The anatomic bases for memory disorders have been widely debated. Lesions of the medial dorsal (MD) and anterior nuclei (AN) of the thalami and lesions of the mammillary bodies (MB) are most commonly involved in amnesic syndromes in humans (1, 2).
In a monkey model, Mishkin (3) conceptualized the existence of a double limbic circuitry supporting the memory function (i.e., the medial limbic [hippocampal] and the basolateral limbic [amygdaloid] circuits). In this functional scheme, the AN and the MD thalamic nuclei act as “nodal” points of convergence where bilateral tissue damage can result in memory function impairment, similar to bilateral lesions of the mammillothalamic tract (MTT), which connects the MB to the AN.
In an article in the June/July issue of the AJNR, Yoneoka et al (4) reported on the onset of a Korsakoff syndrome in a patient with acute ischemic damage of the left MTT assessed by positive diffusion-weighted imaging who had previously suffered from a contralateral right MTT mirror infarction. The authors suggested that bilateral MTT dysfunction may be sufficient to cause severe amnesic state, which matched Mishkin’s hypothesis. Berger highlighted this in an editorial of the same issue of the AJNR, stating that the article added one more site of the hippocampal-limbic system—namely, the MTT—from which bilateral lesion may result in amnesia (5).
The neurosurgical team at our institution has initiated a clinical trial aimed at treating patients with chronic refractory epilepsy by bilateral stereotactic implantation of stimulation electrodes (DBS lead 3389, Medtronic, Minneapolis, MN) within the MB through the longest part as possible of the proximal segment of the MTT (6). We therefore performed a preliminary in vivo anatomic study and derived biometric measurements of the MTT by 3D processing of unenhanced 3D T1-weighted spoiled gradients data in a normative cohort of nine healthy volunteers without structural abnormalities of the brain (7).
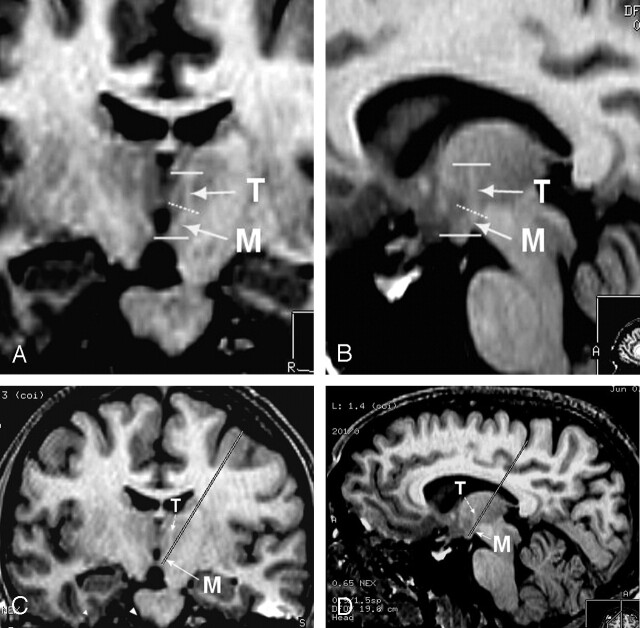
In light of the functional directionality of the MTT projections from the MB to the AN, we clearly delineated 1) a proximal segment, named the “mammillary” segment, which has posterior-cranial orientation in the sagittal plane and lateral-cranial one in the coronal plane, and 2) a distal “thalamic” segment, which has frontal and caudal-cranial orientation (Fig 1A, B). An empirical method using the 3D capabilities of the Advantage Windows, release 4.0, software (GEMS, Milwaukee, WI) running on an off-line Ultra 60 Creator 3D station (Sun Microsystems, Santa Clara, CA) was designed to define the cortical entry point combining targeting of the MB epicenter together with so-called catheterization of the longest possible part of the mammillary segment of the MTT. We obtained the entry point by superimposing the straight line connecting the MB to cortical surface on the longitudinal axis of the mammillary segment of the MTT. It was located in the posterior third of the middle frontal gyrus in the coronal plane (Fig 1C) and in the central sulcus or in the precentral gyrus when drawing the line in the sagittal plane (Fig 1D). The first option was chosen to avoid primary functional areas, and the value of this empirical approach was assessed postoperatively by coregistrating the pre- and postoperative images in the similar juxta/supra-MB section location in all the three patients being recruited up to now (Fig 2). A passage through the most proximal segment of the MTT—at least partially—was demonstrated for the six electrodes, which have four stimulation contacts, the distal one (numbered 0) being located within the MB, and the three proximal ones (numbered 1–3) within the MTT.
Fig 1.
Anatomic study on healthy volunteers (reformatted images from unenhanced 3D T1-weighted SPGR acquisition).
A, Coronal oblique reformat (magnified view) clearly depicts the two segments of the MTT on both sides. The presence of a proximal “mammillary” (M) and of a distal “thalamic” (T) segment separated by an angulation (dotted line) is clearly shown.
B, Sagittal oblique reformat also shows the two segments of the left MTT in an orthogonal plane relative to that in the previous illustration.
C, Electrode trajectory simulation on frontal oblique reformat (similar but demagnified view as 1A) shows “catheterization” of the mammillary segment of the MTT and safe cortical entry point located in the posterior third of the middle frontal gyrus.
D, Electrode trajectory simulation on sagittal oblique reformat (similar but demagnified view as in 1B) showing “catherization” of the proximal MTT, but critical central sulcus entry point.
