Abstract
BACKGROUND AND PURPOSE: It would be useful to have a noninvasive test for correlation with CT findings in patients with intracerebral hemorrhage (ICH). We determined which transcranial Doppler (TCD) variables are related to which CT data in patients with ICH.
METHODS: We prospectively included 51 patients (age ± SD, 66.2 ± 12.4 years; 30 men, 21 women) with first-ever supratentorial, nontraumatic ICH. CT and TCD examination were performed in the acute stage (less than 12 hours from symptom onset). TCD recordings were obtained from the middle cerebral arteries, and the following variables were analyzed: systolic (Vs), diastolic (Vd), mean (Vm) velocities, and pulsatiliy index from the affected (a) and unaffected (u) hemispheres.
RESULTS: PIs obtained for both hemispheres were positively correlated with hematoma volume (aPI, r = 0.43, P = .001; uPI, r = 0.44, P = .001), volume of hypoattenuation (aPI r = 0.64, P < .0001; uPI, r = 0.39, P = .005), total volume (aPI, r = 0.59, P < .0001; uPI, r = 0.48, P < .0001), and midline shift (aPI, r = 0.28, P = .04; uPI, r = 0.29, P = .03). Both PIs were increased in patients with intraventricular hemorrhage (aPI, P = .01; uPI P = .004). No TCD parameter was correlated with ventricular size.
CONCLUSION: Most TCD parameters were correlated with CT data in the acute stage of ICH. An increase in PI probably reflects intracranial hypertension and mass effect. Further studies are needed to determine the clinical application of our findings.
Acute spontaneous intracerebral hemorrhage (ICH) is a disease with a 23–58% mortality rate within 6 months (1). Half of the deaths occur during the first 2 days (2) of the onset of the ICH and are attributable to intracranial hypertension and mass effect. The most important predictors of outcome are the Glasgow Coma Scale score and the volume of hematoma (1). Other CT abnormalities, such as ventricular enlargement and secondary intraventricular hemorrhage, have also been prognostic (3–5).
The sudden eruption of an intracranial mass destroys and displaces brain tissue and can induce an increase in intracranial pressure (ICP). Recent data indicate that ICH is a dynamic disease. During the first hours or days following appearance of the primary lesion, some patients have hematoma growth, perilesional edema, perilesional ischemia, hydrocephalus, or secondary intraventricular hemorrhage (6–8). All of these complications potentially increase ICP and mass effect, finally resulting in neurologic deterioration. However, the information that CT provides is static. As a result, it is not practical to perform frequent control CT studies in these patients. Although invasive devices can be used to monitor the ICP, the procedure has some risks (e.g., infection and tissue lesion) and pitfalls (e.g., those variability inherent to the technique, displacement, unilateral information). Moreover, the value of this approach in the management of ICH is equivocal (9–12). In view of these limitations, it would be useful to have a noninvasive test that can be used for correlation with CT and that provides dynamic information for reliable treatment and prognosis.
Increased ICP and decreased cerebral perfusion pressure (CPP) give rise to typical changes in the Doppler waveform obtained by transcranial insonation (i.e., a decrease of diastolic velocity and an increase in the pulsatility index [PI]) (13–15). Transcranial Doppler (TCD) could be useful for noninvasively and indirectly assessing mass effect and ICP in ICH. Data on the relation of radiologic data to one or more specific TCD variables are sparse. In one study (16), the PI obtained from the unaffected hemisphere was an independent predictor of mortality in acute ICH. In response to the lack of information, we studied the hematoma volume and other CT data, along with TCD findings, in a prospective series of patients in the acute stage of ICH.
Methods
Patients
This study was conducted at a university hospital that provides neurosurgical, neuroradiologic, and intensive care assistance in addition to neurologic evaluation. Therefore, it is a referral center for the care of patients with ICH.
We included patients whose symptoms began within 12 hours from the time they were included in the study. Their symptoms were attributable to a first-ever supratentorial, single, nontraumatic ICH, as diagnosed on cranial CT. We excluded patients who needed emergency surgical evacuation, those with a nonsuitable temporal acoustic bony window, and those with an imprecise time for the onset of symptoms. Also excluded were patients with previous clinical cerebrovascular disease (ischemic or hemorrhagic), epidural or subdural hematoma at initial CT, and those for whom the investigating team was not contacted. As it is difficult to know the cause of such hemorrhage in the first hours, all etiologies were included.
