Abstract
Summary: Patients with malformations of cortical development and epilepsy may have a variety of abnormal brain findings, including abnormal gyral patterns, cortical thickening, decreased volume of white matter, and increased diffusion of white matter. The status of individual white matter fiber tracts, however, is unknown. We present a case of bilateral frontal schizencephaly and subcortical heterotopia and illustrate alterations of white matter fascicles by combined structural and functional diffusion tensor imaging at 3 T.
In the past, malformations of cortical development were thought to be extremely rare disorders, seen primarily in patients at mental institutions. The advent of MR imaging has revolutionized our understanding of these malformations, which are now known to cause about one-third of all cases of intractable partial epilepsy in childhood, up to one-half of cases of congenital hemiplegia, and a significant portion of patients with developmental delay (1–3). Although conventional high-spatial-resolution MR imaging readily identifies the malformed cortex in most cases, recent investigations have begun to focus on the associated abnormalities of the white matter (4–6). Because connectivity problems may be as important as, or more important than, the cortical abnormalities themselves in the clinical outcome of affected patients, the development of new tools to assess brain connectivity is vital to our understanding of affected patients. In this report, we describe our use of high-spatial-resolution anatomic imaging, diffusion tensor imaging, and white matter tractography at 3 T in a patient with schizencephaly and other malformations of cortical development.
Case Report
Our patient, a 34-year-old man, had been suffering medically refractory epilepsy since the age of 12 years. His seizures begin as focal motor epilepsy in the left hand before progressing into complex partial epilepsy and then generalized tonic-clonic convulsions. There was no record of antenatal abnormalities, childhood meningitis, febrile seizures, or family history of epilepsy. On examination, no focal neurologic deficits were identified. He had no visual symptoms, and ophthalmologic examination was normal. He was mildly cognitively impaired, but had finished vocational school. An electroencephalogram revealed sharp waves over the right frontal region, intermittent diffuse slow activity, and a background of slow waves, which suggested an epileptogenic focus in the right frontal region as well as a mild, diffuse encephalopathy.
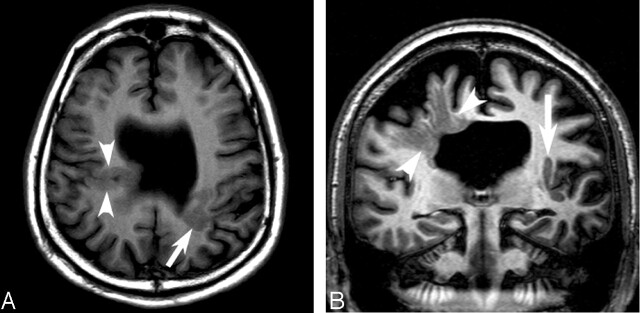
MR imaging at 1.5 T by using volumetric 3D spoiled gradient echo sequence (TR/TE, 6.1/1.4 ms; 24-cm field of view; 256 × 256 acquisition matrix; 1.5-mm partition size) showed absence of the septum pellucidum and three foci of cortical abnormality (Fig 1). An irregular infolding of gray matter extended inward from the right posterior frontal cortex to the ventricular surface, continuing along the ependyma as heterotopic gray matter, consistent with fused lip schizencephaly (Fig 1A, arrowheads). A second closed lip schizencephaly (Fig 1A, arrow) was identified in the contralateral left frontal lobe. Subcortical heterotopia was seen extending medially and parallel to the left insular cortex (Fig 1B, arrow).
Fig 1.
Conventional MR images obtained at 1.5 T and 3 T.
A, Axial T1-weighted image obtained at 1.5 T shows bilateral closed lip schizencephaly (arrow and arrowheads) and absent septum pellucidum.
B, Coronal image obtained at 3T through the schizencephalic cleft reveals individual linear radiations of gray matter signal (arrowheads) extending from the cortex to the ventricle.
The patient also underwent high-spatial-resolution MR imaging at 3 T by using an inversion recovery fast spin-echo sequence (modified driven equilibrium Fourier transformed sequence: TR/TE/TI, 1400/9/700 ms; turbo spin-echo factor 5; 24-cm field of view; 512 × 314 acquisition matrix; 5-mm section thickness with 1-mm intersection gap in the coronal plane), which clearly showed individual strands of gray matter signal, separated by areas of white matter signal, radiating from the subependymal surface to the cortex (Fig 1B, arrowheads). Continuity between the left-sided cleft and subcortical heterotopia was also clearly seen, and the corpus callosum was noted to be very thin.
