Abstract
BACKGROUND AND PURPOSE: Little is known about age-related changes in posterior fossa venous anatomy on 2D time-of-flight MR venography (MRV) or about artifacts that limit its accuracy in diagnosing venous occlusive disease. We evaluated pediatric appearances of posterior fossa venous drainage.
METHODS: One hundred and eight children with normal MR imaging or minimal congenital anomalies underwent 2D MRV. Transverse sinus dominance and absence and the presence of an occipital sinus were correlated with age. Venous structure conspicuity was compared on source and maximum intensity projection images.
RESULTS: Right, left, and codominance of the transverse sinus, respectively, was as follows: at < 25 months, 37%, 21%, and 42%; 25 months to 5 years, 35%, 30%, 35%; and ≥6 years, 50%, 16%, 34%. Transverse sinus dominance was not related to age between the three groups (P = .58, chi-square contingency), but some relationship was observed when patients <6 years were compared to those ≥6 years (P = .032). Chi-square trends showed a mildly positive correlation between age and an absent transverse sinus (P = .026) and a decreasing trend in the presence of an occipital sinus with age (P = .038). Saturation effects due to in-plane/slow flow were worse in patients <25 months; effects in the transverse sinuses or internal jugular veins were miminized with coronal or axial imaging, respectively.
CONCLUSION: 2D TOF MRV shows age-related changes in venous anatomy. Caution should be used before posterior fossa venous occlusive disease is diagnosed on the basis of signal intensity loss, especially in neonates and young infants.
MR venography (MRV) is a widely available, noninvasive means of evaluating venous anatomy. Although other sequences are available, 2D time-of-flight (TOF) MRV is probably the technique most widely used to study the cerebral venous structures, as images can be acquired in <5 minutes on most MR units and intravenous contrast enhancement is not required (1, 2). The use of MRV in the pediatric population has received relatively little emphasis in the literature. Familiarity with normal, age-related findings on 2D TOF MRV is necessary for radiologists who interpret MRV images in children.
The purpose of our study was to retrospectively study the posterior fossa venous anatomy, as depicted by 2D MRV, in infants and children with normal MR imaging or minimal anatomic abnormalities to determine the normal appearance of the posterior fossa venous sinuses and any age-related differences.
Methods
At our institution, routine MR imaging often includes 2D MRV. On review of our MR database, we identified 211 patients who underwent MR imaging with 2D MRV in the preceding 39 months. Three pediatric neuroradiologists (N.R., C.I., T.B.) reviewed the studies in accordance with an institutional investigational review board protocol. Among the patients, 108 had normal MR findings or minimal congenital anomalies and no history of structural or organic brain abnormality, systemic illness or genetic disorder, closed head injury, craniotomy, dural sinus thrombosis, papilledema, or catheterization of the internal jugular vein (IJV). The 108 patients were grouped according to age: Group I consisted of neonates to those aged 24 months (n = 38, 21 boys, 17 girls; mean age, 11 months); group II, patients aged 25 months to 5 years (n = 17; 11 boys, six girls; mean age, 4 years); and group III, patients aged 6–17 years (n = 53, 21 boys, 32 girls, mean age, 11.75 years). Clinical indications for the MR studies were seizures in 55 patients and developmental delay in 51. Two patients had been referred because of tongue masses.
MR imaging was done on 1.5-T units and included sagittal and axial T1 spin-echo, axial fluid-attenuated inversion recovery, and coronal or axial T2 fast spin-echo sequences. Other sequences were performed on the basis on the clinical indication for the MR study and the patient’s age. Parameters for 2D MRV were TR/TE of 23/5.1, flip angle of 50°, 2-mm section thickness, 0.6-mm gap, 320 × 512 matrix, and 24-cm field of view. 2D MRV was acquired in the coronal and/or axial planes by using an inferior saturation slab to saturate arterial inflow. No contrast agent was administered. Source images were reconstructed by using a maximum intensity projection (MIP) algorithm and viewed in multiple oblique and orthogonal projections on a commercially available picture archive and communication system workstation (SUN Microsystems, Santa Clara, CA). Oblique and subvolumetric images were acquired as needed to clarify the venous anatomy.