Our hospital Ethics Research Committee approved the study protocol. In addition, patients or their legal representatives gave written consent to participate. Patients were treated according to a previously approved protocol written jointly by neurologists, neurosurgeons, and intensive care specialists at our hospital. Glasgow Coma Scale and National Institute of Health and Stroke (NIHS) Scale scores were obtained.
CT Evaluation
For consistency and reliability, the same neuroradiologist (E.G.) analyzed all of the CT scans blinded to the clinical and TCD data. Axial 5-mm-thick contiguous sections were obtained from the supratentorial region, while 3-mm-thick contiguous sections were obtained from the infratentorial fossa. CT scans were stored in the hard disk of the CT apparatus (Asteion; Toshiba, Nasu, Japan). Quantitative data were obtained with the aid of computer-assisted measurements provided by the CT apparatus.
In each CT examination, the following information was obtained: 1) topography, which was classified as lobar or deep; 2) hematoma volume, which was calculated according to the (A × B × C)/2 method applied to the hyperattenuating lesion (2), 3) perilesional hypoattenuation, which was also calculated by applying the (A × B × C)/2 method to the hypoattenuating area surrounding the hyperattenuating lesion and by subtracting from this value the hematoma volume; 4) total volume, which was calculated as the sum of the hyperattenuating and hypoattenuating volumes; and 5) midline shift, which was assessed by calculating the lateral displacement (in centimeters) from the midline of the septum pellucidum, the third ventricle, or the pineal gland (A line was traced from the anterior insertion of the falx cerebri to the torcula, and the midline shift was calculated at the most displaced midline structure.); 6) ventricular size, which was calculated according to the Evans ventricular ratio, or the ratio between the maximum spread of ventricular horns to the breadth of cranial cavity (17); and 7) intraventricular hemorrhage. Irrespective of its amount or localization, any intraventricular hyperattenuating image not attributable to calcification or choroid plexus was considered evidence of intraventricular hemorrhage.
TCD Protocol
We used a portable TCD instrument (Multi-Dop2; DWL Elektroniche Systeme GmbH, Baden-Württenberg, Germany). Both middle cerebral arteries (MCAs) were insonated. These arteries were chosen because they provide 80% of the hemispheric blood flow, and it is easy to detect the flow due to the spatial orientation in relation to the probe. TCD examination was performed as soon as possible after the CT diagnosis was available, and always within 12 hours of the onset of symptoms.
The MCA were examined through the temporal acoustic window at a depth of 45–55 mm with the patient in the supine position. The MCA from the unaffected hemisphere was examined first, and then the MCA ipsilateral to the hematoma was examined. When a clearly readable waveform was obtained, it was used in the calculations. The spectra cycles contained in a 6-second frame were analyzed. If all of the spectra were technically interpretable, the time-averaged results automatically calculated by the machine were considered reliable.
The following variables were analyzed: systolic (Vs), diastolic (Vd), mean (Vm) velocities, and PI from the affected (a) and unaffected (u) hemispheres. However, when results of one or more cycles were not interpretable, the measurements were calculated from the best spectra on the printed paper or on the monitor screen. Vm was calculated according to the equation Vm = (Vs − Vd)/3 + Vd, and PI was calculated according to the formula PI = (Vs − Vd)/Vm. Normal values for PI ± SD were 0.98 ± 0.2 (18).
Statistical Analysis
The statistical analyses included the following CT data: hematoma volume, volume of hypoattenuation, total volume, midline shift, ventricular ratio, and intraventricular hemorrhage (yes/no). Continuous variables were correlated with the eight TCD variables: four on the affected side, or aVs, aVm, aVd, and aPI, and four on the unaffected side, or uVs, uVd, uVm, and uPI. Pearson correlation coefficients were obtained. For binary variables, the mean and SD of the eight TCD variables were compared in the two groups with the Student t test. Because of the large SD detected in the CT data, we performed the Kolmogorov-Smirnov test to determine if these variables were normally distributed. In addition, we analyzed the same variables with nonparametric statistical tests (Spearman correlation and Mann-Whitney test). A result was considered statistically significant when P < .05. The statistical analyses were performed with the aid of SPSS version 10 software (SPSS, Chicago, IL).
