Abstract
BACKGROUND AND PURPOSE: The demonstration of communication between arachnoid cysts (ACs) and the adjacent subarachnoid space is a prerequisite for their proper management. CT cisternography (CTC) is the conventional method for functional evaluation of ACs. The sensitivity of MR imaging to CSF flow has been demonstrated, but reports of the clinical usefulness of MR CSF flow techniques in this application are limited. The purpose of our study was to prospectively evaluate the accuracy of MR CSF flow study as an alternative to CTC in this setting.
METHODS: MR CSF flow study with retrospective ECG-gated 2D, fast low-angle shot, phase-contrast (PC), cine gradient-echo sequence was performed in 39 patients with an intracranial AC. Results were compared with intraoperative and CTC findings.
RESULTS: PC cine MR imaging results were compatible with operative or CTC findings in 36 (92.3%) of 39 patients. Twenty-four cysts were noncommunicating, and 15 were communicating. Three cysts were evaluated as being noncommunicating on PC cine MR imaging (false-negative) but demonstrated contrast enhancement on CTC. No false-positive diagnoses occurred. All cysts regarded as being communicating on PC cine MR imaging were also found to be communicating on both confirmation methods.
CONCLUSION: MR CSF flow imaging with a PC cine sequence can be incorporated in the imaging work-up of ACs. This is a reliable alternative to invasive CTC for the functional evaluation of ACs.
Arachnoid cysts (ACs) are widely accepted as developmental anomalies in which splitting or duplication of the primitive arachnoid membrane leads to intra-arachnoid fluid collection (1). ACs constitute 1% of all intracranial masses, but the true incidence of intracranial ACs is unknown because many may be asymptomatic throughout life (2–6).
Intracranial cysts are mostly located along the hemispheric surfaces and cisternal spaces, with 50–60% occurring in the middle cranial fossa (7–10). ACs are classified as communicating or noncommunicating according to their relation to the subarachnoid space (11–13). Inadequate or non-existent communication between the cyst and the subarachnoid space seems to be responsible for mass effect and progressive symptoms (14). Differentiation of communicating cysts and noncommunicating ones is often required if surgical therapy is contemplated (15, 16).
CT cisternography (CTC) is the most widely used diagnostic method to show the communication between the cyst and the subarachnoid space (12, 17–20). In the last decade, flow-sensitive cine MR imaging techniques have been increasingly used to investigate the flow characteristics of CSF, but only a few reports describe the role of flow-sensitive cine MR techniques to show the flow dynamics of intracranial, CSF-related cysts (21, 22). The purpose of this prospective study was to determine whether flow-sensitive phase-contrast (PC) cine MR imaging obviates CTC in demonstrating the communication between ACs and CSF. Our aim was to investigate the accuracy of flow-sensitive PC cine MR imaging as an alternative to invasive CTC.
Methods
Between December 1999 and February 2003, we examined 41 patients with ACs in the preoperative period. In two patients, CTC was unsuccessful and surgery was not performed; they were excluded from our study. The remaining 39 patients (15 female, 24 male; age range, 1–82 years; mean age, 25 years) constituted the study group.
MR imaging was performed with a 1.5-T superconducting magnet (Magnetom Vision Plus; Siemens, Erlangen, Germany) by using a standard head coil. Initially, T2-weighted turbo gradient spin-echo images were obtained in a minimum of two planes before flow-sensitive PC cine MR imaging was done. The imaging parameters were as follows: TR/TE/NEX, 7400/115/1; flip angle, 160°; field of view, 230 mm; matrix 345 × 512; and section thickness, 2 mm. T2-weighted images served as guides for estimating possible communication sites of the cysts and the neighboring cisterns and for determining the extent of the ACs.
To demonstrate possible communication between the cyst and the subarachnoid space, the qualitative flow data were obtained with a 2D fast low-angle shot (FLASH) gradient-echo sequence. The imaging parameters were as follows: TR/TE/NEX, 70/15.8/2; flip angle, 10°; field of view, 250 mm; matrix, 144 × 256; section thickness, 4 mm; and velocity encoding, ±2 cm/s. The measurements were performed in three orthogonal directions at the assumed communication sites.