Image Analysis
Source images from 2D MRV and MIP images were evaluated for transverse sinus dominance. A transverse sinus was considered dominant when it was larger than the contralateral transverse sinus on visual inspection of the coronal and axial MIP images. A transverse sinus was considered absent when it was absent on both the MIP and source images from the torcula to the sigmoid sinus. Areas of signal intensity loss in the transverse sinuses were noted on the source and MIP images. The caliber of the sigmoid sinuses and IJV relative to that of the dominant transverse sinus was noted on the sagittal MIP images, as was the presence of an occipital sinus.
Statistical Analysis
Chi-square contingency analysis was used to explore the relationship between transverse sinus dominance, as seen on the 2D MRV, and age. Chi-square trend analysis was used to determine whether the absence of a transverse sinus on 2D MRV increased with increasing age and whether the incidence of an occipital sinus decreased with patient age.
Results
Of the 38 patients in group I, 32 had normal MR images. Minor anomalies included a small arachnoid cyst of the middle cranial fossa, mega cisterna magna, and unilateral coronal synostosis in one patient each. Three patients had prominent bifrontal extracerebral fluid spaces with normal-sized ventricles. In group I, 14 (37%) (Table) had a right dominant transverse sinus, eight (21%) had a left dominant sinus, and 16 (42%) had codominant sinuses. Two patients had small, codominant transverse sinuses associated with occipital sinuses (Fig 1). Overall, occipital sinuses were observed in five patients (13%) in group I. Two of 38 patients (5%) in group I had absence of a transverse sinus. Absence of a transverse sinus and a persistent occipital sinus together were seen in two patients (Fig 2), while three patients with occipital sinuses had visible transverse sinuses. MIP images showed areas of signal intensity loss in nondominant transverse sinuses in 24 patients in group I; these were consistently longer and more obvious on the MIP images than on the source images (Fig 3). Discrepancy in the conspicuity of the transverse sinuses was most marked in 14 patients <6 months of age. MIP images in 10 of these patients suggested absence of a transverse sinus, while the transverse sinuses were clearly present on the axial source images. The sigmoid sinuses and IJVs were somewhat smaller in caliber than the transverse sinuses. Narrowed midcervical IJVs were seen in four of nine patients in whom the MR venograms included this region.
Variations in posterior fossa venous drainage with age
| Group | Right | Left | Codominant | Atretic Transverse Sinus | Occipital Sinus |
|---|---|---|---|---|---|
| I (n = 38) | 14 (37) | 8 (21) | 16 (42) | 2 (5) | 5 (13) |
| II (n = 17) | 6 (35) | 5 (30) | 6 (35) | 3 (18) | 2 (12) |
| III (n = 53) | 26 (50) | 9 (16) | 18 (34) | 7 (13) | 1 (2) |
| Total (n = 108) | 46 (44) | 22 (20) | 40 (37) | 6 (5) | 8 (7) |
Note.—Data in parentheses are percentages.