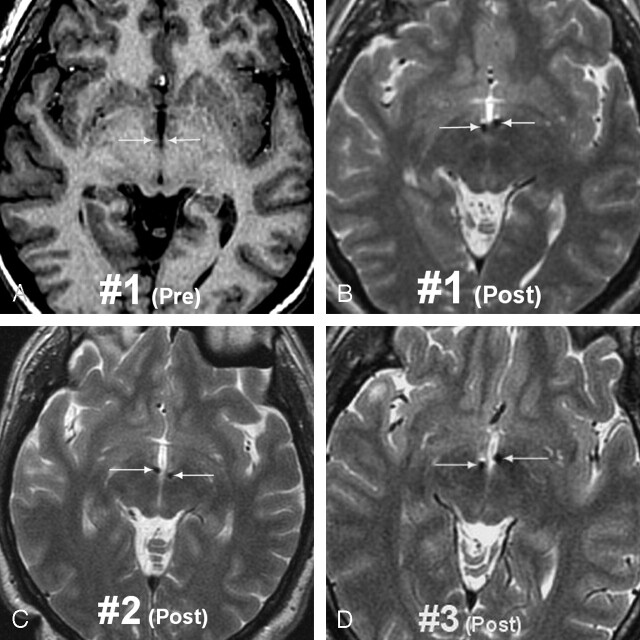
Fig 2.
MR images in the three bilaterally MB implanted patients
A, Preoperative status in patient 1 (T1-weighted 3D SPGR image) showing the mammillary segment of the MTT on both sides (arrows).
B, Postoperative image (T2-weighted 3D fast spin-echo [FSE] image) in patient 1 showing the electrodes through the mammillary segment (arrows). FSE T2 weighting was preferred to minimize susceptibility artifacts due to ferromagnetic components of the electrodes.
C and D, Postoperative status in patients 2 and 3 (T2-weighted 3D FSE images) also showing “catherization” of the mammillary segment of the MTTs in both cases.
None of the three patients experienced any memory deficit, neither immediately after surgical implantation, nor during global or elective stimulations (left side versus right side versus both sides; 0, 1, 2, or 3 only, versus any combination of the four stimulation contacts), which were performed under close neuropsychological monitoring. Additional comprehensive cognitive tests were repeatedly performed, all of which failed to reveal any early or delayed mental decline after implantation.
These data demonstrate the absence of significant memory dysfunction induced by bilateral MB/MTT implantation and electrical stimulation. They agree with the recent experimental work by Vann and Aggleton (8) on a rat model, and with Harding et al (9) on alcoholics with and those without Korsakoff psychosis, who demonstrated that AN degeneration or lesions by far more critically impaired memory than MB or MTT lesions.
The disconnection of the AN from its MB projections through the MTT (the so-called Delay and Brion connection) does not impair the direct fornical route from the hippocampus to the AN. This alternate pathway may explain why MB or MTT lesions, even if bilateral, are not as disruptive as AN lesions. Korsakoff syndrome in the patient reported by Yoneoka et al (4) could have been triggered by bilateral ischemic damage not limited to the MTTs. The newly infarcted left area and the old right lesion seem to extend far beyond the MTTs to involve the anterior thalamic nuclei (Figs 1A–D and 2A, B, p. 965; and Figs 4A, B, p. 967 [AJNR; Vol 25 No 6]). In accordance with the theoretical statement by Vann and Aggleton in their recent review on the topic (10), we think the presence of concurrent anterior thalamic disease to have outweighed the MTT damage in that patient.
References
- 1.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome: a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser 1971;7:1–206 [PubMed] [Google Scholar]
- 2.Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res 1996;20:1489–1495 [DOI] [PubMed] [Google Scholar]
- 3.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 1978;273:297–298 [DOI] [PubMed] [Google Scholar]
- 4.Yoneoka Y, Takeda N, Inoue A, et al. Acute Korsakoff syndrome following mammillothalamic tract infarction. AJNR Am J Neuroradiol 2004;25:964–968 [PMC free article] [PubMed] [Google Scholar]
- 5.Berger JR. Memory and the mammillothalamic tract: editorial. AJNR Am J Neuroradiol 2004;25:906–907 [PMC free article] [PubMed] [Google Scholar]
- 6.Raftopoulos C, van Rijckevorsel K, Abu Serieh B, et al. Chronic electrical stimulation of the mammillary bodies and mammillothalamic tracts in chronic refractory epilepsy [Abstract]. 1st meeting of the Benelux Neuromodulation Society Chapter of the International Neuromodulation Society, November 21–22, 2003, Leuven, Belgium. Neuromodulation 2004;7:148 [Google Scholar]
- 7.Abu Serieh B, Duprez T, van Rijckevorsel K, Raftopoulos C. Anatomical study of mammillothalamic tract in humans using 3D T1-weighted SPGR MR imaging. Abstract, 16th Congress of the European Society for Stereotactic and Functional Neurosurgery. June 23–26, 2004, Vienna, Austria. Acta Neurochir (Wien) 146:878 ,2004 [Google Scholar]
- 8.Vann SD, Aggleton JP. Evidence of spatial encoding deficits in rats with lesions of the mammillary bodies or mammillothalamic tracts. J Neurosci 2003;23:3506–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 2000;123:141–154 [DOI] [PubMed] [Google Scholar]
- 10.Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev/Neurosci 2004;5:35–44 [DOI] [PubMed] [Google Scholar]