Abstract
Summary: We report a case of cauda equina syndrome caused by Gnathostoma spinigerum, which was confirmed by an immunoblotting test. MR imaging of the lumbosacral spine showed long, segmented hyperintensity along the cauda equina with irregular enhancement on the postcontrast study. The conus medullaris was slightly enlarged with abnormal enhancement. The patient was treated with corticosteroids, and her clinical condition improved. MR imaging, 9 months after treatment, showed the condition to be completely resolved.
Gnathostomiasis (Gnathostoma spinigerum infestation) is commonly found in Thailand and Japan and is caused mainly by eating raw infested fish. Most cases present with cutaneous involvement (migratory swelling or urticaria) and neurologic findings (i.e., radiculitis, myelitis, subarachnoid hemorrhage, or intracerebral hemorrhage).
MR imaging of the affected area of the nervous system may help in assessing severity, and revealing the presence of gnathostomiasis and assessing the severity of disease (1). We report a case with involvement of the cauda equina.
Case History
A 56-year-old woman presented with sudden and severe radicular pain from the buttocks to both legs followed by weakness in her legs. She had urinary incontinence 7 days before admission. She recalled no history of migratory swelling, trauma to the back, or having eaten raw fish. On physical examination, she was afebrile and had no migratory swelling or creeping eruption. She was oriented and had paraparesis (hip flexor grade III/V; hip extensor grade II/V; knee flexor grade I/V; knee extensor grade IV/V; ankle flexor and extensor grade IV/V; and extensor hallucis longus grade III/V, bilaterally). She also had decreased pinprick sensation in the perianal area and saddle anesthesia, and the tone of the anal sphincter was decreased with a positive anal wink and bulbocarvernosus reflex. The patient’s quadriceps reflex was 1+ on both sides. The blood count revealed eosinophilia with normal platelet count. Prothrombin time, activated partial thrombin time, and findings on lumbosacral spine radiographs were normal. MR imaging of the lumbosacral spine showed a long, segmental hyperintensity along the cauda equina on T1-weighted (Fig 1A, B) and T2-weighted (Fig 1C, D) images. Postgadolinium injection with fat suppression images showed a long, segmental linear enhancement along the cauda equina and increased enhancement of the conus medullaris (Fig 1E), while the axial images showed irregular enhancement of both the cauda equina (Fig 1F) and the conus medullaris (Fig 1G).
Fig 1.
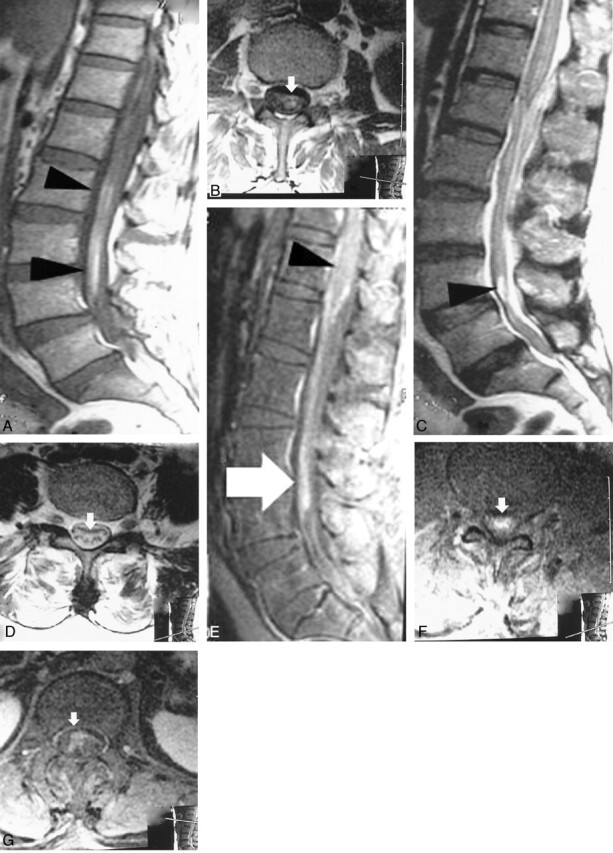
MR imaging of LS spine before treatment.
