Abstract
BACKGROUND AND PURPOSE: Brain has a capacity for reorganization that enables use-dependent adaptations to acquire skills. Previous studies demonstrated morphometric and functional use-dependent changes in the brains of musicians. The purpose of this study was to investigate the differences in metabolite concentrations in the planum temporale, an area strongly associated with the processing of music perception, between trained musicians and non-musicians. We hypothesized that the microscopic changes leading to use-dependent adaptations in brain might cause neurometabolite changes that could be detected with quantitative proton MR spectroscopy.
METHODS: We performed quantitative proton MR spectroscopy in the left planum temporale of 10 musicians (six men and four women; age range, 20–37 years) and in those of 10 age- and sex-matched control subjects who had no musical training. We calculated the major metabolite concentrations in the left planum temporale.
RESULTS: The difference in N-acetylaspartate (NAA) concentrations between the musicians and the non-musician control subjects was statistically significant (P < .01). No significant difference was noted in the choline and creatine concentrations between the musicians and the non-musician control subjects (P > .05). The NAA concentration of the musicians correlated with the total duration of musical training and activity (r = 0.733, P < .05).
CONCLUSION: Long-term, professional musical activity caused significant changes in the neurometabolite concentrations that might reflect the physiologic mechanism(s) of use-dependent adaptation in the brains of musicians.
Brain is an organ that has a high capability of functional reorganization and plasticity. Cortical representation of body parts in the brain is continuously modulated in response to activity, behavior, and skill acquisition (1). After central nervous system (CNS) injury, the plasticity of brain helps with functional recovery of body parts that were previously represented by injured areas of CNS. Functions that were previously performed by damaged regions can be taken over by neighboring areas of brain. Functional reorganization of brain enables not only functional recovery of organs after injury, but also use-dependent adaptation and skill acquirement (2–4).
Music production and perception is a fascinating cognitive function of human brain. Different areas of brain have been reported to be involved in the processing of different components of music perception (5–9). The posterior part of the superior temporal gyrus, especially planum temporale, is an important area for the processing of pitch perception (6, 7). Morphometric MR studies showed a left-sided asymmetry in the planum temporale of musicians compared with non-musicians. The volume and surface area of the left planum temporale in trained musicians, especially those with absolute pitch (an ability to identify a tone in the absence of a reference tone), showed stronger left-sided asymmetry (10–12). Hutchinson et al (13) reported larger cerebellar volumes in musicians compared with those volumes in non-musician control subjects. Also, the cerebellar volumes in musicians correlated with the lifelong intensity of musical performances. Previous functional studies demonstrated enhanced and larger cortical representations of music perception and production in musicians (2, 14). The results of these functional and morphometric studies suggest the presence of use-dependent adaptations in the brains of musicians, which are induced by long-term training and performances.
The morphometric changes reported in the previous studies have been suggested to reflect histologic changes developed in the progression of use-dependent adaptations. The exact mechanism of use-dependent adaptation in brain, to our knowledge, has not yet been defined. In vivo proton MR spectroscopy is a noninvasive method to investigate the changes of metabolite concentrations in brain tissue. N-acetylaspartate (NAA), creatine (Cr), and choline (Cho) are the major metabolites in brain and can be detected with MR spectroscopy. The changes in concentrations of these metabolites represent a variety of histopathologic changes in brain tissue. The purpose of this study was to investigate the differences in metabolite concentrations in the planum temporale, an area strongly associated with the processing of music perception, between trained musicians and non-musicians. We hypothesized that quantitative proton MR spectroscopy would enable the detection of metabolite changes that reflect structural and functional reorganization induced by intense musical training and performances.
Methods
Subjects
We studied 10 right-handed, professionally trained musicians (six men and four women). The musicians were the staff and postgraduate students of a music academy. The mean age of the musicians was 25.2 years (range, 20–37 years). Detailed information about their professional history including the age at which they commenced musical training, total (lifetime) duration of time spent during musical training and musical activities, and time spent for all professional musical activities each week for the previous 6 months. The mean age at which musical training commenced was 6.4 years (range, 5–8 years). The mean total duration of musical training and activity was 18.2 years (range 13–28 years). On average, they spent 22.2 hours for musical performance per week (range, 14–36 hours). The musical performances included instrument playing, concert performances, and composition. Ten age- and sex-matched healthy, right-handed control subjects (six men and four women) were included in the study. The control subjects were chosen among the staff and postgraduate students of medical, economic, and engineering schools of the university. The control subjects did not have formal musical training and could not play any musical instrument. The presence of any metabolic, infectious, or inflammatory neurologic disease in both groups was excluded by detailed clinical history and neurologic examination. Handedness of both the musicians and the control subjects was assessed by using the Edinburgh handedness inventory.