Retrospective cardiac gating was used. Eleven phase images were calculated. MR images for qualitative evaluation of CSF flow were displayed in a closed-loop cine format. The cine mode studies were interpreted directly from monitors by adjusting the window and center measures of the images. The observation of the hyperintense or hypointense areas in the cyst, such as a sign of jetlike flow, was used to judge the presence of communication between the cysts and the cisternal spaces. ACs having the same signal intensity as that of the surrounding tissue were regarded as noncommunicating cysts.
CTC images were ordered at the 2, 6, 12, and 24 hours after the intrathecal administration of contrast material. When early contrast enhancement was visualized in the cyst, the filming procedure was terminated. The criterion for the communication on CTC was the visualization of the intracystic contrast enhancement. If the enhancement was uncertain, we measured the increase (in Hounsfield units) and compared it with the finding on nonenhanced images. A threefold increase on delayed scans was accepted as a sign of communication. Results of the CSF flow studies were compared with operative and CTC findings. The accuracy of PC cine MR imaging was analyzed by using the McNemar test.
Results
The intracranial locations of the ACs were the middle cranial fossa (n = 20), quadrigeminal cistern (n = 6), retrocerebellar cistern (n = 5), cerebral convexity (n = 5), and cerebellopontine space (n = 3). None of the patients had more than one cyst, and none had associated developmental malformations. Headache (n = 18), and epileptic seizures (n = 4) were the most frequent presenting symptoms. The Table summarizes the clinical features of the cases. In four cases, ACs were diagnosed incidentally.
TABLE 1:
Summary of patients with an arachnoid cyst
| Patient/Age (y)/Sex | Location of Arachnoid Cyst | Clinical Findings | Confirmation Method | Communication with CSF* |
||
|---|---|---|---|---|---|---|
| PC Cine MR Imaging | CTC | Surgery | ||||
| 1/8/M | L middle fossa | Seizure | CTC | Yes | Yes | NA |
| 2/53/F | R middle fossa | Headache | CTC | Yes | Yes | NA |
| 3/27/M | L middle fossa | Incidental | CTC | Yes | Yes | NA |
| 4/14/M | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 5/16/M | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 6/26/F | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 7/15/M | R middle fossa | Headache | CTC | Yes | Yes | NA |
| 8/49/M | R cerebellopontine angle | Vertigo | CTC | Yes | Yes | NA |
| 9/66/F | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 10/20/M | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 11/22/M | R middle fossa | Headache | CTC | Yes | Yes | NA |
| 12/50/F | L middle fossa | Headache | CTC | Yes | Yes | NA |
| 13/10/F | R middle fossa | Headache | CTC | No | Yes | NA |
| 14/42/M | L middle fossa | Incidental | CTC | No | Yes | NA |
| 15/37/F | Quadrigeminal cistern | Headache | CTC | No | Yes | NA |
| 16/52/F | Cerebral convexity | Headache | CTC | No | No | NA |
| 17/70/M | Quadrigeminal cistern | Incidental | CTC | No | No | NA |
| 18/11/M | L cerebellopontine angle | Headache | CTC | No | No | NA |
| 19/15/F | Cerebral convexity | Headache | CTC | No | No | NA |
| 20/33/M | Quadrigeminal cistern | Headache | CTC | No | No | NA |
| 21/82/M | L middle fossa | Incidental | CTC | No | No | NA |
| 22/5/M | Cerebral convexity | Seizure | CTC | No | No | NA |
| 23/38/M | L middle fossa | Headache | CTC | No | No | NA |
| 24/33/M | Cerebral convexity | Disarticulation | CTC | No | No | NA |
| 25/12/M | Cerebral convexity | Headache | CTC | No | No | NA |
| 26/34/F | R cerebellopontine angle | Vertigo | Surgery | No | NA | No |
| 27/22/F | Retrocerebellar cistern | Vertigo | Surgery | No | NA | No |
| 28/1/M | Retrocerebellar cistern | Cardiomegaly | Surgery | No | NA | No |
| 29/1/F | Retrocerebellar cistern | Developmental delay | Surgery | No | NA | No |
| 30/21/F | Quadrigeminal cistern | Visual loss | Surgery | No | NA | No |
| 31/10/M | Retrocerebellar cistern | Headache, nausea/vomiting | Surgery | No | NA | No |
| 32/4/M | Retrocerebellar cistern | Ataxia | Surgery | No | NA | No |
| 33/17/M | L middle fossa | Seizure | CTC, surgery | No | No | No |
| 34/1/M | L middle fossa | Tendency to sleep | CTC, surgery | No | No | No |
| 35/3/M | R middle fossa | Seizure | CTC, surgery | No | No | No |
| 36/4/M | L middle fossa | Ataxia | CTC, surgery | No | No | No |
| 37/40/F | Quadrigeminal cistern | Headache, Nausea/vomiting | CTC, surgery | No | No | No |
| 38/11/M | L middle fossa | Temporal bulging | CTC, surgery | No | No | No |
| 39/18/F | Quadrigeminal cistern | Headache | CTC, surgery | No | No | No |
NA indicates not applicable.
