Abstract
BACKGROUND AND PURPOSE: Brain volume and diffusion change during maturation. Quantitation of these changes may be helpful in understanding normal brain development. We used diffusion-weighted imaging to characterize the volumetric and diffusion changes in vivo.
METHODS: We recruited 30 pediatric volunteers (aged 1 month–17 years; 14 male, 16 female). Diffusion was measured in three orthogonal directions with a b value of 1000 s/mm2. The diffusion parameters from the entire brain were calculated and fitted to a triple gaussian model. In addition, region-of-interest measurements were made in caudate, thalamus, genu and splenium of the corpus callosum, and periventricular white matter (PVWM). The brain volume was measured by counting pixels and by using the model.
RESULTS: Water diffusion of the whole brain, caudate, thalamus, genu and splenium of the corpus callosum, and PVWM decreased during maturation, with the most significant change within the first 2 years. Robust negative correlations were found between age and the measured average diffusion constant (Dav) values in each of the measured locations (P < .005). Volumes of different cerebral compartments and the total intracranial volume (ICV) increased rapidly during the first 2 years of life and then had a slower growth process through adolescence. Age was correlated with the ICV and the volume of each brain compartment (P < .005).
CONCLUSION: Brain diffusion decreases and brain volume increases during maturation, with the most significant changes occurring within the first 2 years of life. The brain model used in this study provides a good estimate of the increasing brain volume.
The human brain changes substantially during maturation. Previous studies have shown that cell numbers increase and water content decreases in the whole brain, forebrain, and brainstem during prenatal development to 8 years of age (1). The rapid decrease in water content is considered a good marker for brain development (1, 2). Quantifying these changes on MR imaging may improve our understanding of normal brain development. Disorders of metabolism or malnutrition may be especially vulnerable to impairment in water content; thus, this imaging may help in the early diagnosis of these diseases. Diffusion imaging is a relatively new technique that has been used to noninvasively measure brain water diffusivity in both white matter and gray matter during development (3–11). Previous diffusion imaging studies have shown that brain diffusion decreases during the first decade of life in both gray matter and white matter (4, 7) and that it remains relatively constant throughout adulthood (12).
Brain volume measurement is of neurologic and neuropsychiatric importance in evaluating disorders of development and degeneration (13, 14). It has been reported that human brain volume peaks at approximately 19 years of age and then declines by 1.3–1.6% per decade of life (14). The purpose of our study was to examine diffusion changes in the developing brain in conjunction with volume changes by measuring the diffusion parameters and fitting these to a brain model (4, 12). Intracranial volume (ICV) was calculated by counting pixels and by using the model.
Methods
Thirty children (aged 0.01–17 years; 14 male, 16 female) were included in this study. The subjects were either healthy volunteers or patients undergoing imaging for clinical reasons excluding the assessment of developmental delay. MR images of all subjects were normal. This group included 11 subjects from a previous study (4). The ages of the male and female subjects were not significantly different.
Imaging was performed with a 1.5-T commercial MR machine with a quadrature head coil. The imaging protocol included sagittal and axial T1-weighted images (TR/TE, 500/minimum); axial T2-weighted images (4000/102), axial fluid-attenuated inversion recovery images (TR/TE/TI, 10000/162/2200; matrix, 256 × 192), and diffusion-weighted images (TR/TE, 10,500/minimum; image matrix, 256 × 256; section thickness, 5 mm; field of view, 220 mm).
Diffusion was measured in three orthogonal directions with b = 1000 s/mm2. An additional set of images (So) was obtained with no diffusion weighting. An orientation-independent diffusion image related to the trace of the diffusion tensor was obtained as follows: DWItrace=3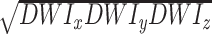 . Maps of the average diffusion constant (Dav) were calculated by using the DWItrace and So images by using this equation: Dav = (1/b)log(So/DWItrace).
. Maps of the average diffusion constant (Dav) were calculated by using the DWItrace and So images by using this equation: Dav = (1/b)log(So/DWItrace).