Axial diffusion tensor imaging was carried out by using the following parameters: single-shot echo planar imaging, TR/TE, 7000/90 ms; 21-cm field of view; 128 × 128 acquisition matrix; 3-mm section thickness; b value, 800 s/mm2 in six noncollinear directions. Off-line postprocessing of diffusion tensor metrics and white matter fiber tracking (Fiber-Tracking version 4, PRIDE, Philips Medical Systems, Best, the Netherlands) showed low fractional anisotropy (Fig 2A, asterisk) within the subcortical heterotopia, including that lining the cleft. Principal eigenvector maps of water diffusion showed deviation in the direction of the corticospinal tracts surrounding the gray matter as compared with normal subjects. The corpus callosum was abnormally thin, and the cingula, which normally lie immediately superior to the corpus with their fibers in an anteroposterior orientation, were distorted (Fig 2C). Fiber tracking, by using the fiber assignment by continuous tracking method (7), of the white matter showed the corticospinal tracts to be smaller and widely separated from the other pyramidal fibers (Fig 3A). The superior longitudinal fasciculus, which runs in an anteroposterior direction, was also deviated and less well developed (Fig 3B). The corpus callosum was thin and poorly developed, with complex orientation of the splenial fibers in different directions as compared with normal control subjects (Fig 3C).
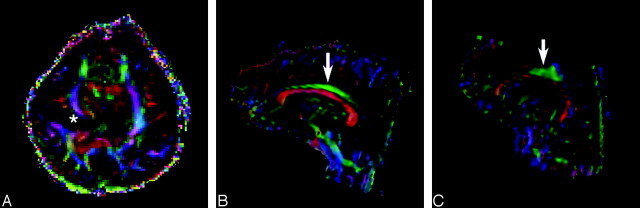
Fig 2.
Principal eigenvector maps obtained from diffusion tensor imaging.
A, Axial color map corresponding to the main direction of the white matter fiber orientation: by convention, blue indicates fibers (e.g., corona radiata) running in the superior to inferior direction, green indicates anterior to posterior (e.g., superior longitudinal fasciculus), and red indicates right-left fibers (e.g., corpus callosum). In our patient, the white matter fibers show deviation in the main direction of the diffusion tensor around the abnormal gray matter of the schizencephalic cleft (asterisk).
B, In a healthy individual, the cingulum (arrow) is a long thin fascicle, lying immediately superior to the corpus callosum, oriented in an anterioposterior direction.
C, The cingulum in our patient (arrow) is shortened and distorted. Note also thinning of the corpus callosum (red) below the cingulum.
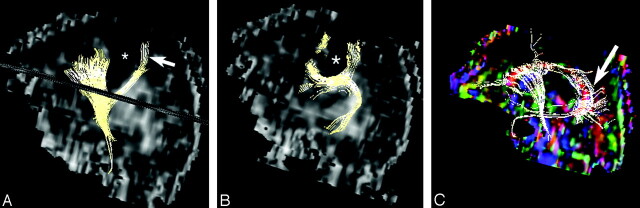
Fig 3.
Fiber tract reconstruction images.
A, 2D projections of fiber tract reconstruction superimposed on sagittal fractional anisotropy map showing the deep schizencephaly (asterisk) separating the corticospinal tract (arrow) from other fibers.
B, In our patient, the superior longitudinal fasciculus is poorly developed and deviated by the schizencephalic cleft (asterisk).
C, Only a few thin fibers are seen in the in the body of the corpus callosum, and the fibers in the splenium (arrow) are oriented in complex directions.
The patient was found to have right frontal lobe epilepsy with schizencephaly, polymicrogyria, and absent septum pellucidum. He was treated with multiple antiepileptic medications, including sodium valproate, lamotrigine, mysoline, carbamazepine, and clonazepam. At the most recent follow-up, he was independent in his activities of daily living, but he continued to experience three to five seizures a month despite treatment.
Discussion
This case demonstrates that malformations of cortical development may be better evaluated by the higher spatial resolution that can be obtained by the increased signal to noise ratio provided by higher field strength. In our patient, standard anatomic neuroimaging at 1.5 T showed the presence of bilateral frontal schizencephaly, polymicrogyria, and subcortical heterotopia, in addition to absence of the septum pellucidum, an anomaly that commonly accompanies frontal schizencephaly (8). Using high-spatial-resolution MR pulse sequences at 3 T, it was possible to see in our patient radiating layers of gray matter signal extending from the ventricular surface to the cortex. To the best of our knowledge, this finding has not been described before in schizencephaly, and we believe these radially oriented transmantle layers may represent columns of immature neurons whose migration along radial glial cells was arrested during embryogenesis. This finding suggests that the added spatial resolution provided by imaging at 3 T may allow further insights into the anatomic details of complex brain malformations.
In addition to the insights provided by high-spatial-resolution imaging of the cortex, the analysis of this patient’s white matter by using diffusion tensor imaging showed the presence of significant alterations in cerebral white matter pathways, both in regions (such as the corticospinal tracts) known to be connected to the malformed areas of cortex and in other tracts (such as the cingulum) as well. Prior studies (2, 4, 6) have shown that the volume of white matter is reduced in cerebral hemispheres that contain malformations of cortical development and that this white matter often has reduced diffusion. In this study, diffusion-weighted imaging was performed by using multiple nonisotropic gradient directions to measure preferential microscopic water movement along highly directional structures such as white matter tracts (9, 10). This information, which cannot be obtained by other means, may be used to generate white matter tractography maps that show functional connections between different regions of the brain. Based on the principal eigenvector of water diffusion, tractography methods may be used to produce maps of white matter connectivity (7). Other authors have used diffusion tensor imaging to study white matter pathways in band heterotopia (4) and holoprosencephaly (11).
