Abstract
Summary: We present a case of serologically proved West Nile virus (WNV) flaccid paralysis of the right upper extremity. Radiologic correlation revealed striking T2 hyperintensities in the anterior horns of the cervical spinal cord, similar to those seen in cases of poliomyelitis. Recognition of the MR imaging findings in cases of WNV flaccid paralysis can provide early evidence of infection.
West Nile virus (WNV), a flavivirus, first garnered attention in the Western Hemisphere during the summer of 1999 when 62 cases of meningoencephalitis were described in New York state (1). The western spread of the virus has resulted in an exponential growth in the number of reported human cases. At the time of this writing, 6957 human cases have been recorded in the continental United States since October 15, 2003 (2). The summer of 2003 also produced a dramatic increase in the number of Canadians infected with the virus. As of October 17, 2003, there were 1280 probable or confirmed cases of WNV infection in Canada, compared with fewer than 200 in 2002. The province of Saskatchewan has seen most human cases, with 731 probable or confirmed cases; this results in a provincial incidence rate of ∼0.7%, by far the highest rate in North America (3).
Case Report
A previously well 59-year-old woman presented to our emergency department with a 3-day history of general malaise, diffuse myalgias, and fever with rigors. She also noted a right-sided headache, nausea, vomiting, and rash. In addition, during the previous day, she noted an isolated and progressive weakness in her right arm.
On examination, she was pyrexic (39.8°C) with rigors, tachycardic (119 beats/min) and slightly hypertensive (146/70 mm Hg). She exhibited meningismus and a fine erythematous rash sparing the palms and soles. Tone and reflexes were diminished in the right arm. Strength testing of this limb revealed diminished power in the proximal (2/5) and distal (3/5) musculature. No fasciculations or wasting was appreciated, and sensory examination, as well as the remainder of the neurologic examination, was normal.
Laboratory analysis showed a mild leukocytosis (11.6 × 109/L). CSF examination was remarkable for a mild leukocytosis (204 × 106/L) consisting of 62% neutrophils and 28% lymphocytes. The CSF glucose was normal, and the protein was mildly elevated (0.87 g/L). Initial WNV acute IgM antibody was equivocal and sent for confirmatory testing, which returned positive for WNV. A CT scan of the head was normal. EMG of the right arm showed preserved motor conduction velocity with few motor unit potentials of the first dorsal interosseus muscle of the right hand. These findings are consistent with subacute denervation, which may indicate anterior horn cell dysfunction.
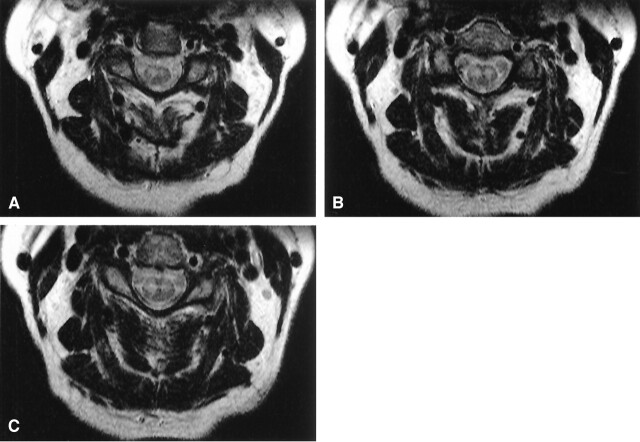
MR imaging displayed increased T2 signal intensity of the cervical spinal cord from the C3–C4 disk space to the cervicothoracic junction (Figs 1 and 2). This hyperintense region was localized to the anterior gray matter and showed right-side predominance, which was consistent with her clinical presentation (Fig 1A–C). The presence of contrast enhancement was not assessed, because the patient was unable to tolerate extended MR imaging examination.
Fig 1.
Axial T2-weighted MR images of the cervical spinal cord. The figures demonstrate bilateral hyperintense signal in the anterior horns. There is a right-side predominance of abnormal signal intensity most apparent in panels A and C. B depicts symmetrical hyperintense signal in the anterior horns.
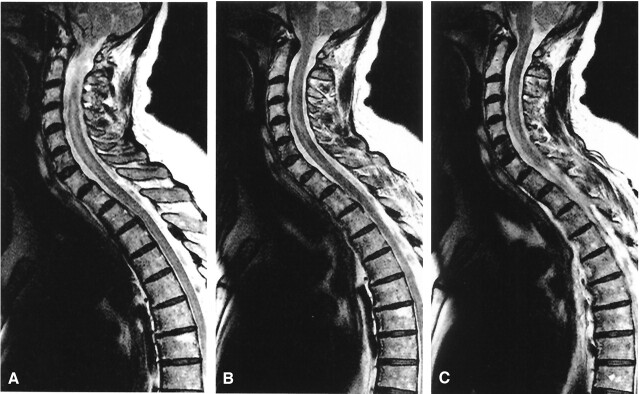
Fig 2.
