared with previous studies about intracranial stent placement (18–24), ours had a greater frequency of acute in-stent thromboses despite sufficient antiplatelet and anticoagulation premedication. Although the causes are unclear, the main causes might have been related to vascular diameter and suboptimal approximation with the multistage balloon technique. Findings from animal studies have demonstrated that platelet activation is the primary mediator in acute in-stent thrombosis (30). Therefore, we assume that the multistage balloon technique with slow inflation may be an important cause of this complication in MCA stent placement. Because the multistage technique may cause a gap between the stent and the vessel (suboptimal approximation) before or after complete deployment, the gap can then result in platelet activation that causes the thrombosis. However, the multistage technique has the most important advantage of preventing the worst complications of dissection or rupture of the parent arteries. In this study, there was no distal embolic infarction, as shown on postprocedural MR imaging.
Other procedure-related complications include stent migration, stent jumping, or vascular rupture or dissection during balloon inflation. The use of a stent with an inadequate diameter or underexpansion of the stent for fear of rupture may account for stent migration from the selected position. In addition, distal jumping of the delivery catheter during balloon inflation is also possible because of increased catheter tension created by it pushing through the tortuous carotid artery. To prevent distal jumping of the stent, catheter tension should be relieved before stent deployment. Vascular rupture or dissection can result from the use of an oversized stent or rapid balloon inflation. As mentioned before, this rupture and dissection can be prevented by using an undersized stent and a multistage balloon technique. In this study, these complications were controlled by placing a second stent, and no vascular dissection occurred during the procedure.
The fourth concern is the preservation of the lateral lenticulostriate arteries arising from the M1 segment. Theoretically, occlusion of these arteries can occur after stent placement. Our results may suggest that, through the ostia of the loose stent strut, the lenticulostriate arteries are not compromised after stent placement. Numerous clinical and experimental studies (18, 31–33) have shown the patency of the small side branches arising from the stented arteries following stent placement.
The last concern is the long-term result of MCA stent placement. To our knowledge, ours series is the only large study of successful, elective stent placement in the symptomatic atherosclerotic MCA. In our study, short-term clinical and angiographic results were encouraging during a mean 10 months of follow-up. Brain SPECT also demonstrated successful flow augmentation after stent placement. Therefore, augmentation of the luminal diameter relieves ischemic symptoms and increases cerebral blood flow because it is proportional to the radius of the lumen to the fourth power (Poiseuille equation) (18–22). However, the long-term restenosis rates of intracranial stent placement have not yet been studied, as they have been with coronary stent placement. The accumulated experience with small-caliber coronary stent placement is important to understand the fate of the MCA stent. The main mechanism of restenosis is intimal hyperplasia and an inflammatory reaction centered at the stent struts (34, 35). In the cardiology literature (36), factors predisposing a patient to in-stent restenosis include patient-related factors (e.g., diabetes, hypertension, smoking), procedure-related factors (e.g., number, length, and overlap of the stent), and lesion-related factors (e.g., vascular size, lesion length, and severity of prestenting and poststenting stenosis). As Fitzgerald et al (37) reported, an important predictor of subsequent restenosis is the postprocedural minimal diameter and the cross-sectional area of the lumen as determined by intravascular sonography. Elezi et al (38) reported the tremendous impact of vascular size on the restenosis rate in coronary stent placement. The restenosis rate of vessels smaller than 2.8 mm in diameter is 38.6% compared with 28.4% for vessels 2.8–3.2 mm and 20.4% for vessels larger than 3.2 mm.
The long-term results of MCA stent placement may differ from those of coronary artery stent placement because of variations in the vascular structure and environment between the coronary artery and the MCA. Therefore, long-term follow-up study is necessary to evaluate in-stent restenosis.
This study had several limitations. First, the number of patients was small. Second, our study had no valid comparison group of patients who underwent only balloon angioplasty or only medical treatment for similar lesions.
Conclusion
Our results suggest that short-term clinical outcomes after MCA stent placement are favorable. However, the safety and efficacy of this technique compared with medical therapy remains to be determined. Greater experience with the technique we describe should allow better definition of the role of stent placement in the treatment of atherosclerotic MCA stenosis.
Acknowledgments
We would like to thank Bonnie Hami, MA, Department of Radiology, University Hospitals of Cleveland, OH, for her editorial assistance in the preparation of this manuscript.
Footnotes
Presented in part at the 12th European Stroke Conference, Valencia, Spain, 2003; the 13th Meeting of the European Neurological Society, Istanbul, Turkey, 2003; and the 7th Congress of the World Federation of Interventional and Therapeutic Neuroradiology, Recife, Brazil, 2003.