In 15 cases, marked jet flow between the cysts and the cisterns were positive on PC cine MR imaging (Fig 1). Twenty-four cases had no alteration in signal intensity within the cysts regarded as being noncommunicating (Fig 2). There was no borderline alteration in signal intensity within cysts with uncertain communication after the PC cine MR examination. In middle cranial fossa and cerebellopontine cistern cysts, jet flow was most reliably depicted on axial and coronal images. Axial and sagittal images were more useful for cysts in the quadrigeminal cistern.
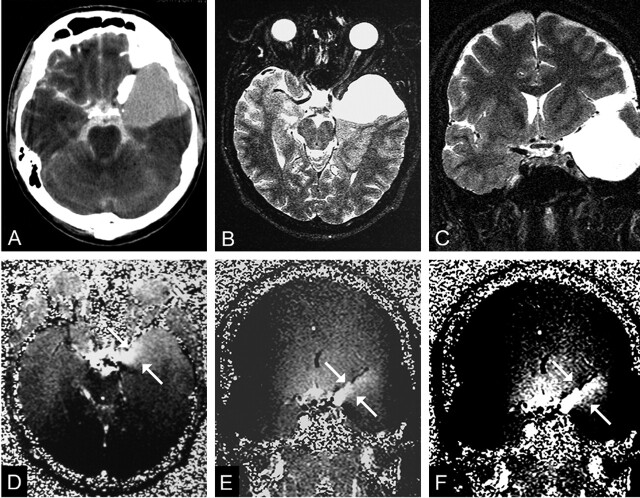
Fig 1.
A 20-year-old man with headaches and a communicating left middle cranial fossa AC (patient 10).
A, CTC shows homogeneous contrast enhancement in the cyst.
B and C, Transverse (B) and coronal (C) T2-weighted images (TR/TE/NEX, 7400/115/1) obtained before PC cine MR imaging helps in proper section orientation.
D and E, Transverse(D) and coronal (E) PC cine MR images (TR/TE/flip angle, 70/15.8/10°) show hyperintensity (arrows) arising from left chiasmatic cistern, which represents communication with the subarachnoid space.
F, Adjusting the windowing makes the flow jet (arrows) more clear.
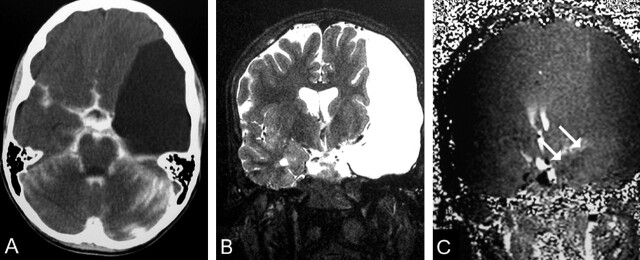
Fig 2.
A 17-year-old male adolescent with convulsions and a left middle cranial fossa noncommunicating AC (patient 33).
A, CTC shows no contrast enhancement on delayed scan.
B, Coronal T2-weighted image (TR/TE/NEX, 7400/115/1) shows the cyst causing a mild midline shift.