Sagittal T2-weighted MR images with hyperintense signal in the anterior horns. A, Midline sagittal section through spinal cord. B, slightly right of midline sagittal section. C, sagittal section through anterior horn on right side.
The patient noted improvement in her flulike symptoms within a few days of admission. After 8 days, she was discharged. At that time, the patient still had residual myalgias and her right-arm strength was unchanged from admission. Outpatient physiotherapy was arranged.
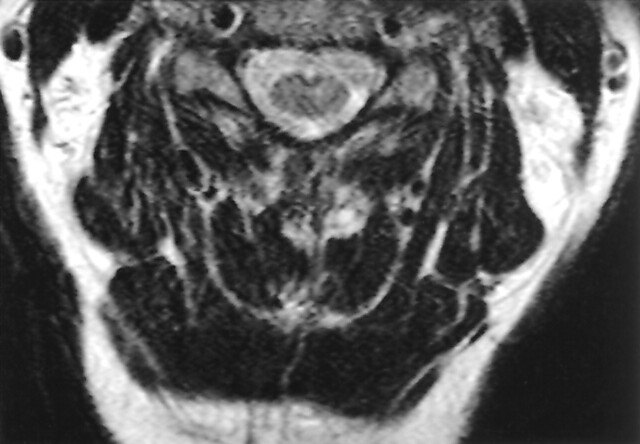
Follow-up at 6 weeks after discharge revealed the patient to be experiencing moderate left-arm weakness (4/5) in addition to the original right-arm weakness, which remained unchanged. EMG examination revealed increased amplitude and duration of motor unit potentials in the left 1st dorsal interosseus muscle. At this time there were no motor unit potentials in the right dorsal interosseus. Again, sensory conduction was normal and findings indicated motor neuron disease. Follow-up MR images were obtained 6 months after presentation. (Fig 3) The study showed resolution of the T2 hyperintense regions seen previously and the contrast-enhanced images were unremarkable.
Fig 3.
Axial T2-weighted MR image taken 6 months following infection. The hyperintense signal noted previously has resolved.
Discussion
WNV infection has been associated with a myriad of clinical features ranging from a subclinical febrile illness to severe neurologic deficit. Common symptoms include fever, headache, nausea, meningismus, myalgias, rash, and tremor (4). In addition, a subset of patients exhibits features of an acute, usually asymmetric, flaccid paralysis. First described more than 2 decades ago, this rapidly progressive motor deficit, accompanied with a flulike prodrome, is characterized by a preservation of sensory function (5). The recent North American epidemic has produced multiple cases of this unique neuromuscular presentation of WNV infection (4, 6–8).
The relative paucity of reported WNV flaccid paralysis cases has provided few published reports of MR imaging correlation specific to this subset of WNV infection. Six patients have exhibited abnormalities on MR images of the brain. Most of these patients had areas of increased signal intensity on T2-weighted images in the cortex and subcortical white matter. These regions also tended to demonstrate restricted diffusion (6). In addition, five patients with lower extremity weakness displayed leptomeningeal enhancement of the conus medullaris or cauda equina or both (6, 9–10).
Less extensively described are signal intensity abnormalities within the parenchyma of the spinal cord. Only two reported cases display this finding. The first case was a middle-aged woman with severe (1–2/5) right-leg weakness and moderate weakness (3–4/5) of the remaining extremities. Her MR imaging findings were remarkable for nonenhancing T2 hyperintensities bilaterally in the anterior horns of the lumbar spinal cord (7). The second example consisted of a representative sagittal image displaying gadolinium-enhancing lesions involving the cervical spinal cord (6). We present a case of serologically proved WNV infection resulting in right-arm flaccid paralysis and the MR imaging correlate of asymmetric hyperintensities within the anterior horns of the cervical spinal cord. Follow-up imaging revealed a resolution of MR findings despite clinical progression. Thus, early imaging is advised to assess the degree and extent of spinal cord abnormalities.
T2-weighted spinal cord parenchymal abnormalities can be caused by vasculitides, infection, inflammation, edema, neoplasia, and autoimmune processes such as multiple sclerosis (MS). Although the specificity of MR imaging examination of the cord is relatively low, a few imaging patterns suggest different causes. Specifically, multiple, smaller enhancing areas of hyperintensity suggest MS, whereas extensive multilevel abnormalities may be seen in the vasculitides (11).