A, Midsagittal, noncontrast T1-weighted MR image reveals a long segmental hyperintense signal along the cauda equina at the lower lumbar region (arrows).
B, Axial, noncontrast T1-weighted MR image shows hyperintense signal at the same level of as that of A.
C, Midsagittal T2-weighted MR image shows hyperintense signal along cauda equina (arrow).
D, Axial noncontrast T2-weighted MR image reveals hyperintense signal of cauda equina at the same level as that of C (arrow).
E, Midsagittal postgadolinium injection T1-weighted with fat suppression MR image reveals a long segmental linear enhancement along the cauda equina at the lower lumbar region (white arrow). The conus medullaris is slightly enlarged with increase enhancement (black arrow).
F, Axial postgadolinium injection, T1-weighted MR image shows irregular hyperintense signal of cauda equina (at the level of white arrow in E).
G, Axial postgadolinium injection, T1-weighted MR image reveals irregular hyperintense signal of conus medullaris (at the level of black arrow in E).
Lumbar puncture showed xanthochromic CSF. The CSF opening pressure was 180 mm H2O; the white blood cell count, 750 cells/mm3 (lymphocytes 63%, eosinophils 27%, neutrophils 7%); the red blood cell count, 5650 cells/mm3; protein, 182 mg/dL; and glucose, 11 mg/dL (plasma glucose, 119 mg/dL), or 9% compared with plasma glucose. No bacteria or fungi were cultured. The serum was found to be positive for Gnathostoma spinigerum after immunoblotting by using the 24-kDa diagnostic band and negative for Angiostrongylus cantonensis (the same technique but by using the 31-kDa diagnostic band).
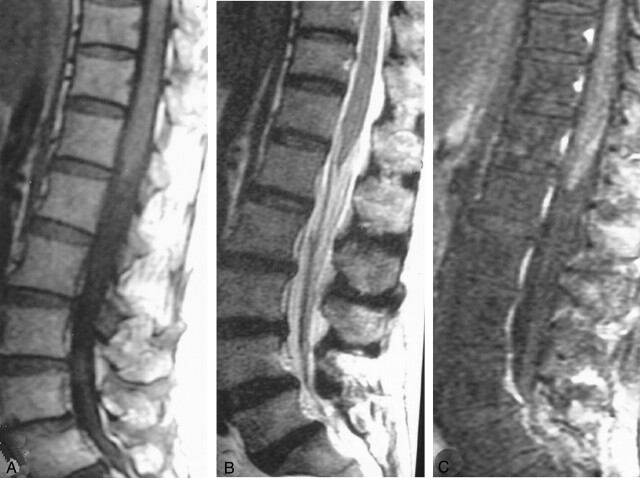
The patient received prednisolone 1 mg/kg/dose for 3 weeks and felt relief from the severe radicular pain. One month after treatment with rehabilitation and bladder training, the patient gradually improved motor control and urinary continence. After 6 months, she had no noticeable motor weakness or incontinence. MR imaging of the lumbosacral spine at 9-month follow-up showed improvement (Fig 2). The long hyperintensity along the cauda equina disappeared on T1- and T2-weighted images (Fig 2A, B). The roots of the cauda equina appeared clumped together on T2-weighted images (Fig 2B). Postgadolinium imaging showed contrast enhancement of cauda equina (Fig 2C).
Fig 2.
MR imaging of LS spine following treatment.
A, Sagittal T1-weighted MR image shows normal finding of cauda equina.
B, Sagittal T2-weighted MR image reveals clumping of the roots of cauda equina without a hyperintense signal.
C, Midsagittal postgadolinium T1-weighted image with fat suppression shows the enhancement of cauda equina.