Written informed consent was obtained from all the subjects before the MR examinations, and the study was approved by the local human subjects committee.
Proton MR spectroscopy
MR imaging and single-voxel proton MR spectroscopic studies were performed with a 1.5-T superconducting whole-body MR imaging and spectroscopic system (Symphony; Siemens Medical Systems, Erlangen, Germany) by using a quadrature head coil. Motion artifacts were minimized by supporting the head with foam cushions.
Anatomic images were acquired before MR spectroscopy and included sagittal, axial, and coronal T1-weighted sequences. Sagittal T1-weighted images (530/20/3 [TR/TE/NEX]) were obtained with a section thickness of 3 mm and a intersection distance of 0.3 mm. Coronal T1-weighted images (530/20/3) were acquired perpendicular to the plane of the sylvian fissure with a section thickness of 2 mm and intersection distance of 0.2 mm. Axial T1-weighted anatomic images (530/20/3) were acquired parallel to the plane of the sylvian fissure with a section thickeness of 3 mm and a intersection distance of 0.3 mm. The fields of view used for the anatomic images were 225–220 × 200–180 mm.
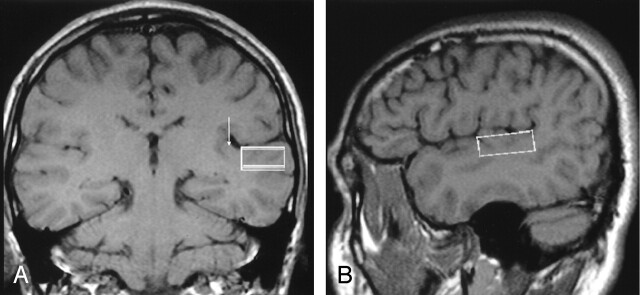
In the single-voxel proton MR spectrocopic examinations, the voxels were placed in the cortex and subcortical white matter of the planum temporale by using the T1-weighted anatomic images (Fig 1). The planum temporale was defined by criteria reported by Shapleske et al (15). The posterior border of the planum temporale was defined as the point where the posterior descending ramus of the sylvian fissure flips into the ascending ramus on the sagittal anatomic images. The lateral border of the planum temporale was defined as the lateral rim of the superior temporal plane on coronal images. Although the subcortical white matter of the superior temporal gyrus was involved in the voxel, cortex of the superior temporal gyrus was not included. The anterior border was defined as the bottom of the Heschl sulcus on sagittal, axial, and coronal images. In the cases with more than one Heschl sulcus, we included the additional Heschl gyrus into the planum temporale. The medial border was defined as the retroinsular origin of the Heschl gyrus on coronal and sagittal images. Voxel sizes ranged from 7.13 cm3 (36 × 18 × 11 mm) to 4.95 cm3 (30 × 15 × 11 mm). Quantification of proton MR spectroscopy was performed by the internal-water standard method (16). An automated shimming algorithm was used before water suppression to maximize the homogeneity of the magnetic field within the voxel of interest. Localization of both water-suppressed and unsuppressed proton spectra was performed by using a stimulated echo acqusition mode, or STEAM, sequence. Water suppression was performed with three chemical shift selective saturation, or CHESS, pulses at the water resonance. The numbers of acqusitions for water-suppressed and unsuppressed spectra were 246 and 16 times, respectively. A total of 1024 points were sampled. To correct the signal decays caused by T2 (transverse) relaxations, T2 values of the metabolites had to be calculated. For T2 corrections, we obtained proton spectra with TE values of 30, 48, 84, 135, and 230 ms. Spectral postprocessing included zero filling to 2056 points, Fourier transformation, and zero-order phase and baseline corrections. The MR system manufacturer’s software package was used for curve fitting and measurement of peak integrals. Curve fittings were performed by an experienced neuroradiologist (K.A.) and a biomedical engineer (K.C.) together. Major metabolite peaks were assigned to NAA at 2.02 ppm, Cr at 3.02 ppm, and Cho at 3.22 ppm.
Fig 1.
T1-weighted MR images (530/20/3) depict examples of voxel placement.
A, Coronal MR image shows a voxel placed in the left planum temporale, which is located between the Heschl gyrus medially (arrow) and superior rim of the superior temporal gyrus.