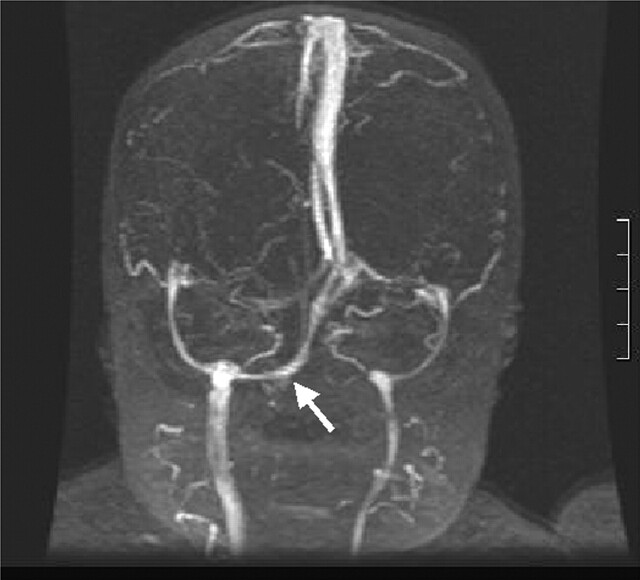
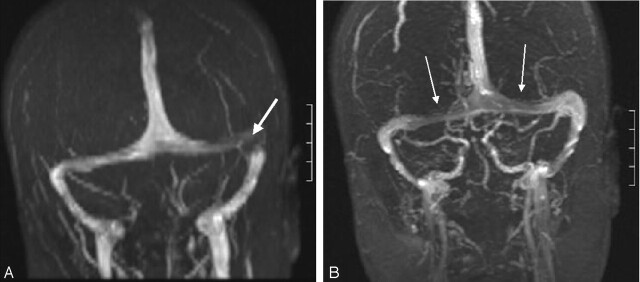
Fig 1.
Coronal MIP from coronal MRV shows symmetric hypoplasia of the transverse sinuses associated with a persistent occipital sinus (arrow).
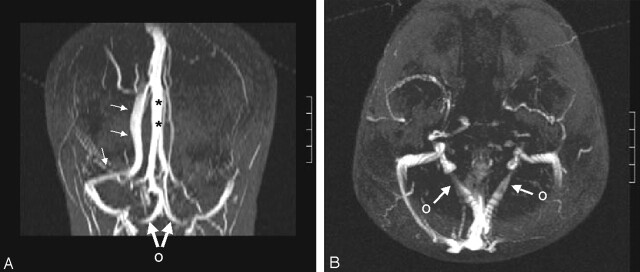
Fig 2.
Absence of the left transverse sinus and a persistent occipital sinus.
A, Coronal MIP from axial MRV shows that the superior sagittal sinus continues as the right transverse sinus (small arrows). Straight sinus (asterisks) courses upward to the high-riding torcula, which drains downward into the occipital sinus. Large arrows and O indicate bifurcation of the occipital sinus at the foramen magnum.
B, Axial MIP shows the occipital sinus bifurcating (arrows, O) and draining into the IJVs.
Fig 3.
Saturation effects due to in-plane flow in the transverse sinuses.
A, Oblique sagittal MIP from axial MRV in shows diminished signal intensity from the transverse and sigmoid sinuses.
B, Axial image shows patent transverse sinuses.
Of the 17 patients in group II, 16 had normal MR imaging, and patient had a small arachnoid cyst in the middle cranial fossa. In group II, the right transverse sinus was dominant in six (35%), and the left was dominant in five (30%), while codominant transverse sinuses were seen in six patients (35%). One patient with a right dominant transverse sinus underwent sequential MR imaging at 5 and 6 years. The venous anatomy remained unchanged. Absence of a transverse sinus, as defined by absence of signal intensity in the transverse sinus on the source images (Fig 4), was seen in three patients (18%). Occipital sinuses were seen in two (12%); both had absence of a transverse sinus (Fig 5). Signal intensity loss in the nondominant transverse sinuses was seen in 49% of patients; as in group I, this was consistently longer and more apparent on MIPs than on the source images. Signal intensity loss in the transverse sinuses was more pronounced on axial MRV than on coronal MRV, whereas signal intensity loss in the IJVs was more pronounced on coronal MRV (Fig 6). Focal narrowing at the junction of the sigmoid and the IJV was observed in >60% of the patients (Fig 7). Narrowed midcervical IJVs were seen in five of 11 patients in whom 2D MRV included this region; this sometimes simulated venous occlusive disease.