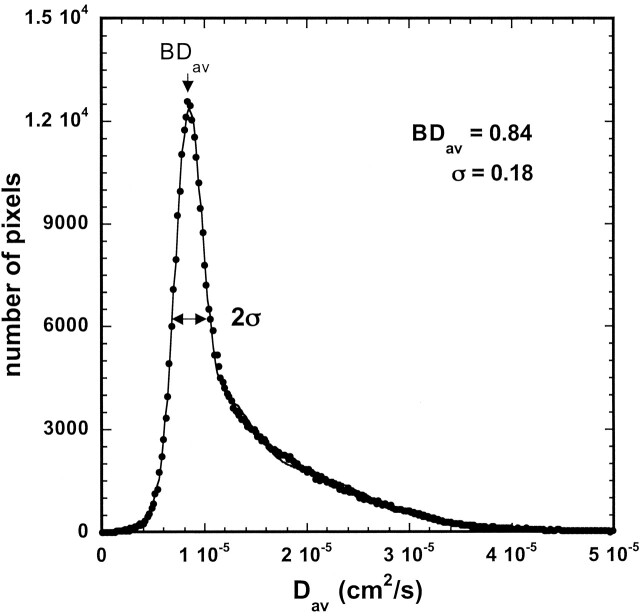
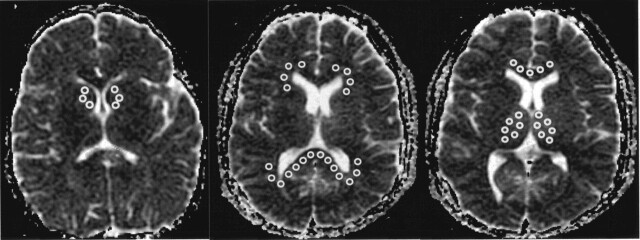
A computer program was used to calculate diffusion distribution histograms (Fig 1) with a bin width of 0.02 × 10−5 cm2/s. This histogram was fitted to a triple gaussian curve by using commercial software. This curve was given by C1exp{−[(Dav − BDav)/σ]2 } + C2exp{−[(Dav − D2)/σ2]2} + C3exp{−[(Dav − D3)/σ3]2}, where BDav is the diffusion constant for the whole brain. The curve represented a brain model with three compartments: 1) brain tissue compartment, 2) compartment of brain tissue mixed with CSF, 3) high-diffusion compartment of CSF and non-brain tissue. The mean of the brain tissue compartment was recognized as a mean diffusion constant for the whole brain BDav. The details of this model were reported previously (4, 10). A representative histogram fitted to the triple gaussian model is shown in Figure 1. Using the region-of-interest (ROI) analysis, we also measured Dav in the bilateral caudate, thalamus, genu and splenium of the corpus callosum, and periventricular white matter (PVWM) (Fig 2).
Fig 1.
Representative diffusion distribution histogram in a 3.5-year-old girl with triple gaussian fit. BDav = 0.839 10−5cm2/s, σ = 0.183 10−5cm2/s.
Fig 2.
Locations of ROIs in a 2-year-old boy. Round ROIs were placed on Dav maps to measure the diffusion constants of the PVWM, caudate, thalamus, and genu and splenium of the corpus callosum in all subjects.
For each subject, total ICV was calculated by multiplying the individual pixel volume by the number of pixels in the brain. The brain model was also used to calculate the individual brain compartments by analytically integrating the model function for each of the three compartments. The volume of each compartment was Ciσi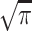 multiplied by the pixel volume. In this way, the volume of different brain compartments could be calculated without segmenting the images. The measured diffusion parameters were plotted against age and fitted to a double exponential curve: m1exp(−m2age) + m3exp(−m4age) + m5, where mi are the fitted parameters. The measured ICV and compartmental volumes were plotted against age.
multiplied by the pixel volume. In this way, the volume of different brain compartments could be calculated without segmenting the images. The measured diffusion parameters were plotted against age and fitted to a double exponential curve: m1exp(−m2age) + m3exp(−m4age) + m5, where mi are the fitted parameters. The measured ICV and compartmental volumes were plotted against age.
Pearson product correlation and the Student t test were used for statistical analysis. P < .05 was used as the significance level.
Results
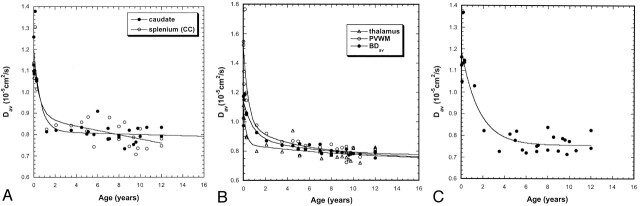
The average Dav values measured in the entire population are summarized in Table 1. Water diffusion of the whole brain (BDav), caudate, thalamus, genu, splenium of the corpus callosum, and PVWM decreased during maturation, with the most significant change within the first 2 years (Table 2, Fig 3). Robust negative correlations were found between age and measured Dav values in all of the anatomic locations evaluated (P < .01).