Results
During a 2-year period, 138 consecutive patients received a diagnosis of spontaneous supratentorial ICH. Patients were excluded for the following reasons: emergency evacuation of hematoma (n = 7), admission after 12 hours from the onset of symptoms or an unknown time of onset (n = 29), deficient acoustic window (n = 5), previous cerebrovascular disease (n= 11), or contact with the investigator team after the 12-hour window (n = 17). In addition, 16 patients were excluded because they died before TCD study was performed, and two patients were excluded because of diagnostic errors. Therefore, this study consisted of 51 patients (30 men, 21 women), whose mean age was 66.2 ± 12.4 years.
First CT was performed a mean of 190 ± 173 minutes (range, 15–702 minutes) after the onset of symptoms, while the first TCD study was performed after a mean of 340 ± 219 minutes (range, 60–720 minutes) after the onset of symptoms. Suspected etiologies were high blood pressure (n = 29), anticoagulant therapy (n = 2), other coagulation abnormalities (n = 2), arteriovenous malformation (n = 1), tumor (n = 1), other etiologies (n = 2), and unknown (n = 14). The median Glasgow Coma Scale score was 14, while the median NIHS Scale score was 17. The mortality rate at 30 days was 33% (n = 17).
Data from acute CT are shown in Table 1. About 25% of the patients had hematomas of more than 50 mL, and the range of hematoma volume was wide (0.05–344 mL). Secondary intraventricular hemorrhage was present in 55% of the patients. The TCD data are shown in Table 2. Values were comparable in the affected and unaffected hemispheres (Student t test).
TABLE 1:
CT data from the acute stage of ICH
| A: Topography and hemorrhage | n | Percentage (%) |
|---|---|---|
| Topography | ||
| Lobar | 19 | 37 |
| Deep | 32 | 63 |
| Intraventricular hemorrhage | 28 | 55 |
| B: Volume, shift, and ratio | Mean ± SD | Range |
| Hematoma volume (mL) | 51.7 ± 70.4 | 0.05–344 |
| Edema volume (mL) | 28.4 ± 38.1 | 0–210.5 |
| Total volume (mL) | 80.1 ± 93.7 | 0.28–348 |
| Midline shift (cm) | 0.56 ± 0.79 | 0–4 |
| Ventricular ratio | 0.23 ± 0.04 | 0.11–0.33 |
TABLE 2:
TCD results in the acute stage of ICH
| Result | Mean ± SD |
|---|---|
| aVs (cm/s) | 79.2 ± 36.0 |
| aVd (cm/s) | 19.7 ± 11.1 |
| aVm (cm/s) | 35.6 ± 16.5 |
| aPI | 1.85 ± 0.87 |
| uVs (cm/s) | 81.2 ± 34.2 |
| uVd (cm/s) | 22.8 ± 13.6 |
| uVm (cm/s) | 39.6 ± 18.0 |
| uPI | 1.62 ± 0.93 |
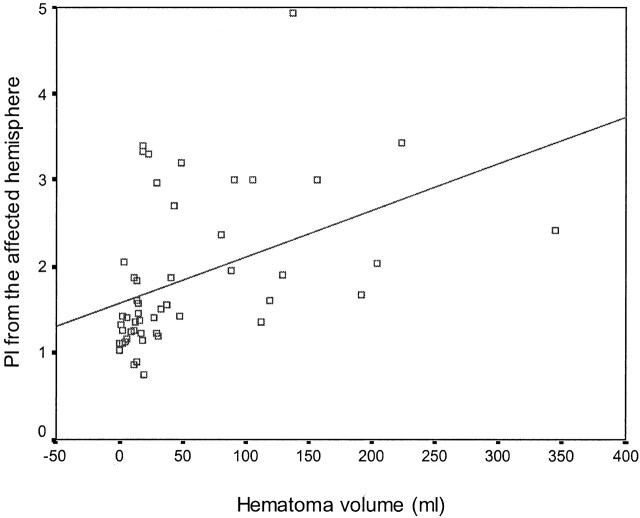
Mean PI in the acute stage was clearly above normal values in both hemispheres. Table 3 and Figure 1 shows the relationships between quantitative CT variables and TCD data obtained with Pearson correlation coefficient. We found a positive correlation between the PI from both hemispheres and the volume of hematoma (aPI, r = 0.43, P = .001; uPI, r = 0.44, P = .001), the hypoattenuating volume surrounding the hematoma (aPI, r = 0.64, P < .0001; uPI, r = 0.39, P = .005), total volume (aPI, r = 0.59, P < .0001; uPI, r = 0.48, P < .0001), and midline shift (aPI, r = 0.28, P = .04; uPI, r = 0.29, P = .03). Vd from the affected hemisphere was negatively correlated with the volume of hypoattenuation (r = −0.32, P = .02) and the total volume (r = −0.32, P = .02). No statistically significant correlations were found between systolic or mean velocities and any of the CT data. No TCD variable was correlated with the ventricular ratio, and TCD results were equivalent in patients with deep or lobar ICH. Level of consciousness measured with the Glasgow Coma Scale was negatively correlated with aPI (r = −0.53, P < .0001) and uPI (r = −0.61, P < .0001). Neurologic severity measured with the NIHS scale showed a positive correlation with the PI from both hemispheres (aPI, r = 0.52, P < .0001; uPI, r = 0.55, P < .0001).