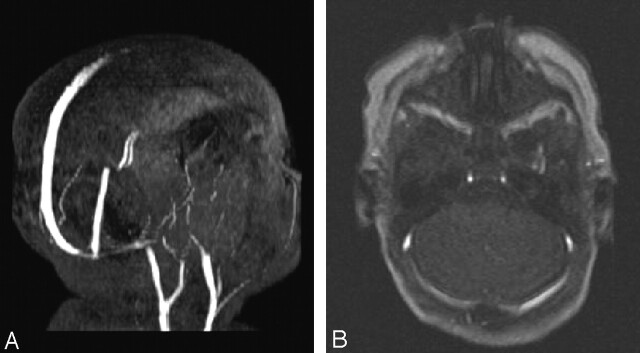
Fig 4.
Artifactual signal intensity loss due to the MIP algorithm.
A, Axial MIP from the axial images shows diminished signal intensity from the medial two-thirds of the left transverse sinus.
B, Axial image shows nearly equal signal intensity in both transverse sinuses.
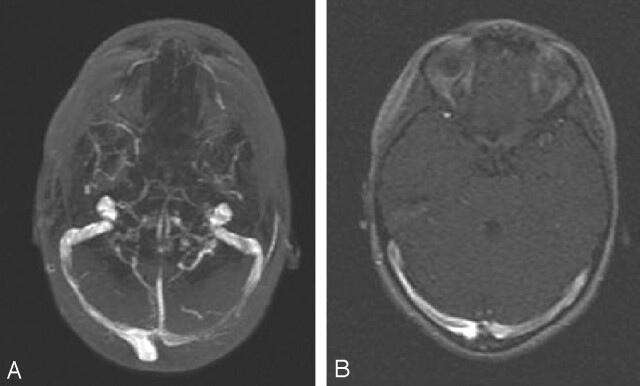
Fig 5.
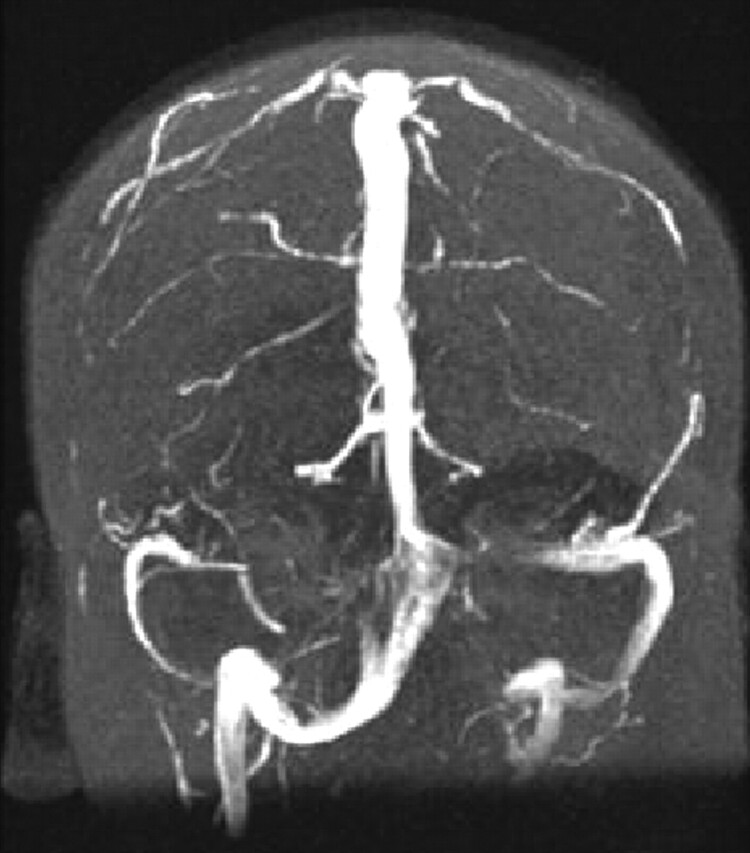
Asymmetric transverse sinus hypoplasia associated with a large persistent occipital sinus. Coronal MIP from coronal MRV.