B, Sagittal T1-weighted image shows location of the voxel.
Calculating T2 values and T2-corrected signal amplitudes for water-suppressed and unsuppressed spectra is an optimization problem that involves minimizing the squared error between the actual values of the measurement and the estimated function values evaluated at measurement points. The routines of MATLAB software (The Mathworks Inc., Sherborn, MA) were used for finding optimum model parameters. A monoexponential curve was fit to the metabolite spectra (met) by the following equation:
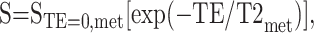 |
where STE=0,met is the signal amplitude at TE = 0. STE=0,met and T2met were calculated by using the Levenberg-Marquardt algorithm. For unsuppressed water spectra, CSF contamination was measured by using the difference in T2 decay between the brain tissue and CSF. The following equation was used for double-exponential curve fitting:
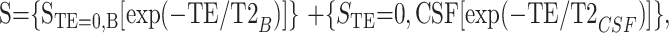 |
where STE=0,B and STE=0,CSF are the signal amplitudes at TE = 0 from brain water and CSF, respectively. During curve fitting, T2CSF was set at 800 ms and T2B was allowed to vary. So, optimization was performed over three parameters: STE=0,B, STE=0,CSF, and T2B.
After the T2 corrections, metabolite concentrations were calculated by using the following equation:
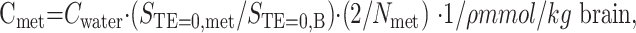 |
where Cmet and Cwater (55 × 103 mmol/L) are the concentrations of the metabolite and pure water, respectively. STE = 0,met and STE = 0,B are amplitudes of T2 corrected signals (signal amplitudes at TE = 0) obtained from the metabolite and brain water, respectively. Nmet is the number of protons contributing to the metabolite peaks. ρ represents the specific gravity of the brain tissue (ρ = 1.04 kg/L) (17). It was reported that T1 (longtidunal) relaxation did not affect the metabolite concentrations obtained with TR values above 3 seconds. Therefore, we used TR = 3.5 seconds and did not do T1 correction.
We performed phantom studies to demonstrate the reproducibility of the spectral quantification. We used a spherical phantom containing 0.1 mol/L sodium acetate and 0.1 mol/L lactate (Fig 2A). We measured acetate concentration with the internal water reference method by using the same TR and TE values (Fig 2B). We repeated the measurements on different days.
Fig 2.
A, Image shows voxel placement for the phantom study.
B, Spectrum obtained from the phantom shows the acetate peak and lactate doublet.
Data Analysis
We tested the difference in the metabolite concentrations between the musicians and the non-musician control subjects by using the Student t test (independent samples). We tested the association of the metabolite concentrations with the age of commencement of musical training, duration of total musical training and activity, and time spent for musical activities per week, by using linear regression analysis. The differences in metabolite concentrations between men and women in both the musician and the non-musician groups were tested by using the Student t test. A one-sample t test was used to test the measured concentrations in the phantom studies. A P value less than .05 was selected to indicate a statistically significance difference. All statistics in our study were calculated by using SPSS 11.0 for Microsoft Windows (SPSS Inc., Chicago, IL).
Results
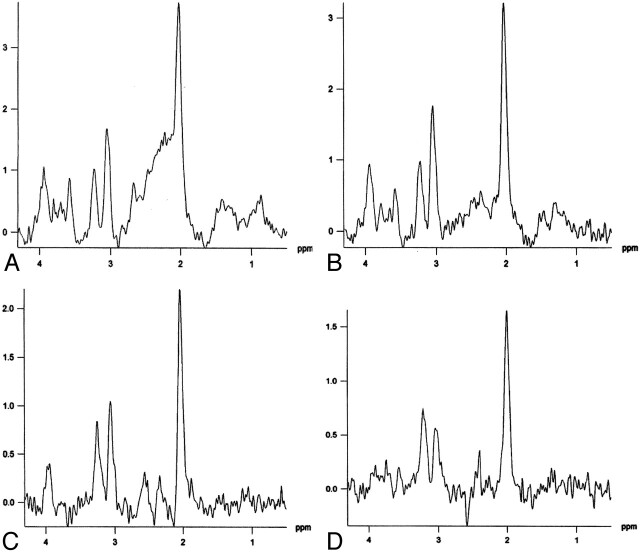
The anatomic MR images revealed no abnormality in any of the subjects. All spectra obtained from the musicians and control subjects were deemed to be of good quality (Figs 3 and 4). The calculated T2 values of brain tissue water (mean T2 value = 81.6 ms) were similar to those of the previous studies in literature. The calculated metabolite concentrations and professional information of the musicians are given in Table 1.