TABLE 1:
Mean diffusion measurements in all subjects
| Location | Measurement (10−5 cm2/s) |
|---|---|
| Caudate | 0.885 ± 0.16 |
| Thalamus | 0.844 ± 0.11 |
| Corpus callosum | |
| Genu | 0.870 ± 0.18 |
| Splenium | 0.880 ± 0.15 |
| PVWM | 0.952 ± 0.27 |
| Whole brain (BDav) | 0.880± 0.14 |
Note.—Data are the mean ± standard deviation.
TABLE 2:
Age-dependent diffusion changes for the study subjects
| Location | Dav (10−5 cm2/second) | R |
|---|---|---|
| Thalamus | Dav = 0.239 exp(−4.072 × age) + 0.0123 exp(−0.0821 × age) + 0.724 | 0.920 |
| PVWM | Dav = 0.527 exp(−2.301 × age) + 0.212 exp(−0.221 × age) + 0.771 | 0.942 |
| Whole brain (BDav) | BDav = 0.251 exp(−1.139 × age) + 0.157 exp(−0.102 × age) + 0.732 | 0.959 |
| Caudate | Dav = 0.241 exp(−1.583 × age) + 0.201 exp(−0.243 × age) + 0.704 | 0.921 |
| Corpus callosum | ||
| Genu* | Dav = 0.431 exp(−0.608 × age) + 0.748 | 0.912 |
| Splenium | Dav = 0.398 exp(−1.490 × age) + 0.061 exp(−0.0209 × age) + 0.743 | 0.929 |
Note.—Subjects’ age range was 0.01–17 years.
Single exponential decay describes the age dependency of diffusion changes in the genu of the corpus callosum.
Fig 3.
Biexponential curves of Dav versus age.
A, Caudate and splenium of the corpus callosum.
B, Thalamus, PVWM, and whole brain (BDav).
C, Data for the genu are fitted by using a single exponential curve. Brain diffusion decreases fastest in the first 2 years, with slower changes afterward.
When the subjects were grouped and compared according to sex, no diffusion difference were observed between boys and girls, and no age difference was noted between the groups.
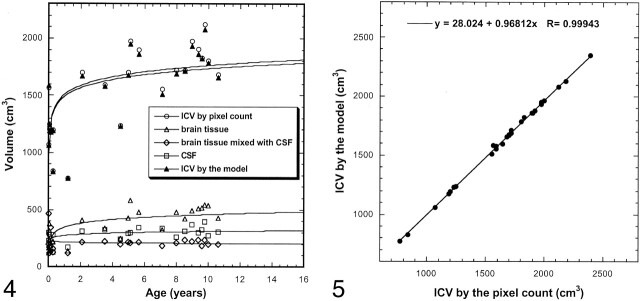
The average ICV measured by means of pixel counting and the brain model was 1639 and 1614 cm3, respectively (Table 3). Volume changes during maturation were fitted to a logarithmic function. The volumes of different cerebral compartments and total ICV increased rapidly during the first 2 years of life; this change was followed by a slower growing process through adolescence (Table 4, Fig 4). A robust positive correlation was found between the volume measurement based on a simple pixel count and the volume calculated from the brain model (R = 0.999, P < .001) (Fig 5). Age was robustly correlated to the volume of the three compartments and the ICV (P < .001).
TABLE 3:
Summary of ICV and compartmental volumes
| Volume | Boys | Girls | Average | Increase (%)* | P Value |
|---|---|---|---|---|---|
| By pixel count | |||||
| ICV | 1803 | 1495 | 1639 | 17 | .03 |
| By the model | |||||
| Brain tissue | 741 | 628 | 681 | 15 | .23 |
| CSF and brain tissue | 460 | 355 | 404 | 23 | .03 |
| CSF | 574 | 491 | 530 | 14 | .09 |
| ICV | 1775 | 1474 | 1614 | 17 | .03 |
In boys versus girls.