Fig 6.
Comparison of coronal MIPs from 2D TOF MRV in the axial (A) and coronal planes (B).
A, Focal area of diminished signal intensity in the left transverse sinus (arrow). Internal jugular veins are well depicted.
B, Diminished signal intensity in the medial thirds of both transverse sinuses (arrows), while the lateral aspect of the left transverse sinus is well depicted. Loss of signal intensity in the IJVs is due to in-plane flow. Lack of a caudal presaturation pulse accounts for visualization of arterial structures.
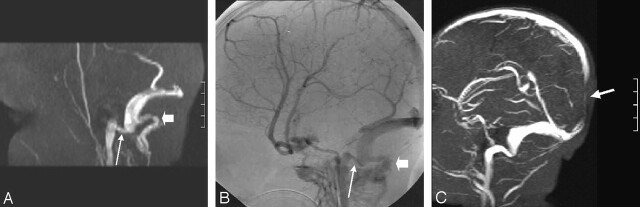
Fig 7.
Narrowing at the junction of the sigmoid sinus and the IJV seen in >60% of patients older than 24 months.
A, Parasagittal subvolume MIP from coronal MRV shows marked luminal narrowing at this junction (long arrow). Note the prominent posterior condylar vein (short arrow).
B, Comparative digital subtraction angiogram, venous phase. Long arrow indicates the narrowed junction; short arrow, a prominent posterior condylar vein.
C, Narrowed junction not associated with a prominent posterior condylar vein in another patient. Arrow indicates loss of signal intensity in the posterior aspect of the superior sagittal sinus due to in-plane flow; coronal MRV was performed.
Of the 53 patients in group III, 52 had normal MR images, and one had a small venous angioma in the right temporal lobe. In this group, the right transverse sinus was dominant in 26 patients (50%), and the left, in nine (16%). Codominant transverse sinuses were seen in 18 patients (34%). Absence of a transverse sinus was noted in seven (13%), while signal intensity loss was seen in almost 50% of those with nondominant transverse sinuses. An occipital sinus was seen in one patient (2%) and associated with absence of a transverse sinus. Narrowing of the sigmoid-IJV junction was seen in 23 patients (43%), while a narrowed midcervical IJV was observed in 12 of 19 patients in whom the midcervical region was included on 2D MRV.
On chi-square contingency analysis, we observed no significant relationship between transverse sinus dominance, as seen on 2D MRV, and age when the three age groups were compared (P = .58). Chi-square contingency analysis showed some relationship between transverse sinus dominance and age when groups I and II were combined and when patients <6 years of age were compared with those 6 years of age and older (P = .032). Chi-square trend analysis showed a mildly positive correlation between age and the absence of a transverse sinus (P = .026) and a decreasing trend in the presence of an occipital sinus with increasing age (P = .038).
Discussion
MR methods of evaluating patency of dural venous sinuses include 2D and 3D phase-contrast, 3D contrast-enhanced thin-section gradient-echo, and 2D MRV techniques (3–6). Phase-contrast venography is limited by a potential lack of sensitivity to flow because of the selection of an inappropriate velocity-encoding value (5, 6) and its relatively lengthy acquisition times. At our institution, phase-contrast MRV is reserved for patients with subacute dural sinus thrombus having hyperintense signal intensity on T1-weighted images; this finding can falsely suggest a patent sinus on 2D TOF MRV (6). Compared with 2D TOF MRV, 3D contrast-enhanced gradient-echo techniques are more sensitive to dural sinus thrombosis, they are less affected by saturation effects, and they enable better differentiation of atretic and hypoplastic sinuses (3). Findings from one comparison of gadolinium-enhanced 3D MRV with 2D MRV in patients with idiopathic intracranial hypertension suggest that this method may be preferred to 2D TOF techniques in some patients with nonthrombotic stenoses of the transverse and sigmoid sinuses (4).