Fig 3.
A–D, Proton spectra from the planum temporale in a musician, obtained with TE values of 30 ms (A), 48 ms (B), 135 ms (C), and 230 ms (D).
Fig 4.
A–D, Proton spectra from the planum temporale in a non-musician control subject, obtained with TE values of 30 ms (A), 48 ms (B), 135 ms (C), and 230 ms (D).
TABLE 1:
Personal information and metabolite concentrations of the musicians
| Musician No./Age (y)/Sex | Age (y) at Commencement of Musical Training | Total Duration of Musical Training | Time Spent on Musical Activity per week (h) | Metabolite Concentration (mmol/kg brain) |
||
|---|---|---|---|---|---|---|
| NAA | Cr | Cho | ||||
| 1/20/F | 6 | 14 | 18 | 14.52 | 6.92 | 1.27 |
| 2/37/M | 7 | 28 | 16 | 15.58 | 7.39 | 1.42 |
| 3/22/F | 7 | 15 | 22 | 12.78 | 7.67 | 1.14 |
| 4/24/M | 5 | 19 | 24 | 13.34 | 5.32 | 1.14 |
| 5/33/M | 7 | 24 | 20 | 15.86 | 6.33 | 0.97 |
| 6/23/F | 5 | 18 | 14 | 15.22 | 5.91 | 1.04 |
| 7/24/M | 6 | 16 | 28 | 13.82 | 8.35 | 1.24 |
| 8/21/F | 8 | 13 | 24 | 11.26 | 7.46 | 0.94 |
| 9/23/M | 6 | 17 | 36 | 13.72 | 5.34 | 1.34 |
| 10/25/M | 7 | 18 | 20 | 13.91 | 6.96 | 1.32 |
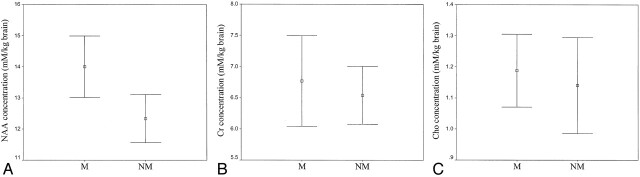
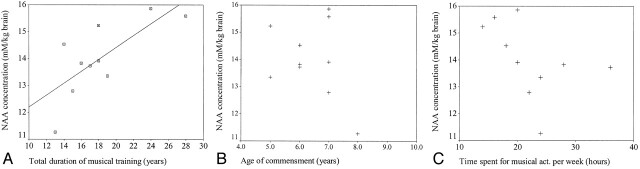
The mean NAA concentration in the left planum temporale of the musicians was 14.00 mmol/kg brain (range, 15.86–11.26 mmol/kg brain) compared with a value of 12.33 mmol/kg brain (range, 14.09–10.79 mmol/kg brain) in the control subjects (Table 2) (Fig 5). The difference in NAA concentrations between the musicians and the non-musician control subjects was statistically significant (P < .01). The calculated NAA concentrations of the musicians were associated with total duration of musical training and activity (r = 0.733, P = .016) (Fig 6). However, no significant association was noted between NAA concentrations and age of commencement (r = −0.463, P = .178) or time spent for musical activities per week (r = −0.327, P = .357) (Fig 6).
TABLE 2:
Metabolite concentrations of study participants
| Metabolite | Metabolite Concentrations (mmol/kg brain) |
P Value | |
|---|---|---|---|
| Musicians | Nonmusicians | ||
| NAA | 14.00 ± 1.38 | 12.33 ± 1.08 | .008 |
| Cr | 6.76 ± 1.01 | 6.54 ± 0.65 | .559 |
| Cho | 1.18 ± 0.16 | 1.14 ± 0.21 | .583 |
Note.—Data are mean ± standard deviation.
Fig 5.
A–C, Graphs show metabolite concentrations in musicians (M) and the non-musician control group (NM) for NAA concentration (A), Cr concentration (B), and Cho concentration (C). Error bars show 95% confidence intervals for the means of the metabolite concentrations.
Fig 6.
A, Graph demonstrates the relationship between NAA concentration in the musicians and total duration of musical training and activity. NAA concentration increases significantly as the duration of total musical training and activity increases (r = 0.733 [Pearson correlation coefficient], P = .016).
B, NAA concentration aganist age of commencement (r = −0.463, P = .178).