C, Coronal PC cine MR image (TR/TE/flip angle, 70/15.8/10°) shows no signal intensity alteration in the cyst (arrows). Cyst and brain have the same signal intensity pattern.
PC cine MR imaging results were confirmed with CTC findings in 25 cases, with surgical findings in seven cases, and with both CTC and surgical results in seven cases. In 36 (92.3%) of 39 cysts, PC cine MR results corresponded well with the results of the CTC or surgery (Table 1). There were three false-negative MR studies, which failed to demonstrate the communication established on CTC. There was no discrepancy in cases invovling both CTC and surgery. The sensitivity and specificity of PC cine MR imaging in demonstrating the communication between the ACs and the CSF were 80% and 100%, respectively.
Discussion
Although there has been considerable controversy regarding the indications for the surgical treatment of asymptomatic ACs, the literature seems to show some consensus that patients with symptomatic cysts causing seizures, hydrocephalus, increased intracranial pressure, or neurologic impairment should be treated. Basically, two surgical approaches have been used: 1) Craniotomy with cyst excision or fenestration into the subarachnoid space, basilar cisterns, or ventricles, and 2) cystoperitoneal shunt placement (23–26). The determination of whether the AC is communicating to the CSF space is important in the preoperative evaluation. Because conventional MR images and CT fail to solve this problem, CTC is generally included in the diagnostic work-up (12, 17, 20, 27–29). However, the major disadvantages of CTC are its invasiveness, considerable amount of x-ray exposure, long imaging time, and cost.
The issue we face is whether PC cine MR imaging may be a part of the routine in the evaluation of ACs. The evaluation of CSF flow with flow-sensitive MR images was reported previously. Most of these reports have focused on physiologic flow patterns of CSF or abnormal flow patterns, especially in obstructed CSF pathways (30–33). Only a few studies concern the flow dynamics in ACs. Hoffmann et al (22) reported that, by using ECG-gated cine-mode steady-state precession sequence (PSIF), they increased the diagnostic certainty regarding the communication between cysts and CSF spaces in 18 of 19 cysts. They used the depiction of a jetlike flow void at the assumed communication site as the most convincing criterion to confirm communication of the cysts. Eguchi et al (21) used PC cine MR with a 2D FLASH sequence (similar to the sequence used in our study) in 10 patients with middle cranial fossa cysts. They found jet flow in the cyst to be a reliable finding indicating communication of ACs.
We obtained PC cine MR images with a 2D FLASH sequence. The flow sensitivity of this sequence is achieved by the interleaved flow-compensated and flow-sensitive measurements performed in a frequency-encoding direction. Marked signal intensity alteration in the cyst, representing the flow between cyst and CSF, was observed in all communicating cysts. None of the ACs in our study were regarded as “probably communicating.” Although we suggest that the appearance of the jet flow within the cyst definitely proves that the cyst has communication, more reliable results will be achieved as the cohort expands. We did not observe any slight intensity alterations in the noncommunicating cysts that might be interpreted as consequences of CSF fluctuation. This can be explained with the insensitivity of the PC 2D FLASH sequence to these kind of complex, low-magnitude flow patterns. PC sequences are more suitable for evaluating laminar flow. The PC 2D FLASH sequence used in our study has sensitivity to velocity as low as 2 cm/s. Brooks et al (34) reported that a Steady-State Free Precession (SSFP) imaging technique (PSIF sequence) is sensitive to velocities in the range of 1 mm/s. In the SSFP imaging technique, slight CSF flow or even CSF pulsation decreases the steady state of transverse magnetization and leads to a signal void. Hoffmann et al (22) suggest that SSFP imaging is better than other sequences (FLASH, gradient-recalled acquisition in the steady state [GRASS]) to detect dephasing due to complex flow. However we do not think that increasing the sensitivity to slow flow has a substantial effect on evaluating ACs in clinical practice; in fact, it may increase false-positive findings.