The initial goal of this study was the evaluation of anatomic variations in posterior fossa venous drainage rather than the detection of dural sinus thrombosis. As our patients did not necessarily have a clinical indication for gadolinium enhancement, 2D MRV was used to study the posterior fossa venous anatomy. However, we noted problems with saturation effects during 2D MRV, especially in infants. These effects were most often observed in the transverse sinuses of neonates. The effects were due to slow flow, low volume of flow, or in-plane flow (5) and were most pronounced when the 2D MRV was performed in the axial plane. In-plane flow was minimized by orienting the section slab perpendicular to the expected direction of flow, and better visualization of the transverse sinuses, in addition to greater anatomic coverage, was achieved with coronal imaging. However, acquisition in the coronal plane resulted in loss of signal intensity due to saturation effects in the IJVs in some patients. In all age groups undergoing 2D MRV, the MIP array tracing resulted in some underestimation of vascular caliber such that transverse sinuses that were hypoplastic on source images often appeared to be absent on MIP images. This occurrence stresses the need to examine the source images before one concludes that a transverse sinus is absent.
We are not the first to analyze the posterior fossa venous anatomy using 2D TOF MRV. Ayanzen et al (2) reported 100 patients with normal MR imaging who underwent 2D MRV. Their patient population was aged 9 days to 83 years, and they reported no correlation between patient age and visibility of the venous structures. The authors noted that the distribution of transverse sinus dominance, as seen on 2D MRV, was in accordance with the distribution of transverse sinus dominance, as seen on conventional angiography. Transverse sinuses were right dominant in 59%, left dominant in 25%, and codominant in 16%. Our pattern of transverse sinus dominance on 2D MRV differs from somewhat. In our study, patients were separated by age to determine if any age-related changes in posterior fossa venous anatomy could be seen on 2D MRV. Among the patients aged 0–24 months, 37% had a right dominant transverse sinus, 21% had a left dominant sinus, and 42% had codominant sinuses. Between 25 months and 5 years, right transverse sinus dominance was seen in 35%; left dominance, in 30%; and codominance, in 35%. From 6 years of age and upward, right dominance was seen in 50%; left dominance, in 16%; and codominance, in 34%. These differences may be potentially attributable to patient age. Statistical analysis showed a relationship between transverse sinus dominance and age when patients under 6 years of age were compared with those 6 years of age and older (P = .032), suggesting that transverse sinus dominance does vary with patient age and is more like the adult pattern after 5 years of age. The frequency with which codominant transverse sinuses were observed decreased with increasing age, while right transverse sinus dominance increased with increasing age, approaching the distribution reported in the literature.
Anatomic studies of fetal specimens have shown an extensive collateral network connecting the posterior fossa venous sinuses with the cervical and vertebral veins (7). Toward the end of the first trimester of gestation, the sigmoid sinus and the IJVs are connected by small caliber jugular sinuses, which remain small until the third trimester. Marked increase in venous flow from the rapidly growing cerebral hemispheres leads to ballooning of the transverse sinuses in the absence of an increase in the diameters of the sigmoid and jugular sinuses. As seen on MRV, the diameters of the sigmoid sinuses and IJVs are somewhat smaller than those of the dominant transverse sinuses in early infancy. In our study, the disparity in luminal caliber became more pronounced in later infancy and childhood and persisted through adolescence, and marked narrowing at the sigmoid-IJV junction was common. In one patient, both 2D MRV and conventional angiography showed narrowing at the sigmoid-IJV junction; this indicated that the finding on MRV was anatomic rather than artifactual and flow related.