In patients with cortical malformation and epilepsy, dysgenesis of the white matter tracts may be important in epileptogenicity as well as in functionality of the patient. Although dysgenesis of the cortex and misconnection secondary to neuronal malpositioning is commonly held to cause epilepsy (12), a modified hypothesis has been proposed that the primary cause might be abnormal interneuronal connectivity, rather than simply neuronal malpositioning (5, 6). Because epileptic patients with malformations of cortical development respond poorly to both medical and surgical treatment (13), more work needs to be done to understand the normal connections of the brain and their aberrations in cortical malformations. Our demonstration of aberrations of white matter tracts by using rather crude diffusion tensor imaging methods illustrates the potential power of this technique if signal to noise ratio could be improved or voxel size reduced by the use of higher-spatial-resolution phased array coils, higher field strength, or longer imaging times.
Disrupted or abnormal axonal connections with their cortical destinations could also be responsible, to an unknown degree, for the neurologic impairments suffered by many of the affected patients. In our patient, diminished volume of the corpus callosum, cingulum, and corticospinal tracts was accompanied by a demonstration of unusual pathways of axonal fascicles not seen in normal patients. By using current methods, however, we were unable to determine the nature, the origin, or the destination of these fiber bundles. Thus, it is uncertain whether these tracts might represent 1) normal axons with aberrant pathways due to pathfinding difficulties; 2) abnormal axons that persisted because of the lack of normal axons and connected with other cortical centers to assume the functional roles normally performed by cortical regions that are dysplastic in this patient; or 3) other pathways with currently unknown and unsuspected functions.
Although we were able to visualize the pyramidal tracts, we were unable in this patient, whose schizencephalic cleft had replaced the normal central sulcus, to identify the precentral gyrus and to determine confidently which of the pyramidal tracts were the sensory and motor pathways. The combination of diffusion tensor imaging with task-activated functional MR imaging would have been useful to demonstrate these functional systems in the brain (14).
Conclusion
This report demonstrates the enormous potential of high-spatial-resolution of MR imaging at 3 T in revealing anatomic details and the potential of diffusion tensor imaging at high field strength to visualize unsuspected white matter anomalies in patients with cerebral malformations. Such combinations of high-spatial-resolution anatomic and functional imaging hold the key to understanding these diseases and, one hopes, will lead to new possibilities in diagnosis and treatment.
Acknowledgments
This study was partly funded by National Medical Research Council grant 03/139. The authors thank J. T. Tan and X. Golay, for their valuable technical assistance.
References
- 1.Yakovlev PI, Wadsworth RC. Schizencephalies: a study of the congenital clefts in the cerebral mantle. 1. Clefts with fused lips. J Neuropathol Exp Neurol 1946;5:116–130 [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Norman D. MR imaging of schizencephaly. AJR Am J Roentgenol 1988;150:1391–1396 [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Kjos BO. Schizencephaly: correlation of clinical findings with MR characteristics. AJNR Am J Neuroradiol 1992;13:85–94 [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson SH, Rugg-Gunn FJ, Symms MR, et al. Diffusion tensor imaging in patients with epilepsy and malformations of cortical development. Brain 2001;124:617–626 [DOI] [PubMed] [Google Scholar]
- 5.Sisodiya SM. Wiring, dysmorphogenesis and epilepsy: a hypothesis. Seizure 1995;4:169–185 [DOI] [PubMed] [Google Scholar]
- 6.Sisodiya SM, Free SL. Disproportion of cerebral surface areas and volumes in cerebral dysgenesis. MRI-based evidence for connectional abnormalities. Brain 1997;120:271–281 [DOI] [PubMed] [Google Scholar]
- 7.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–269 [DOI] [PubMed] [Google Scholar]
- 8.Raybaud C, Girard N, Levrier O, et al. Schizencephaly: correlation between the lobar topography of the cleft(s) and absence of the septum pellucidum. Childs Nerv Syst 2001;17:217–222 [DOI] [PubMed] [Google Scholar]
- 9.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–219 [DOI] [PubMed] [Google Scholar]
- 10.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 11.Albayram S, Melhem ER, Mori S, et al. Holoprosencephaly in children: diffusion tensor MR imaging of white matter tracts of the brainstem: initial experience. Radiology 2002;223:645–651 [DOI] [PubMed] [Google Scholar]
- 12.Caviness VS Jr. Mechanical model of brain convolutional development. Science 1975;189:18–21 [DOI] [PubMed] [Google Scholar]
- 13.Cho WH, Seidenwurm D, Barkovich AJ. Adult-onset neurologic dysfunction associated with cortical malformations. AJNR Am J Neuroradiol 1999;20:1037–1043 [PMC free article] [PubMed] [Google Scholar]
- 14.Janszky J, Ebner A, Kruse B, et al. Functional organization of the brain with malformations of cortical development. Ann Neurol 2003;53:759–765 [DOI] [PubMed] [Google Scholar]