C, NAA concentration aganist time spent for musical activities per week (r = −0.327, P = .357).
The mean Cr concentration of the musicians was 6.76 mmol/kg brain (range, 5.32–8.35 mmol/kg brain) compared with 6.54 mmol/100 g brain (range, 5.46–7.33 mmol/kg brain) in the control subjects. The increase in Cr concentrations of the musicians did not reach statistical significance (P > .05). No significant association was noted between Cr levels of the musicians and age of commencement (r = 0.563, P = .09), total duration of musical training and activity (r = −0.150, P = .68), or time spent for musical performances per week (r = −0.155, P = .669).
The mean Cho concentration of the musicians was 1.18 mmol/kg brain (range, 0.94–1.42 mmol/kg brain) compared with 1.14 mmol/kg brain (range, 0.86–1.48 mmol/kg brain) in the control subjects. No significant difference was noted between the Cho concentrations of musicians and that of control subjects (P > .05). No significant association was noted between the Cho concentrations in the musicians and age of commencement (r = -0.116, P = .751), total duration of musical training and activity (r = 0.270, P = .451), and time spent for musical activities per week (r = 0.159, P = .662).
No significant difference was noted in metabolite concentrations between women and men in both the musician and non-musician groups (P > .05).
In the phantom studies, the mean value of the measured acetate concentration was 0.1 ± 0.005 mol/L. The results of the phantom studies demonstrated that there was no significant difference between the measured values and the real acetate concentration.
Discussion
Brain has a capacity for functional and structural reorganization, known as plasticity, that allows it to adapt to changing intrinsic or environmental conditions. Owing to this ability, functional recovery after an injury to the CNS is possible, to some extent. Function(s) of the injured area of brain may be taken over by the adjacent cortical areas, which can lead to the functional recovery. Cortical reorganization might also have a role in recovery after a peripheral injury, such as amputation. After the amputation of an extremity, the cortical areas representing the motor functions of the muscles adjacent to the amputation invade the cortical areas previously representing the amputation, to improve the motor control of the stump and compensate for the loss of the extremity.
The reorganization capacity of brain not only helps with functional recovery and compensation after CNS or peripheral nervous system injuries, but also causes the use-dependent adaptations in brain, which help in acquiring behavioral skills. Use-dependent adaptation causes not only functional but also structural changes in brain (3, 18). Maguire et al (3) reported that taxi drivers had larger volumes of gray matter in their right posterior hippocampus, which was shown to be the center of spatial memory and navigation in previous studies, compared with the healthy control subjects who did not drive taxis. The results of their study revealed that the volume of the right posterior hippocampus of the taxi drivers correlated with time spent as a taxi driver. Maguire et al proposed that the need for storage of detailed and extensive navigation information led to internal reorganization in the posterior hippocampus of the taxi drivers. Similar use-dependent structural and functional adaptations in the brains of musicians have been reported. In a voxel-based MR morphometry study, Sluming et al (19) reported increased gray matter density and volume in the Broca area of musicians compared with IQ-matched non-musicians. There was a significant correlation between gray matter density in the Broca area of the musicians and number of years of instrument playing. They interpreted this result of their study as the use-dependent adaptation caused by the musical training and professional activities. In another study (20), the gray matter volume in the Heschl gyrus of professional musicians was 130% larger than that of non-musicians. In a magnetoencephalographic study, Pantev et al (14) reported larger cortical reorganization in the recognition of tones among musicians who began their musical training before the age of 9 years. The results of all these functional and morphometric studies about music perception in musicians indicated a use-dependent reorganization capacity of the brain in musicians.
Several studies have been conducted to investigate the neuronal networks involved in processing musical perception (5–7). The Heschl gyrus, planum temporale, planum polare, superior temporal gyrus, inferior frontal gyrus, and dorsolateral prefrontal area have been reported to process musical perception. The perceptions of different components of music are processed in different regions of brain (6). The location of processing music perception also changes with musical training. Music perception is processed in the left hemisphere in musicians, whereas non-musicians show a right hemispheric dominance (7, 21, 22). In the previous neuroimaging studies, left planum temporale was reported to be the area that was responsible for pitch processing in musicians (6). Absolute pitch is the ability to identify the pitch of a tone without the need for a reference tone. Absolute pitch is associated with beginning musical training at an early age. Sergeant (23) reported that musicians with absolute pitch started their musical training before the age of 7 years. In an MR morphometry study conducted by Schlaug et al (10), the planum temporale in right-handed musicians was lateralized to the left compared with that in right-handed non-musicians. The degree of asymmetry between the left and right planum temporale of musicians showed a correlation with the ability of absolute pitch. Musicians with perfect pitch discrimination had stronger left planum temporale asymmetry (11, 12). The degree of activation in the left planum temporale correlated with absolute pitch ability (7). Ohnishi et al (7) also reported that the activation in the left planum temporale during music perception was enhanced in musicians compared with that in non-musicians. Also, a significant correlation existed between the degree of activation in the left planum temporale of musicians and age of commencement of musical training. Because use-dependent functional and structural adaptations have been demonstrated in previous studies, we studied the left planum temporale of musicians to investigate the possible metabolite changes induced by professional musical training and activities.