In the present study, PC cine MR imaging failed to show communication in three ACs. One was a quadrigeminal cistern cyst in which contrast enhancement was observed in the late phases of CTC (Fig 3). The other two cysts were located in the middle cranial fossa and showed rapid filling of contrast material on CTC. The inability to determine the true communication between ACs and CSF spaces may be related to an unsuitable section orientation. Appropriate section orientation is a prerequisite to make a reliable measurement. Almost all ACs occur in relation to an arachnoid cistern. In addition the operator must carefully define the assumed communication sites on T2-weighted images. Operator dependence can be regarded a weakness of the technique. Cysts with restricted communication may be another cause of technical insufficiency. However, therapeutic decisions generally do not change in cases of slowly communicating cysts, since they have a tendency to expand like noncommunicating cysts.
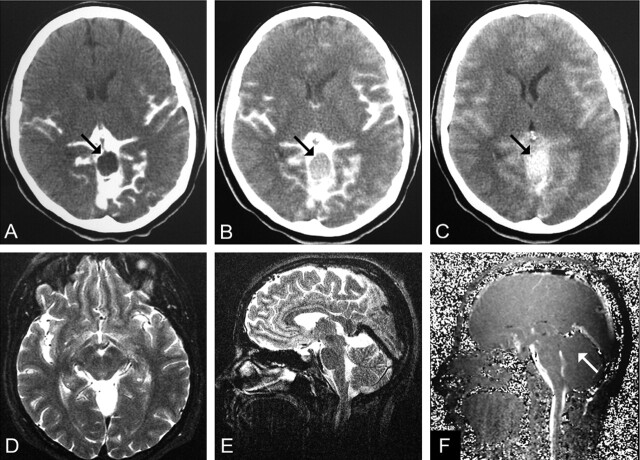
Fig 3.
A 37-year-old woman with headaches and a communicating quadrigeminal cistern AC (patient 15).
A, CTC performed 2 hours after an intratechal injection of contrast agent shows no enhancement (arrow). Intracystic attenuation was 9 HU.
B, CTC at 12 hours shows mild enhancement (51 HU, arrow).
C, CTC at 24 hours shows clear enhancement (88 HU, arrow).
D and E, Transverse (D) and coronal (E) T2-weighted images (TR/TE/NEX, 7400/115/1).
F, Midsagittal PC cine MR image (TR/TE/flip angle, 70/15.8/10°) shows no evidence of communication (arrow). No signal intensity alterations were seen on transverse and coronal images (not shown).
We performed PC cine MR imaging in three orthogonal planes. However, in our experience, the identification of jet flow in one of the orthogonal planes makes it unnecessary to obtain images in other planes; eliminating these others helps to reduce the imaging time.
The differential diagnosis of the posterior fossa cysts has always been a challenge for radiologists. Five of the cysts in this series were located in the retrocerebellar cistern. These cysts were noncommunicating on PC cine MR images. The operative findings showed that all were true cysts with no communication. Beyond these five cases, we performed PC cine MR and CTC in 13 patients with the presumptive diagnosis of retrocerebellar ACs on cranial MR examination. All of these cysts were communicating on both PC cine MR and CTC images. These lesions were accepted as mega cisterna magna and beyond the scope of this study. However, a considerable number of ACs in this series were communicating, and some of these posterior fossa lesions, regarded as mega cisterna magna, might have been communicating ACs. We could not find any data in the literature concerning the radiologic differentiation of mega cisterna magna from communicating ACs. Can-flow sensitive cine MR techniques help in the characterization of posterior fossa cysts? This question may be answered as flow-sensitive MR techniques are included in the diagnostic work-up of posterior fossa cysts.
In this series, the most common location of ACs was the middle cranial fossa. According to the widely accepted classification system by Galassi et al (35) and others (36–37), middle cranial fossa cysts are categorized into three types. Type I cysts are located in the sylvian fissure, posterior to the sphenoid ridge without any mass effect; these freely communicate with the subarachnoid space. Type II cysts are larger, rectangular, and localized at proximal and middle part of the sylvian fissure. These slowly communicate with the subarachnoid space. Type III cysts are the largest and lenticular shaped. They generally cause midline shift without communication to the subarachnoid space. In this study, it was interesting to observe that two type I cysts of the middle cranial fossa showed no communication on both PC cine MR and CTC, and one type III cyst demonstrated clear communication on both PC cine MR and CTC (Fig 4).
Fig 4.