TABLE 3:
Relationship between TCD and quantitative CT variables
| Variable | Hematoma Volume |
Hypoattenuating Volume |
Total Volume |
Midline Shift |
Ventricular ratio |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | |
| aVd (cm/s) | −0.25 | .07 | −0.32 | .02 | −0.32 | .02 | −0.24 | .08 | 0.01 | .93 |
| aPI | 0.43 | .001 | 0.64 | <.0001 | 0.59 | <.0001 | 0.28 | .04 | −0.23 | .09 |
| uVd (cm/s) | 0.19 | .16 | −0.24 | .08 | −0.24 | .07 | −0.14 | .29 | −0.11 | .41 |
| uPI | 0.44 | .001 | 0.39 | .005 | 0.48 | <.0001 | 0.29 | .03 | −0.05 | .70 |
Note.—Data were analyzed by using the Pearson correlation coefficient.
Fig 1.
Scatterplot shows the correlation between hematoma volume, volume of hypoattenuation, and total volume with PI in the affected and unaffected hemispheres of the brain.
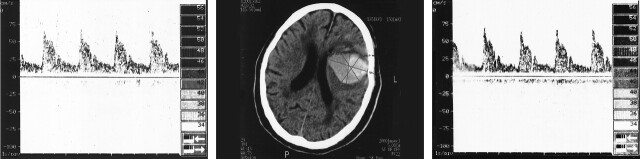
Table 4 shows the comparison of TCD variables between the group with and the group without intraventricular hemorrhage. Both aPI and uPI were significantly higher in the group with intraventricular hemorrhage (aPI, P = .01; uPI P = .004). Vd from the affected hemisphere was lower in patients with intraventricular hemorrhage (P = .03). Mean and systolic velocities did not show differences in patients with or without intraventricular hemorrhage. Figure 2 presents an example of the TCD and CT results in one representative patient.
TABLE 4:
Comparison of mean ± SD TCD parameters with binary CT variables
| Intraventricular Hemorrhage |
|||
|---|---|---|---|
| Yes (n = 28) | No (n = 23) | P Value | |
| aVd (cm/s) | 16.7 (10.7) | 23.4 (10.6) | .03 |
| aPI | 2.12 (0.96) | 1.53 (0.62) | .01 |
| uVd (cm/s) | 20.9 (15.2) | 25.2 (11.2) | >.05 |
| uPI | 1.94 (1.13) | 1.24 (0.33) | .004 |
Fig 2.
Patient with a high bilateral PI (aPI, 1.96; uPI, 1.72) with symmetrical flow velocities: (aVm, 28 cm/s; uVm, 29 cm/s). Center, CT scan shows a large hematoma (87.8 mL), a hypoattenuating lesion of 36.3 mL, and a midline shift of 0.2 cm; Left, Doppler recording corresponding to the right hemisphere; Right, Doppler recording corresponding to the left hemisphere.