References
- 1.Fisher CM, Gore I, Okabe N, White PD. Atherosclerosis of the carotid and vertebral arteries: extracranial and intracranial. J Neuropathol Exp Neurol 1965;24:455–476 [Google Scholar]
- 2.Caplan LR, Gorelick PB, Hier DB. Race, sex, and occlusive cerebrovascular disease: a review. Stroke 1986;17:648–655 [DOI] [PubMed] [Google Scholar]
- 3.Moossy J. Pathology of cerebral atherosclerosis. Influence of age, race, and gender [Suppl]. Stroke 1993;24:I22–23, I31–32 [PubMed] [Google Scholar]
- 4.Marzewski DJ, Furlan AJ, St Louis P, Little JR, Modic MT, Williams G. Intracranial internal carotid artery stenosis: long-term prognosis. Stroke 1982;13:821–824 [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR, Babikian V, Helgason C, et al. Occlusive disease of the middle cerebral artery. Neurology 1985;35:975–982 [DOI] [PubMed] [Google Scholar]
- 6.Bogousslavsky J, Barnett HJ, Fox AJ, Hachinski VC, Taylor W. Atherosclerotic disease of the middle cerebral artery. Stroke 1986;17:1112–1120 [DOI] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease study. Neurology 1995;45:1488–1493 [DOI] [PubMed] [Google Scholar]
- 8.The EC/IC Bypass study group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med 1985;313:1191–1200 [DOI] [PubMed] [Google Scholar]
- 9.North American Symptomatic Endarterectomy Trial collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Eng J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kwon SU, Lee JH, Suh DC, Kim JS. Percutaneous transluminal angioplasty for symptomatic middle cerebral artery stenosis: long-term follow-up. Cerebrovasc Dis 2003;15:90–97 [DOI] [PubMed] [Google Scholar]
- 11.Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998;19:1525–1533 [PMC free article] [PubMed] [Google Scholar]
- 12.Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke 1999;30:1065–1069 [DOI] [PubMed] [Google Scholar]
- 13.Connors JJ III, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg 1999;91:415–423 [DOI] [PubMed] [Google Scholar]
- 14.Nahser HC, Henkes H, Weber W, Berg-Dammer E, Yousry TA, Kuhne D. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol 2000;21:1293–1301 [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997. :95 ;376–381 [DOI] [PubMed] [Google Scholar]
- 16.Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J 2001;142:314–323 [DOI] [PubMed] [Google Scholar]
- 17.Betriu A, Masotti M, Serra A, et al. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J Am Coll Cardiol 1999;34:1498–1506 [DOI] [PubMed] [Google Scholar]
- 18.Shin YS, Kim SY, Bang OY, et al. Early experiences of elective stenting for symptomatic stenosis of the M1 segment of the middle cerebral artery: reports of three cases and review of the literature. J Clin Neurosci 2003;10:53–59 [DOI] [PubMed] [Google Scholar]
- 19.Levy EI, Hanel RA, Bendok BR, et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg 2002;97:1294–1301 [DOI] [PubMed] [Google Scholar]
- 20.Lylyk R, Cohen JE, Ceratto R, Ferrario A, Miranda C. Angioplasty and stent placement in intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol 2002;23:430–436 [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez CR, Misra VK, Campbell MS, Soto RD. Elective stenting of symptomatic middle cerebral artery stenosis. AJNR Am J Neuroradiol 2000;21:971–973 [PMC free article] [PubMed] [Google Scholar]
- 22.Nakahara T, Sakamoto S, Hamasaki O, Sakoda K. Stent-assisted angioplasty for intracranial atherosclerosis. Neuroradiology 2002;44:706–710 [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Kazita K, Chokyu K, Mima T, Mori K. Short-term arteriographic and clinical outcome after cerebral angioplasty and stenting for intracranial vertebrobasilar and carotid atherosclerotic occlusive disease. AJNR Am J Neuroradiol 2000;21:249–254 [PMC free article] [PubMed] [Google Scholar]
- 24.Malek AM, Higashida RT, Halbach VV, Phatouros CC, Meyers PM, Dowd CF. Tandem intracranial stent deployment for treatment of an iatrogenic, flow-limiting, basilar artery dissection: technical case report. Neurosurgery 1999;45:919–924 [DOI] [PubMed] [Google Scholar]
- 25.Blankenship JC, Krucoff MW, Werns SW, et al. Comparison of slow oscillating versus fast balloon inflation strategies for coronary angioplasty. Am J Cardiol 1999;83:675–680 [DOI] [PubMed] [Google Scholar]
- 26.Morris PP, Martin EM, Regan J, Braden G. Intracranial deployment of coronary stents for symptomatic atherosclerotic disease. AJNR Am J Neuroradiol 1999;20:1688–1694 [PMC free article] [PubMed] [Google Scholar]
- 27.Moussa I, Oetgen M, Roubin G, et al. Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary stent implantation. Circulation 1999;99:2364–2366 [DOI] [PubMed] [Google Scholar]
- 28.Cheng JW. Efficacy of glycoprotein IIb/IIIa-receptor inhibitors during percutaneous coronary intervention [Suppl]. Am J Health Syst Pharm 2002;59:S5–S14 [DOI] [PubMed] [Google Scholar]
- 29.Abciximab in Ischemic Stroke investigators. Abciximab in acute ischemic stroke: a randomized, double-blind, placebo-controlled, dose-escalation study. Stroke 2000;31:601–609 [DOI] [PubMed] [Google Scholar]
- 30.Jeong MH, Owen WG, Staab ME, et al. Porcine model of stent thrombosis: platelets are the primary component of acute stent closure. Cathet Cardiovasc Diagn 1996;38:38–43 [DOI] [PubMed] [Google Scholar]
- 31.Wakhloo AK, Tio FO, Lieber BB, Schellhammer F, Graf M, Hopkins LN. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol 1995;16:1043–1051 [PMC free article] [PubMed] [Google Scholar]
- 32.Masuo O, Terada T, Walker G, et al. Study of the patency of small arterial branches after stent placement with an experimental in vivo model. AJNR Am J Neuroradiol 2002;23:706–710 [PMC free article] [PubMed] [Google Scholar]
- 33.Lopes DK, Ringer AJ, Boulos AS, et al. Fate of branch arteries after intracranial stenting. Neurosurgery 2003;52:1275–1279 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–274 [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann R, Mintz GS, Dussaillant GR, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation 1996;94:1247–1254 [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann R, Mintz GS. Coronary in-stent restenosis: predictors, treatment and prevention. Eur Heart J 2000;21:1739–1749 [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation 2000;102:523–530 [DOI] [PubMed] [Google Scholar]
- 38.Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schomig A. Vessel size and long-term outcome after coronary stent placement. Circulation 1998;98:1875–1880 [DOI] [PubMed] [Google Scholar]