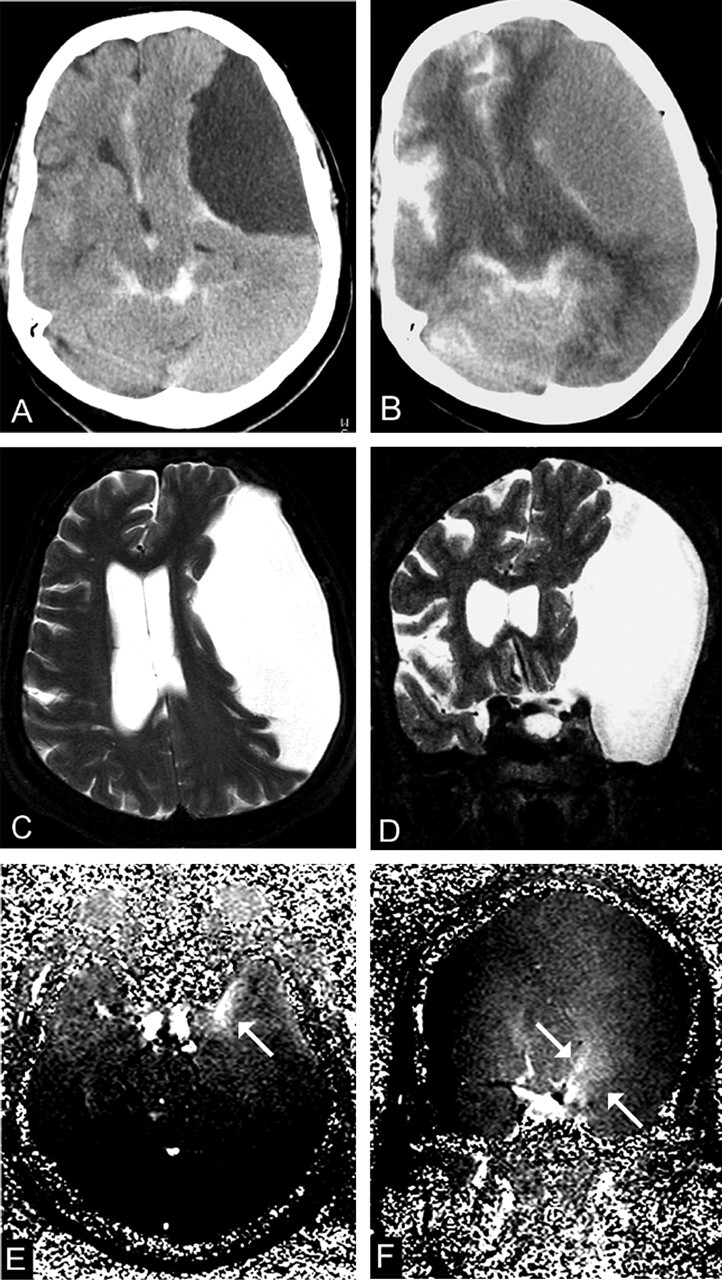
A 66-year-old woman with a type III cyst in the left middle cranial fossa (patient 9).
A, CTC 2 hours after intratechal contrast injection shows no enhancement (8 HU).
B, CTC at 12 hours shows intracystic enhancement of 46 HU.
C and D, Transverse (C) and coronal (D) T2-weighted images (TR/TE/NEX, 7400/115/1) show marked midline shift.
E and F, Transverse (E) and coronal (F) PC cine MR images (TR/TE/flip angle, 70/15.8/10°) shows evidence of a flow jet (arrows), although communication with the cisternal space is unlikely in type III cysts.
CTC is generally accepted as a criterion standard method to verify communication between the subarachnoid space and ACs. However, we performed 31 CTC procedures and experienced technical failure in two. In both cases, we performed the intratechal injection and then observed the column of contrast agent above the lower thoracic vertebrae on fluoroscopic control; however, no contrast enhancement was seen on cranial CT. We investigated these cases and found spontaneous intracranial hypotension in one case, which might have explained the absence of contrast enhancement on CT, but we could not explain the other case. In both, PC cine MR imaging showed communicating cysts. We excluded these two cases as we could not repeat the CTC study.