The occipital sinus, which may be solitary, duplicated, or composed of a mesh of venous collaterals, is contained within leaves of dura and connects the torcula with the IJV (8). The occipital venous network is thought to become involuted once most of the venous flow passes through the large dural sinuses as the child achieves an upright position (7). Ayanzen et al (2) reported occipital sinuses in 10% of their patients, but they made no comments about the ages of the patients in whom persistent occipital sinuses were observed. In our study, persistent occipital sinuses were seen in 13% of patients <25 months of age but only 2% of children older than 5 years. As >50% of the occipital sinuses were seen in children < 25 months of age, an age-related regression in the occipital sinuses may occur. In addition to an apparent age-related regression of occipital sinuses, we found an apparent age-related increase in the frequency of an absent transverse sinus; a transverse sinus was absent in 5% of patients <25 months of age and in 13% of patients ≥25 months. This apparent increase in the frequency of absence of a transverse sinus may be due to alterations in posterior fossa venous flow patterns rather than structural involution of the transverse sinus, as a decrease in the volume of flow through a transverse sinus may render a patent sinus unapparent on 2D MRV.
Most of the venous drainage of the brain, as depicted with the patient in a supine position, is into the IJVs via the transverse and sigmoid sinus. Collateral venous drainage between the posterior fossa and vertebral venous system, as seen on cast corrosion studies in adult cadavers, is provided via the anterior, posterior, and lateral condylar veins as well as the anterior condylar confluence (8). The posterior condylar vein originates from the posterior-superior surface of the sigmoid-IJV junction and drains into the deep cervical venous plexus, providing drainage from the sigmoid sinus in presence of stenosis or occlusion of the jugular veins. The posterior condylar vein was the collateral venous structure most often identified seen on 2D MRV. Although we did not tabulate the frequency with which posterior condylar veins were observed or the range in size, as seen on 2D MRV, the posterior condylar veins were routinely observed in patients of all ages and varied in size, sometimes approaching the size of the sigmoid-IJV junction.
Conclusion
Age-related changes in the posterior fossa venous anatomy are visible on 2D MRV. These include an increasing frequency of right transverse sinus dominance with age, involution of the occipital sinuses, and increasing frequency of an absent transverse sinus. Caution should be used before posterior fossa venous occlusive disease is diagnosed, on the basis of signal intensity loss on 2D MRV, especially in neonates and young infants. Further investigation into the use of gadolinium-enhanced MRV in infants and children is indicated.
Acknowledgments
Statistical analysis was provided by Joan Reisch, PhD, Department of Biostatistics; manuscript preparation, by Rayna Ross; and image processing, by Tony Reyes, MS.
References
- 1.Mattle HP, Wentz KU, Edelman RR, et al. Cerebral venography with MR. Radiology 1991;178:453–458 [DOI] [PubMed] [Google Scholar]
- 2.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobold MR, Heiserman JE. Cerebral venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol 2000;21:74–78 [PMC free article] [PubMed] [Google Scholar]
- 3.Liang L, Korogi Y, Sugahara T, et al Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. AJNR Am J Neuroradiol 2002;23:1739–1746 [PMC free article] [PubMed] [Google Scholar]
- 4.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension. Neurology 2003;60:1418–1424 [DOI] [PubMed] [Google Scholar]
- 5.Rippe DJ, Boyko OB, Spritzer CE, et al. Demonstration of dural sinus occlusion by the use of MR angiography. AJNR Am J Neuroradiol 1990;11:199–201 [PMC free article] [PubMed] [Google Scholar]
- 6.Nadel L, Braun IF, Kraft KA, Fatouros PP, Laine FJ. Intracranial vascular abnormalities: value of MR phase imaging to distinguish thrombus from flowing blood. AJNR Am J Neuroradiol 1990;11:1133–1140 [DOI] [PubMed] [Google Scholar]
- 7.Okudera T, Huang YP, Ohta T, et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol 1994;15:1871–1883 [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz DSM, Gailloud P, Rufenacht DA, Delaville J, Henry F, Fasel J. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002;23:1500–1508 [PMC free article] [PubMed] [Google Scholar]