The Kolmogorov-Smirnov test showed that the distribution of all CT data were not normal. Therefore, we evaluated the data by using nonparametric analyses that also showed the following correlations: volume of the hematoma (aPI, r = 0.61, P < .0001; uPI, r = 0.56, P < .0001); volume of hypoattenuation (aPI, r = 0.56, P < .0001; uPI, r = 0.45, P = .001; and aVd, r = −0.31, P = .02); the total volume (aPI, r = 0.66, P < .0001; uPI, r = 0.58, P < .0001; aVd, r = −0.36, P = .008; and uVd, r = −0.30, P = .03); and the midline shift (aPI, r = 0.50, P < .0001; uPI, r = 0.50, P < .0001; and aVd, r = −0.34, P = .01). Patients with intraventricular hemorrhage had significantly higher aPI (P = .01) and uPI (P = .03) than those of patients without intraventricular hemorrhage.
Discussion
By insonating the MCA in patients during the acute stage of ICH, we found that TCD data are related to CT data. Considering all of the patients, we found an increase of mean PI in both hemispheres in the acute stage. We also found that the PI obtained from the affected and unaffected hemispheres were correlated with those CT signs associated with mass effect: volume of the hematoma, volume of surrounding edema, total volume, midline shift, and intraventricular hemorrhage. TCD measurements that were most often correlated with the CT data were either an increase of the PI (aPI or uPI) or a decrease in the Vd. In fact, as the PI was obtained by the ratio (Vs − Vd)/Vm, the decrease in Vd made the numerator higher and the denominator lower, increasing the value of the PI.
Early mortality in patients with ICH is sometimes related to an increase of ICP and mass effect, whereas death in the chronic stage is often attributed to respiratory infections or other consequences of immobility (19, 20). Accordingly, hematoma volume (the main cause of increased ICP) and decreased level of consciousness (a consequence of increased ICP) are the main predictors of outcome in ICH. Experimental studies have found immediate and sustained increases in ICP after an ICH (21, 22). These findings have been confirmed in clinical studies in which high ICP and mass effect were recorded after stroke (12, 23).
Our results agree with those of several authors who found that the progressive increase in ICP and decrease in CPP induce characteristic changes in the Doppler waveform (10, 13–15, 24, 25): a progressive decrease of Vd and a progressive increase in PI, which is more marked with ICP above 60 mmHg (15). The reduction in Vd is accounted for by the increase in peripheral cerebrovascular resistance, which is determined mainly by the ICP and the diameter of the arterioles (26). As a consequence, TCD has been used to indirectly estimate ICP and CPP in patients with severe head injuries (10, 14, 25–29). These experimental studies demonstrate a high correlation between ICP and PI, either linear or exponential, and an inverse correlation between CPP and PI (14, 15, 29). Therefore, some authors have used TCD to monitor the effect of different therapies (30, 31). An alternative explanation for an increase in PI is vasospasm, but this is unlikely in spontaneous ICH. In our study, the PI from both hemispheres was correlated with the hypoattenuating area surrounding the hematoma. Although the exact composition of this perihematoma hypoattenuation in the acute stage is controversial, our finding indicates that it also contributes to intracranial hypertension (as it is correlated with an increase in PI).
Few groups have analyzed the correlations between TCD and CT data in spontaneous ICH. Mayer et al (32) examined 30 patients with supratentorial ICH and found an elevation of aPI, the aPI/uPI ratio, and uPI in hematomas larger than 25 mL. We also found that the PI obtained from both hemispheres were directly correlated with the volume of the hematoma, the volume of edema, and the total volume and that the PI was higher in patients with secondary intraventricular hemorrhage. Our study is not entirely comparable to that of Mayer et al, because they included patients admitted within 24 hours of symptom onset (vs. 12 hours in our series), they excluded patients with Glasgow Coma Scale scores between 3 and 5, and TCD examination was performed after considerable delay (on either the first or second day of hospitalization). Moreover, our series included larger hematomas, and we did not dichotomize the hematoma volumes. Despite these differences, it is conspicuous that our results agree with those of Mayer et al.
Our study has some limitations. We studied only the MCA, but we do not know how relevant the TCD findings from other arteries of the anterior or posterior circulation are. We chose the MCA because findings were easily obtainable and because MCA carries 80% of the blood flow to the hemispheres of the brain. TCD values change depending on the hematocrit, pCO2, systemic blood pressure, and autoregulatory capacity. However, these variables affect mainly flow velocities and their ratios (such as PI), although to a lesser degree. Recent findings suggest that autoregulation is preserved in ICH (33). Our data are based on a single TCD examination, but because of the broad interindividual variability of measured TCD parameters, it would be interesting to follow up individual subjects over time to see if a change in any CT and clinical parameter is correlated with a change in a TCD parameter. We assumed that the TCD changes provoked by ICH were related to intracranial hypertension. However, we did not measure the ICP, realizing that TCD really measures the flow dynamics related to intracranial compliance. Finally, we acknowledge that the variance of most CT data explained by the TCD results is rather low.