Proton MR spectroscopy is a sensitive, noninvasive, in vivo method used in the detection of metabolite changes. It gives clues about the histopathologic changes in tissues. In our study, NAA concentration in the left planum temporale of the musicians was significantly increased compared with that in the non-musicians. The correlation of NAA concentration with total duration of musical training and activity is compatible with the results of previous functional and morphometric studies about the adaptational changes in the brains of musicians. This correlation suggests that increased NAA concentration in the left planum temporale reflects the functional and structural changes stimulated by the long-lasting, intensive musical training. Nonassociation of NAA concentrations with the time spent for musical activities per week suggested that the changes in NAA levels were not developed in the short term. In a commentary article about the study conducted by Maguire et al (3), Terrazas and McNaughton (24) argued that morphometric MR imaging studies could not reflect the subtle cellular changes leading to brain adaptation. Changes in metabolite concentrations may be a more sensitive and precise tool to follow the trace of cellular changes in the process of brain adaptation.
NAA is a free amino acid that is biosynthesized from acetyl coenzyme A and aspartate by D-aspartate N-acetyltransferase in the mitochondria of neurons (25). NAA is expected as a marker of neuroaxonal integrity in proton MR spectroscopy (26, 27). The exact function of NAA is not known, yet. However, NAA is known to be involved in the intercellular communication of the neurons with glial cells (28). Abnormalities that cause neuronal loss or impairment of neuroaxonal functions decrease the level of NAA at proton MR spectroscopy. In neurodegenerative diseases, an association exists between the degree of neuronal loss and level of NAA at proton MR spectroscopy. In a recent study, a correlation between neuronal density and NAA concentration was demonstrated (29). In our study, NAA concentrations of musicians were not associated with time spent for musical activities per week, although the association of NAA concentration with total duration of musical training and activity was significant. These two findings demonstrate that increased NAA levels in musicians represent a long-term process induced by musical training. In our study, increased NAA concentration might be caused by increased neuronal density in the left planum temporale of musicians.
Until recently, it was believed that neuronal proliferation in brain was not possible after birth. However, neurogenesis in adult hippocampus has been demonstrated (30, 31). Kempermann et al (31) reported the experience-dependent regulation of adult neurogenesis in the hippocampi of mice. In an animal study conducted by Nillson et al (32), it was shown that an enriched environment increased the neurogenesis in the dentate gyrus. The possibility of neurogenesis in the adult brain is not restricted to the hippocampi (33, 34). Palmer et al (35) reported the presence of multipotent precursor cells in the various regions of adult rat brains that could differentiate into neurons. Today, it is known that human brain has neuron-generating capacity. All the musicians in our study had started musical training before 9 years of age. Intensive musical training and performances during the developmental periods of brains in the musicians might stimulate neurogenesis by acting as an “environment enriching activity.”
Kempermann (36) proposed that the addition of even small numbers of new neurons can dramatically increase the complexity of a neuronal network to adapt to increased functional stimuli. Maguire et al (3) suggest that the increased cortical volume in the right posterior hippocampus of taxi drivers might have been caused by neurogenesis that might be induced by a high need for spatial memory. Similarly, a long-term need to process the pitch perception of highly complex musical performances may induce neurogenesis in the planum temporale of musicians. Addition of new neurons may increase the complexity of neuronal networks and make the processing of highly complex data possible. Cecchi et al (37) proposed a mathematics model to explain the role of neurogenesis and incorporation of new neurons into the synaptic networks in experience-induced adaptations.
Neural branching and formation of new synapses are associated with long-term uses in many species. Long-term sensory stimulation causes increased numbers of central synapses that can modify the sensitivity of sensory perception (38, 39). Increased number of synapses, glial cells, and capillary density occur after long-term motor learning in the motor cortex (40–43). Increased number of synapses, which is one of the mechanisms proposed to explain the use-dependent adaptations, might contribute to the increased NAA concentrations in our study. The musical experience beginning in early childhood might have led to the formation of new synapses in the left planum temporale of musicians, to be able to process highly complex data during music perception.