Conclusion
The results of our study indicate the utility of PC cine MR imaging as a reasonable noninvasive alternative to CTC for the functional evaluation of ACs. Following the conventional MR procedure, PC cine MR imaging can be performed easily, especially in candidates of surgery. CTC should be reserved for cases in which PC cine MR imaging fails to demonstrate the connection between the cyst and the subarachnoid space.
References
- 1.Artico M, Cervoni L, Salvati M, et al. Supratentorial arachnoid cysts: clinical and therapeutic remarks on 46 cases. Acta Neurochir 1995;132:75–78 [DOI] [PubMed] [Google Scholar]
- 2.Passero S, Filosomi G, Cioni R, et al. Arachnoid cysts of the middle cranial fossa: a clinical, radiological and follow-up study. Acta Neurol Scand 1990;82:94–100 [DOI] [PubMed] [Google Scholar]
- 3.von Wild K. Arachnoid cysts of the middle cranial fossa. Neurochirurgia 1992;35:177–182 [DOI] [PubMed] [Google Scholar]
- 4.Parsch CS, Krauss J, Hofmann E, et al. Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow-up, and review of the literature. Neurosurgery 1997;40:483–490 [DOI] [PubMed] [Google Scholar]
- 5.Leo JS, Pinto RS, Hulvat GF, et al. Computed tomography of arachnoid cysts. Radiology 1979;130:675–680 [DOI] [PubMed] [Google Scholar]
- 6.Aicardi J, Bauman F. Supratentorial extracerebral cysts in infants and children. J Neurol Neurosurg Psychiatry 1975;38:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol 1981;40:61–83 [PubMed] [Google Scholar]
- 8.Spaziante R, Cirillo S, Constans JP, et al. Arachnoid cyst of the quadrigeminal cistern. Neurochirurgia 1986;29:117–123 [DOI] [PubMed] [Google Scholar]
- 9.Garcia Santos JM, Martinez-Lage J, Gilabert Ubeda A, et al. Arachnoid cysts of the middle cranial fossa: a consideration of their origins based on imaging. Neuroradiology 1993;35:355–358 [DOI] [PubMed] [Google Scholar]
- 10.Lange M, Oeckler R. Results of surgical treatment in patients with arachnoid cysts. Acta Neurochir 1987;87:99–104 [DOI] [PubMed] [Google Scholar]
- 11.Gentry LR, Menezes AH, Turski PA, et al. Suprasellar arachnoid cysts, II: evaluation of CSF dynamics. AJNR Am J Neuroradiol 1986;7:87–96 [PMC free article] [PubMed] [Google Scholar]
- 12.Crisi G, Calo M, De Santis M, et al. Metrizamide-enhanced computed tomography of intracranial arachnoid cysts. J Comput Assist Tomogr 1984;8:928–935 [DOI] [PubMed] [Google Scholar]
- 13.Galassi E, Tognetti F, Pozzati E, et al. Extradural hematoma complicating middle fossa arachnoid cyst. Childs Nerv Syst 1986;2:306–308 [DOI] [PubMed] [Google Scholar]
- 14.Koch CA, Voth D, Kraemer G, et al. Arachnoid cysts: does surgery improve epileptic seizures and headaches? Neurosurg Rev 1995;18:173–181 [DOI] [PubMed] [Google Scholar]
- 15.Becker T, Wagner M, Hofmann E, et al. Do arachnoid cysts grow? A retrospective CT volumetric study. Neuroradiology 1991;33:341–345 [DOI] [PubMed] [Google Scholar]
- 16.Santamarta D, Aguas J, Ferrer E. The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg 1995;38:133–137 [DOI] [PubMed] [Google Scholar]
- 17.Wolpert SM, Scott RM. The value of metrizamide CT cisternography in the management of cerebral arachnoid cysts. AJNR Am J Neuroradiol 1981;2:29–35 [PMC free article] [PubMed] [Google Scholar]
- 18.Miyajima M, Arai H, Okuda O, et al. Possible origin of suprasellar arachnoid cysts: neuroimaging and neurosurgical observations in nine cases. J Neurosurg 2000;93:62–67 [DOI] [PubMed] [Google Scholar]