Our results suggest that TCD indirectly assesses intracranial hypertension and mass effect. Except for ventricular size, all CT parameters indicating mass effect or intracranial hypertension were correlated to some degree with TCD measurements. Obviously, TCD is not a substitute for urgent scanning in patients in deteriorating condition, but it has the advantage of high temporal resolution, as well as noninvasiveness. It is relatively easy to perform at the bedside and at frequent intervals (or with continuous monitoring) without interfering with the treatment of the patient. However, because of the low variance explained by the TCD data and because we did not perform longitudinal studies, our results should be viewed as preliminary and without definitive implications regarding the treatment of patients with ICH. Further studies with serial examinations should evaluate whether TCD is useful to monitor ICP and CPP, to evaluate the effect of therapy (e.g., surgical evacuation [34] or osmotic therapy [31]), and to analyze its prognostic value (16) (as suggested by the high correlation found between PI and Glasgow Coma Scale and NIHS scale scores). Increased PI could lead to placement of an ICP measurement device a patient who is clinically deteriorating. Studies that include simultaneous recording of ICP and TCD at frequent intervals and that correlate the findings with corresponding CT results are likely to provide important information.
Conclusion
In acute ICH, the TCD waveform is affected by many factors that are components of the CT study. TCD results do not replace information provided on CT, but TCD gives complementary data. Although our results are not definitive, they do provide a firm background for future studies. We hope that these findings will further the understanding of the relation between TCD and CT and ultimately translate to clinical practice.
Acknowledgments
We thank Professor William Stone for reviewing the manuscript.
Footnotes
Supported by a grant from Fondo de Investigaciones Sanitarias (00/0029), Ministerio de Sanidad, Spain.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–1460 [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993 [DOI] [PubMed] [Google Scholar]
- 3.Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage: a prospective study of 166 cases using multivariate analysis. Stroke 1991;22:1–6 [DOI] [PubMed] [Google Scholar]
- 4.Rosenow F, Hojer C, Meyer-Lohmann C, Hilgers RD, Mühlhofer H. Spontaneous intracerebral hemorrhage: prognostic factors in 896 cases. Acta Neurol Scand 1997;96:174–182 [PubMed] [Google Scholar]
- 5.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 1998;29:1352–1357 [DOI] [PubMed] [Google Scholar]
- 6.Diringer MN. Intracerebral hemorrhage: pathophysiology and management. Crit Care Med 1993;21:1591–1603 [DOI] [PubMed] [Google Scholar]
- 7.Brott T, Broderick T, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5 [DOI] [PubMed] [Google Scholar]
- 8.Mayer SA, Lignelli A, Fink ME, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage. A SPECT study. Stroke 1998;29:1791–1798 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt EA, Czosnyka M, Gooskens I, et al. Preliminary experience of the estimation of cerebral perfusion pressure using transcranial Doppler ultrasonography. J Neurol Neurosurg Psychiatry 2001;70:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt B, Klingelhöfer J, Schwarze JJ, Sander D, Wittich I. Noninvasive prediction of intracranial pressure curves using transcranial Doppler ultrasonography and blood pressure curves. Stroke 1997;28:2465–2472 [DOI] [PubMed] [Google Scholar]
- 11.Wijman CAC, Kase CS. Intracerebral hemorrhage: medical considerations. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, eds. Stroke. Pathophysiology, Diagnosis, and Management. Philadelphia: Churchill Livingstone;1998. :1359–1372
- 12.Janny P, Colnet G, Georget AM, Chazal J. Intracranial pressure with intracerebral hemorrhages. Surg Neurol 1978;10:371–375 [PubMed] [Google Scholar]