TABLE 4:
Age dependent volume of the study subjects
| Volume or Compartment | Volume (cm3) | R |
|---|---|---|
| ICV | ||
| By pixel count | V = 1510 + 252 log(age) | 0.680 |
| By the model | V = 1492 + 242 log(age) | 0.671 |
| Brain tissue | V = 348 + 111 log(age) | 0.773 |
| Brain tissue and CSF | V = 218 − 11 log(age) | 0.159* |
| CSF | V = 276 + 36 log(age) | 0.548 |
Note.—Subjects’ age range was 0.01–17 years.
Correlation is not significant.
Fig 4.
Age dependence of brain volume. The most significant volume changes occur within the first 2 postnatal years. Lines are the logarithmic fit.
Fig 5.
ICV measured by using the pixel count and the brain model. Correlation was excellent (R = 0.999).
When the subjects’ brains were analyzed according to sex, ICV was significantly larger in boys than in girls (P < .05), with an average ICV of 1803 ± 441 cm3 in boys compared with 1495 ± 327 cm3 in girls, as measured by using the pixel count. There was no statistically significant volume difference in the brain tissue compartment between boys and girls (P > .2), although the average brain tissue compartment of the boys was 741 ±161 cm3, which was 15% higher than the 627 ±116 cm3 found in girls.
Discussion
In this study, we determined the diffusion and volume changes during brain development in children by using quantitative diffusion imaging and a mathematical model. Diffusion analysis showed that diffusion values decrease in both the gray matter and white matter of the brain during maturation. The greatest diffusion changes occurred within the first 2 years after birth, when a large drop in the water content of the brain tissue occurs (1). The biexponential age-dependent diffusion decay is consistent with previous quantitative autopsy brain measurements (1), brain water content, and results of other diffusion studies (8–10, 13). Many mechanisms are thought to contribute to decreased diffusion. These mechanisms include increased intercellular tortuousity; new membrane-barrier formation as a result of myelination and the formation of new axons, dendrites, and glial cells; and an increase in macromolecular concentrations (2–6).
The correlations between Dav values and age in the measured areas were all robust, indicating that the water content changes throughout the maturation process in these areas were parallel in pattern, although they were possibly different in magnitude.
In the splenium of the corpus callosum, the measured Dav was 0.880 ± 0.147 (10−5cm2/s), which is comparable that described in previous reports (10, 14). Previous work in adults has shown that the splenium has higher anisotropy (8, 15) and higher Dav (15) than the rest of the corpus callosum. There was no difference in Dav between the splenium and the genu in this study (P > .05). The ongoing diffusional development of the corpus callosum may be the reason for this finding. A larger sample size could improve recognition of the diffusion characteristics of the corpus callosum.
The PVWM had the highest Dav value. This might have been due to the inadvertent inclusion of CSF in the ROI close to ventricles. CSF-suppressed diffusion-weighted imaging may enable better diffusion characterization in the regions close to the ventricles (15, 16).
When we compared the biexponential decrease of diffusion from Table 2 with data from our previous study (Table 1 of reference 4), the slower component of diffusional change from both studies agree well with each other. However, there is a considerable difference in the fast component, which is dominant before age 2. This observation suggests that a larger data set with more subjects younger than 2 years is necessary for more accurate description of diffusion at this early age.
Previous studies revealed that the ICV varies from 1300 to 2000 cm3 at full growth (17). The average ICV measured by using the pixel count and brain model in this study were 1639 and 1614 cm3, respectively. ICV increased from 0.01 to 17 years with a sharper increase during the first 2 years. These findings are in agreement with those of previous studies (12, 17–20). In our study, pixels from the extracranial tissue might have affected the volume measurement during automatic data processing. However, the curve of volume change can still serve as a guideline for estimating brain volume.
In this study, the average ICV for boys and girls was 1803 and 1495 cm3, respectively, by pixel count. Statistically, ICV was larger in the boys than in the girls (P < .05), similar to previously reported findings (17, 19). However, the volume difference in the brain tissue compartment was not significantly different between boys and girls.
Many factors can affect brain growth and shrinkage. Examples include nutritional status, delivery complications, stressful life events, psychiatric or neurologic disorders, Alzheimer disease, and endocrine diseases such as Cushing disease (21, 22). Evidence about reductions in brain volume following child abuse and neglect has also been outlined (23). The brain modeling in this study may provide good estimates in disorders related to brain development, including the neurologic aftermath of brain trauma, mood disorders, and child abuse.