- 19.Shigemori M, Okura A, Takahashi Y, et al. New surgical treatment of middle fossa arachnoid cyst. Surg Neurol 1996;45:189–192 [DOI] [PubMed] [Google Scholar]
- 20.Arai H, Sato K, Wachi A, et al. Arachnoid cysts of the middle cranial fossa: Experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery 1996;39:1108–1112 [DOI] [PubMed] [Google Scholar]
- 21.Eguchi T, Taoka T, Nikaido Y, et al. Cine-magnetic resonance imaging evaluation of communication between middle cranial fossa arachnoid cysts and cisterns. Neurol Med Chir 1996;36:353–357 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann KT, Hosten N, Meyer BU, et al. CSF flow studies of intracranial cysts and cyst-like lesions achieved using reversed fast imaging with steady-state precession MR sequences. AJNR Am J Neuroradiol 2000;21:493–502 [PMC free article] [PubMed] [Google Scholar]
- 23.Raffel C, McComb JG. To shunt or to fenestrate: Which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery 1988;23:338–342 [DOI] [PubMed] [Google Scholar]
- 24.Schroeder HW, Gaab MR, Niendorf WR. Neuroendoscopic approach to arachnoid cysts. J Neurosurg 1996;85:293–298 [DOI] [PubMed] [Google Scholar]
- 25.Furuta S, Hatakeyama T, Nishizaki O, et al. Usefulness of neuroendoscopy in treating supracollicular arachnoid cysts–case report. Neurol Med Chir 1998;38:107–109 [DOI] [PubMed] [Google Scholar]
- 26.Ruge JR, Johnson RF, Bauer J. Burr hole neuroendoscopic fenestration of quadrigeminal cistern arachnoid cyst: technical case report. Neurosurgery 1996;38:830–837 [PubMed] [Google Scholar]
- 27.Samii M, Carvalho GA, Schuhmann MU, et al. Arachnoid cysts of the posterior fossa. Surg Neurol 1999;51:376–382 [DOI] [PubMed] [Google Scholar]
- 28.Tamas LB, Wyler AR. Intracranial mucocele mimicking arachnoid cyst: case report. Neurosurgery 1985;16:85–86 [PubMed] [Google Scholar]
- 29.Yamakawa H, Ohkuma A, Hattori T, et al. Primary intracranial arachnoid cyst in the elderly: a survey on 39 cases. Acta Neurochir 1991;113:42–47 [DOI] [PubMed] [Google Scholar]
- 30.Greitz D, Franck A, Nordell B. On the pulsatile nature of intracranial and spinal CSF-circulation demonstrated by MR imaging. Acta Radiol 1993;34:321–328 [PubMed] [Google Scholar]
- 31.Greitz D, Wirestam R, Franck A, et al. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging: the Monro-Kellie doctrine revisited. Neuroradiology 1992;34:370–380 [DOI] [PubMed] [Google Scholar]
- 32.Nitz WR, Bradley WG Jr, Watanabe AS, et al. Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology 1992;183:395–405 [DOI] [PubMed] [Google Scholar]
- 33.Citrin CM, Sherman JL, Gangarosa RE, et al. Physiology of the CSF flow-void sign: modification by cardiac gating. AJR Am J Roentgenol 1986;148:205–208 [DOI] [PubMed] [Google Scholar]
- 34.Brooks ML, Jolesz FA, Patz S. MRI of pulsatile CSF motion within arachnoid cysts. Magn Reson Imaging 1988;6:575–584 [DOI] [PubMed] [Google Scholar]
- 35.Galassi E, Tognetti F, Gaist G, et al. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 1982;17:363–369 [DOI] [PubMed] [Google Scholar]
- 36.Callaway MP, Renowden SA, Lewis TT, et al. Middle cranial fossa arachnoid cysts: not always a benign entity. Br J Radiol 1998;71:441–443 [DOI] [PubMed] [Google Scholar]
- 37.Arai H, Sato K, Wachi A, et al. Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery 1996;39:1108–1112 [DOI] [PubMed] [Google Scholar]