Discussion
The clinical manifestation of this patient corresponded with a cauda equina syndrome, and MR imaging showed a long segmental hyperintensity along the cauda equina. Postgadolinium injection showed irregular-shaped enhancement at the cauda equina and conus medullaris. These findings suggested a subacute hemorrhage at the cauda equina and an inflammatory process at the terminus of the cord.
Differential causes of bleeding in the cauda equina include bleeding diathesis (2), parasitic infestation (gnathostomiasis), or bleeding tumor (3). The patient did not have any other bleeding disorder or evidence of malignancy. The sudden sacral pain with radicular pain to both legs is not characteristic of tumor pain (4, 5) and was consistent with gnathostomiasis infection. The mechanisms of bleeding caused by Gnathostoma spinigerum include tissue and vascular injury (6, 7).
A lumbar puncture, which demonstrated xanthochromic CSF with eosinophils (>10%) suggested gnathostomiasis (1, 8). The diagnosis was confirmed through the positive serum immunoblotting test by using the 24-kDa diagnostic band.
The presence of eosinophils in the CSF can be caused by Angiostrongylus cantonensis. We therefore performed immunoblotting for A. cantonensis as well, but the result was negative. There is, in fact, no report of any case of spinal cord lesion or bleeding tracts from Angiostronyliasis, probably because it is a smaller organism than Gnathostoma spinigerum. The enhancement of the terminal lumbar cord with enhancement was similar with a previous report of spinal gnathostomiasis (1). The eosinophilia at the initial presentation supported the diagnosis of this tissue parasite.
Antihelmintics, such as albendazole, or corticosteroids, have not been proved effective against neurologic gnathostomiasis (6, 9). Corticosteroids are believed to reduce the inflammatory process in damaged tissue (6). We therefore treated the patient with prednisolone (1 mg/kg/d) for 3 weeks, and she gradually improved. In 1 month, her severe radicular pain was relieved followed by recovery of motor strength and sensory acuity, and, after 4 months, she regained urinary continence. At 9 months, the MR imaging of lumbosacral region improved (Fig 2) and she had no residual neurologic deficit.
Acknowledgments
The authors thank the patient for allowing us to report the case and Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript.
References
- 1.Sawanyawisuth K, Tiamkao S, Kanpittaya J, et al. MR Imaging findings in cerebrospinal gnathostomiasis. AJNR Am J Neuroradiol 2004;25:446–449 [PMC free article] [PubMed] [Google Scholar]
- 2.Willems J, Anne A, Herregods P, et al. A cauda equina syndrome in a patient treated with oral anticoagulants: case report. Paraplegia 1994;32:277–280 [DOI] [PubMed] [Google Scholar]
- 3.Cordan T, Bekar A, Yaman O, Tolunay S. Spinal subarachnoid hemorrhage attributable to schwannoma of the cauda equina. Surg Neurol 1999;51:373–375 [DOI] [PubMed] [Google Scholar]
- 4.Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression: occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir 1990;107:37–43 [DOI] [PubMed] [Google Scholar]
- 5.Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastasis with neurological manifestations: review of 600 cases. J Neurosurg 1983;59:111–118 [DOI] [PubMed] [Google Scholar]
- 6.Miriam LC, David TD. Helminthic infections. In: Scheld WM, Whitley RJ, Durack DT, eds. Infections of the Central Nervous System. 2nd ed. Philadelphia: Lippincott-Raven;1997. :863–864
- 7.del Brutto OH. Cerebrovascular disease in the tropics. Rev Neurol 2001;33:750–762 [PubMed] [Google Scholar]
- 8.Daengsvang S. Gnathostomiasis in Southeast Asia. Southeast Asian J Trop Med Public Health 1981;12:319–332 [PubMed] [Google Scholar]
- 9.Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis 2001;32:E154–E157 [DOI] [PubMed] [Google Scholar]