Modulation of synaptic transmission is another theory proposed to explain the mechanisms of brain reorganization and adaptation (44, 45). Intracortical γ-aminobutyric acid (GABA)ergic inhibitory neurons have been reported to play a role in the plasticity and the exercise-induced rapid adaptations of brain. In an MR spectroscopy study, Levy et al (46) showed rapid reduction of GABA levels in the motor cortex within 10 minutes after ischemic deafferentation of the contralateral hand. GABAergic mechanism has been reported to be rapid and short-term, and it cannot explain the morphometric changes induced by functional stimuli, which were reported in the previous studies. Because the resolution of minor metabolites such as GABA is low with 1.5-T MR systems, we could not study the effects of musical training on GABA concentration in the planum temporale.
Because of the long examination times, we could not study the metabolite concentrations in other cortical areas not associated with music perception. This can be accepted as a limitation of our study. One may argue about the possibility of significant differences in metabolite concentrations of non-music-associated cortical areas of musicians and those of the control subjects. However, metabolite mapping of musical perception in the brain was not the aim of this study. The significant correlation of metabolite concentrations with total duration of musical training has demonstrated that long-term, intensive musical training and activities induce significant changes in the concentrations of neurometabolites, whether specific to the left planum temporale or not.
Significant associations between age of commencement of musical training and morphometric measurements in the brains of musicians were reported in the previous studies. All the musicians in our study had started musical training before 9 years of age. Because the ages of commencement were not wide spread among the musicians, we did not find any association between metabolite concentrations and age of commencement in our study.
An auditory functional MR imaging study might be performed together with quantitative spectroscopy. Correlation of the neurometabolite changes with the results of the functional MR imaging study might be investigated. We could not perform an auditory functional MR imaging study because of the lack of technical background. However, the combination of these studies would increase examination time, which was already long enough.
Conclusion
Structural and morphometric changes in brain that are created by use-dependent adaptation and reorganization have been shown in previous studies. However, the results of our study demonstrated use-dependent changes in neurometabolite concentrations that might be created by the cellular changes developed in the process of brain adaptation. The increase of NAA concentration in regions of brain that show use-dependent adaptation may be created by the increase in neuronal or synaptic density.
References
- 1.Chen R, Cohen LG, Hallet M. Nervous system reorganization following injury. Neuroscience 2002;6:761–773 [DOI] [PubMed] [Google Scholar]
- 2.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science 1995;270:305–307 [DOI] [PubMed] [Google Scholar]
- 3.Maguire E, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A 2000;97:4398–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci 2003;23:9240–9245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platel H, Price C, Baron JC, et al. The structural components of music perception: a functional anatomical study. Brain 1997;120:229–243 [DOI] [PubMed] [Google Scholar]
- 6.Chauvel CL, Peretz I, Babai M, Laguitton V, Chauvel P. Contribution of different cortical areas in the temporal lobes to music processing. Brain 1998;121:1853–1867 [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi T, Matsuda H, Asada T, et al. Functional anatomy of musical perception in musicians. Cereb Cortex 2001;11:754–760 [DOI] [PubMed] [Google Scholar]
- 8.Janata P, Birk JL, Van Horn JD, et al. The cortical topography of tonal structures underlying Western music. Science 2002;298:2167–2170 [DOI] [PubMed] [Google Scholar]
- 9.Koelsch S, Gunter TC, Cramon DYV, et al. Bach speaks: a cortical “language -network” serves the processing of music. Neuroimage 2002;17:956–966 [PubMed] [Google Scholar]
- 10.Schlaug G, Jancke L, Huang Y, Steinmetz H. In vivo evidence of structural brain asymmetry in musicians. Science 1995;267:699–701 [DOI] [PubMed] [Google Scholar]
- 11.Zatorre RJ, Perry DW, Beckett CA, Westbury CF, Evans AC. Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci U S A 1998;95:3172–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan JP, Thangaraj V, Halpern AR, Schlaug G. Absolute pitch and planum temporale. Neuroimage 2001;14:1402–1408 [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson S, Lee LH, Gaab N, Schlaug G. Cerebellar volumes of musicians. Cereb Cortex 2003;13:943–949 [DOI] [PubMed] [Google Scholar]