The limited experience thus far with WNV flaccid paralysis shows a preferential inflammation of the anterior gray matter of the spinal cord. Evidence for this localization is suggested clinically, electromyographically, radiologically, and pathologically. Clinically, weakness and areflexia in the absence of sensory findings suggest an anterior localization. EMG patterns in reported cases of WNV flaccid paralysis, including our own, demonstrate isolated anterior horn cell dysfunction (6). In addition, imaging studies have now demonstrated abnormal signal intensity within the anterior gray matter of the spinal cord (6, 7). In fact, the MR imaging findings in WNV flaccid paralysis are strikingly similar to the reported spinal cord abnormalities in patients with polio (12–13). Finally, recent postmortem examination of patients with WNV myelitis has revealed pathologic correlation of anterior horn cell inflammation (14).
The province of Saskatchewan displayed a remarkable increase in the incidence of WNV infection during the summer of 2003. It is postulated that both environmental and political factors contributed to the magnitude of new cases. Biologically, the Culex tarsalis species of mosquito, which is primarily responsible for transmission of the virus in the west, is more likely to feed on mammals than is its eastern counterpart, the C. pipiens species. In addition, C. tarsalis mosquitoes reproduce best during hot, dry weather; the early spring and warm summer provided ideal conditions for the species to thrive. Politically, Saskatchewan spent only $1.2 million on WNV prevention. This was an amount far less than its neighbor to the east, Manitoba, spent. This may explain why Manitoba, which has a similar climate, had fewer than one-fifth the number of WNV cases (15).
Conclusion
We have presented a case of WNV flaccid paralysis. Our case demonstrates early EMG anterior horn cell dysfunction with accompanying right-side-dominant hyperintensity in the parenchyma of the cervical spinal cord. The importance of this finding is that, in the case of our patient, MR imaging provided definitive evidence of spinal cord involvement, whereas EMG findings were subtler. In addition, confirmatory serology is often delayed. It was our experience that the MR imaging findings were present 2 weeks before the return of definitive serology and, thus, can serve as a valuable practice aid in the face of the growing incidence of this disease.
References
- 1.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 2001;14:1807–1814 [DOI] [PubMed] [Google Scholar]
- 2.West Nile virus activity: United States, October 9–15, 2003. MMWR Morb Mortal Wkly Rep 2003;52:996–967 [PubMed] [Google Scholar]
- 3.Health Canada website. WNV Human Surveillance Data;2003. . http://www.hc-sc.gc.ca/pphb-dgspsp/wnv-vwn/pdf_sr-rs/2003/surveillance_table_101703_hm.pdf. Accessed November 1, 2003
- 4.Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA 2003;290:511–515 [DOI] [PubMed] [Google Scholar]
- 5.Gadoth N, Weitzman S, Lehmann EE. Acute anterior myelitis complicating West Nile fever. Arch Neurol 1979;36:172–173 [DOI] [PubMed] [Google Scholar]
- 6.Jeha LE, Sila CA, Lederman RJ, et al. West Nile virus infection: a new acute paralytic illness. Neurology 2003;61:55–59 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Loeb JA, Shy ME, et al. Asymmetric flaccid paralysis: a neuromuscular presentation of West Nile virus infection. Ann Neurol 2003;53:703–710 [DOI] [PubMed] [Google Scholar]
- 8.Sejvar JJ, Leis AA, Stokic DS, et al. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis 2003;9:788–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsan AD, Milburn JM, Baumgarten KL, Durham HL. Leptomeningeal enhancement in a patient with proven West Nile virus infection. AJR Am J Roentgenol 2003;181:591–592 [DOI] [PubMed] [Google Scholar]
- 10.Acute flaccid paralysis syndrome associated with West Nie infection: Mississippi and Louisiana, July–August 2002. MMWR Morb Mortal Wkly Rep 2002;51:825–828 [PubMed] [Google Scholar]
- 11.Scotti G, Gerevini S. Diagnosis and differential diagnosis of acute transverse myelopathy: the role of neuroradiological investigations and review of the literature [Suppl]. Neurol Sci 2001;22:S69–S73 [DOI] [PubMed] [Google Scholar]
- 12.Kornreich L, Dagan O, Grunebaum M. MRI in acute poliomyelitis. Neuroradiology 1996;38:371–372 [DOI] [PubMed] [Google Scholar]
- 13.Rao D, Bateman D. Hyperintensities of the anterior horn cells on MRI due to poliomyelitis. J Neurol Neurosurg Psychiatry 1997;63:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley TW, Prayson RA, Ruiz AI, et al. The neuropathology of West Nile virus meningoencephalitis: a report of two cases and review of the literature. Am J Clin Pathol 2003;119:749–753 [DOI] [PubMed] [Google Scholar]
- 15.Canadian Medical Association Journal website. Sibbald B. Sakatchewan’s West Nile Surge Baffles Experts. http://www.cmaj.ca/news/10_09_03.shtml. Accessed November 1,2003