Conclusion
This study was a combined investigation of diffusion and volumetric changes of the developing brain. The brain model used herein provided a good estimate of brain growth, which may be helpful for in vivo monitoring of the brain without a need for segmentation.
Footnotes
Supported in part by grants National Institute for Child Health and Human Development R03-HD39796 and National Institute for Mental Health R01-MH63255.
References
- 1.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Childhood 1973;48:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smart JL, Dobbing J. Vulnerability of developing brain, IX: the effect of nutritional growth retardation on the timing of the brain growth-spurt. Biol Neonate 1971;19:363–378 [DOI] [PubMed] [Google Scholar]
- 3.Normura O, Sakuma H, Takeda K, Tagami T, Okuda Y, Nakagawa T. Diffusional anisotropy of the human brain assessed with diffusion weighted MR: Relation with normal brain development and aging. AJNR Am J Neuroradiol 1994;15:231–238 [PMC free article] [PubMed] [Google Scholar]
- 4.Uluğ AM. Monitoring brain development with quantitative diffusion imaging. Dev Sci 2002;5:286–292 [Google Scholar]
- 5.Huppi PS, Maier SE, Eeled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 1998;44:584–590 [DOI] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D. Normal brain maturation of the neonatal and infant brain: MR imaging at 1.5T. Radiology 1988;166:173–180 [DOI] [PubMed] [Google Scholar]
- 7.Chepuri NB, Yen YF, Brudette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol 2002;23:803–808 [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P, Miller JH,. Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 2002;23:1445–1456 [PMC free article] [PubMed] [Google Scholar]
- 9.Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Paediatric neuroradiology: changes in brain water diffusion during childhood. Neuroradiology 1999;41:929–924 [DOI] [PubMed] [Google Scholar]
- 10.Chun T, Filippi CG, Zimmerman RD, Ulug AM. Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 2000;21:1078–1083 [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long term cognitive outcome in preterm infants. JAMA 2000;284:1939–1947 [DOI] [PubMed] [Google Scholar]
- 12.Kumra S, Giedd JN, Vaituzis AC, et al. Childhood onset psychotic disorders: magnetic resonance imaging of volumetric differences in brain structure. Am J Psychiatry 2000;157:1467–1474 [DOI] [PubMed] [Google Scholar]
- 13.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlations of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology 2002;222:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uluğ AM, van Zijl PC. Orientation-independent diffusion imaging without tensor diagonalization: anisotropy definitions based on physical attributes of the diffusion ellipsoid. J Magn Reson Imaging 1999;9:804–813 [DOI] [PubMed] [Google Scholar]
- 15.Kwong KK, McKinstry RC, Chien D, Crawley AP, Pearlman JD, Rosen BR. CSF-suppressed quantitative single shot diffusion imaging. Magn Reson Med 1991;21:157–163 [DOI] [PubMed] [Google Scholar]
- 16.Falconer JC, Narayana PA. Cerebrospinal fluid suppressed high resolution diffusion imaging of the human brain. Magn Reson Med 1997;37:119–123 [DOI] [PubMed] [Google Scholar]
- 17.Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K. Intracranial volume change in childhood. J Neurosurg 1999;91:610–616 [DOI] [PubMed] [Google Scholar]
- 18.Jäncke L, Staiger JF, Schlaug G, Huang YX, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cerebral Cortex 1997;7:48–56 [DOI] [PubMed] [Google Scholar]
- 19.Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 1–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex 1996;6:726–736 [DOI] [PubMed] [Google Scholar]
- 20.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000;216:672–682 [DOI] [PubMed] [Google Scholar]
- 21.Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D. Negative association of neuroticism with brain volume ratio in healthy humans. Biol Psychiatry 2001;50:685–690 [DOI] [PubMed] [Google Scholar]
- 22.Simmons NE, Do HM, Lipper MH, Laws ER Jr. Cerebral atrophy in Cushing’s disease. Surg Neurol 2000;53:72–76 [DOI] [PubMed] [Google Scholar]
- 23.Glaser D. Child abuse and neglect and the brain: a review. J Child Psychol Psychiatry Allied Discipl 2000;41:97–116 [PubMed] [Google Scholar]