- 14.Pantev C, Oostenveld R, Engelien A, et al. Increased cortical representation in musicians. Nature 1998;392:811–814 [DOI] [PubMed] [Google Scholar]
- 15.Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev 1999;29:26–49 [DOI] [PubMed] [Google Scholar]
- 16.Keevil SF, Barbiroli B, Brooks JCW, et al. Absolute metabolite quantification by in vivo NMR spectroscopy: multicenter trial of protocols for in vivo localised proton studies of human brain. Magn Reson Imaging 1998;16:1093–1106 [DOI] [PubMed] [Google Scholar]
- 17.Michaelis T, Merboldt KD, Bruhn H, et al. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology 1993;187:219–227 [DOI] [PubMed] [Google Scholar]
- 18.Zilles K. Neuronal plasticity as an adaptive property of the central nervous system. Ann Anat 1992;174:383–391 [DOI] [PubMed] [Google Scholar]
- 19.Sluming V, Barrick T, Howard M, et al. Voxel-based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage 2002;17:1613–1622 [DOI] [PubMed] [Google Scholar]
- 20.Schneider P, Scherg M, Dosch HG, et al. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 2002;5:688–694 [DOI] [PubMed] [Google Scholar]
- 21.Bevers TG, Chiarello RJ. Cerebral dominance in musicians and nonmusicians. Science 1974;9:537–539 [DOI] [PubMed] [Google Scholar]
- 22.Evers S, Dannert J, Rodding D, Rotter G, Ringelstein EB. The cerebral haemodynamics of music perception: a transcranial Doppler sonography study. Brain 1999;122:75–85 [DOI] [PubMed] [Google Scholar]
- 23.Sergeant D. Experimental investigation of absolute pitch. J Res Music Educ 1969;17:135–143 [Google Scholar]
- 24.Terrazas A, McNaughton B. Brain growth and the cognitive map. Proc Natl Acad Sci U S A 2000;25:4414–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neale JH, Bzdega T, Wroblewska B. N-acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 2000;75:443–452 [DOI] [PubMed] [Google Scholar]
- 26.Miller BL. A review of chemical issues in H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991;4:47–52 [DOI] [PubMed] [Google Scholar]
- 27.Simmons M, Frondoza C, Coyle J. Immunocytochemical localization of N-acetyl aspartate with monoclonal antibodies. Neuroscience 1991;45:37–45 [DOI] [PubMed] [Google Scholar]
- 28.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem 2000;75:453–459 [DOI] [PubMed] [Google Scholar]
- 29.Chenga LL, Newell K, Mallory AE, Hyman BT, Gonzalez RG. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging 2002;20:527–533 [DOI] [PubMed] [Google Scholar]
- 30.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 1998;18:3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kempermann G, Gast G, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 2002;52:135–143 [DOI] [PubMed] [Google Scholar]
- 32.Nillson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 1999;39:569–578 [DOI] [PubMed] [Google Scholar]
- 33.Kuhn HG, Palmer TD, Fuchs E. Adult neurogenesis: a compensatory mechanism for neuronal damage. Eur Arch Psychiatry Clin Neurosci 2001;251:152–158 [DOI] [PubMed] [Google Scholar]
- 34.Gage FH. Neurogenesis in the adult brain. J Neurosci 2002;22:612–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic programin neural stem cell from diverse regions of the adult CNS. J Neurosci 1999;19:8487–8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 2002b;22:635–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cecchi GA, Petreannu LT, Alvarez-Buylla A, Magnasco MO. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci 2001;11:175–182 [DOI] [PubMed] [Google Scholar]
- 38.Acebes A, Ferrus A. Increasing the number of synapses modifies olfactory perception in Drosophila. J Neurosci 2001;15:6264–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devaud JM, Acebes A, Ferrus A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci 2001;15:6274–6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black JE, Isaac KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis whereas motor activity causes angiogenesis in cerebellar cortex of the adult rats. Proc Natl Acad Sci U S A 1990;87:5568–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaacs KR, Anderson BJ, Alacantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 1992;12:110–119 [DOI] [PubMed] [Google Scholar]
- 42.Anderson BJ, Li X, Alcantara A, et al. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia 1994;11:73–80 [DOI] [PubMed] [Google Scholar]
- 43.Kleim JA, Lussing E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 1996;16:4529–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 1993;3:361–372 [DOI] [PubMed] [Google Scholar]
- 45.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 2001;124:1171–1181 [DOI] [PubMed] [Google Scholar]
- 46.Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol 2002;52:755–761 [DOI] [PubMed] [Google Scholar]