- 13.Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg 1988;68:745–751 [PubMed] [Google Scholar]
- 14.Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol 1988;235:159–162 [DOI] [PubMed] [Google Scholar]
- 15.Ursino M, Giulioni M, Lodi CA. Relationship among cerebral perfusion pressure, autoregulation, and transcranial Doppler waveform: a modeling study. J Neurosurg 1998;89:255–266 [DOI] [PubMed] [Google Scholar]
- 16.Martí-Fàbregas J, Belvís R, Guardia E, et al. Prognostic value of pulsatility index in acute intracerebral hemorrhage. Neurology 2003;61:1051–1056 [DOI] [PubMed] [Google Scholar]
- 17.Evans WA Jr. An encephalographic ratio for estimating the size of the cerebral ventricles; a comparative anatomical study. Am J Dis Child 1942;64:820–830 [Google Scholar]
- 18.Segura T, Serena J, Plaza I, Monforte C, Figuerola A, Dávalos A. Valores de normalidad del estudio Doppler transcraneal en nuestro medio. Neurología 1999;14:437–443 [PubMed] [Google Scholar]
- 19.Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry 1990;53:824–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oppenheimer S, Hachinski V. Complications of acute stroke. Lancet 1992;339:721–724 [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Hung YC, Lee MY. Early alterations in cerebral hemodynamics, brain metabolism, and blood-brain barrier permeability in experimental intracerebral hemorrhage. J Neurosurg 1999;91:1013–1019 [DOI] [PubMed] [Google Scholar]
- 22.Nath FP, Jenkins A, Mendelow AD, Graham DI, Path FRC, Teasdale G. Early hemodynamic changes in experimental intracerebral hemorrhage. J Neurosurg 1986;65:697–703 [DOI] [PubMed] [Google Scholar]
- 23.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke 1999;30:1167–1173 [DOI] [PubMed] [Google Scholar]
- 24.Nagai H, Moritake K, Takaya M. Correlation between transcranial Doppler ultrasonography and regional cerebral blood flow in experimental intracranial hypertension. Stroke 1997;28:603–608 [DOI] [PubMed] [Google Scholar]
- 25.McQuire JC, Sutcliffe JC, Coats TJ. Early changes in middle cerebral artery blood flow velocity after head injury. J Neurosurg 1998;89:526–532 [DOI] [PubMed] [Google Scholar]
- 26.Homburg AM, Jakobsen M, Enevoldsen E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol Scand 1993;87:488–493 [DOI] [PubMed] [Google Scholar]
- 27.Newell DW, Aaslid R, Stooss R, Seiler RW, Reulen HJ. Evaluation of hemodynamic responses in head injured patients with transcranial Doppler monitoring. Acta Neurochir 1997;139:804–817 [DOI] [PubMed] [Google Scholar]
- 28.Shiogai T, Sato E, Tokitsu M, Hara M, Takeuchi K. Transcranial Doppler monitoring in severe brain damage: relationships between intracranial hemodynamics, brain dysfunction and outcome. Neurol Res 1990;12:205–213 [DOI] [PubMed] [Google Scholar]
- 29.Chan KH, Miller JD, Dearden NM, Andrews PJD, Midgley S. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg 1992;77:55–61 [DOI] [PubMed] [Google Scholar]
- 30.Chan K-H, Dearden NM, Miller JD, Andrews PJD, Midgley S. Multimodality monitoring as a guide to treatment of intracranial hypertension after severe brain injury. Neurosurgery 1993;32:547–553 [DOI] [PubMed] [Google Scholar]
- 31.Treib J, Becker SC, Grauer MT, Haass A. Transcranial Doppler Monitoring of intracranial pressure therapy with mannitol, sorbitol and glycerol in patients with acute stroke. Eur Neurol 1998;40:212–219 [DOI] [PubMed] [Google Scholar]
- 32.Mayer SA, Thomas CE, Diamond BE. Asymmetry of intracranial hemodynamics as an indicator of mass effect in acute intracerebral hemorrhage: a Transcranial Doppler study. Stroke 1996;27:1788–1792 [DOI] [PubMed] [Google Scholar]
- 33.Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology 2001;57:18–24 [DOI] [PubMed] [Google Scholar]
- 34.Lee EJ, Chio CC, Lin HJ, Yang LH, Chen HH. Application of transcranial Doppler sonography in surgical aspects of hypertensive putaminal haemorrhage. Acta Neurochir 1996;138:60–67 [DOI] [PubMed] [Google Scholar]