Abstract
BACKROUND AND PURPOSE: Our purpose was to investigate the association between cervicomedullary neuroschisis and mirror movements in patients with Klippel-Feil syndrome (KFS).
METHODS: We conducted a retrospective analysis of 23 patients with KFS who were seen at our institution during a 10-year period. Sixteen of the 23 patients had undergone adequate axial view cross-sectional imaging of the upper cervical spine. The degree of neuroschisis was assessed for each patient, using an objective scoring system. Twelve patients were evaluated for the presence or absence of mirror movements.
RESULTS: A high percentage of female patients with KFS was noted (17 [74%] of 23 patients). Adequate cross-sectional images were available for 16 of the 23 patients, six (38%) of whom had some form of cervicomedullary neuroschisis. Five of the six patients had been clinically evaluated, and all were shown to have mirror movements. One patient with Chiari II malformation, which obscured evaluation for neuroschisis, also had mirror movements. Of the remaining nine patients without cervicomedullary neuroschisis, six were evaluated, and none of the six had mirror movements. A review of the theoretical neuroanatomic basis of mirror movements is presented herein, and neurosurgical management concerns for patients with KFS are discussed.
CONCLUSION: A strong association exists between cervicomedullary neuroschisis and mirror movements in cases of KFS. Screening of patients with mirror movements may help identify clinically unsuspected KFS and may also help stratify risk within this patient population, identifying patients who might benefit from early neurosurgical intervention.
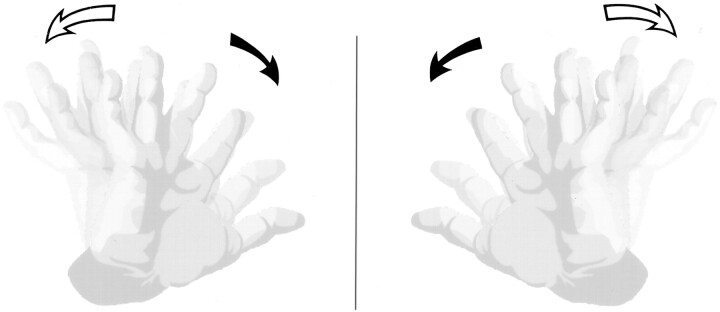
The initial report by Klippel and Feil (1) described a tailor with the triad of short neck, low hairline, and limited neck mobility found to have only four cervical vertebrae at autopsy. Twenty years later, in 1932, Bauman (2) described a series of six patients with Klippel-Feil syndrome (KFS), four of whom displayed abnormal mirror movements. These were defined as movements in which the voluntary (active) movements in one extremity were mimicked by the involuntary (passive) movements in the other with a central plane of symmetry (Fig 1). Mirror movements have since been recognized as a common association with KFS (3–12). Associated neuropathologic findings were first described in 1936 by Avery and Rentfro (13), who reported a case of incomplete neuroschisis of the cervical spinal cord and lack of pyramidal decussation in a patient who had KFS and mirror movements. The exact anatomic basis of mirror movements has been studied but remains in question (11, 12, 14–19). We conducted a retrospective analysis of 23 patients with KFS who were seen at our institution during a 10-year period, to investigate the association of mirror movements and neuroschisis in this group of patients. We herein present a review of the literature regarding these entities and the theoretical neuroanatomic basis of mirror movements.
Fig 1.
Illustration of mirror movements. With mirror movements, voluntary (active) movements of one extremity are mimicked by involuntary (passive) movements in the opposite extremity with a central plane of symmetry. Thus, movements of the hand and individual digits also occur in the opposite hand as if a central mirror were reflecting its image to the opposite side.
Methods
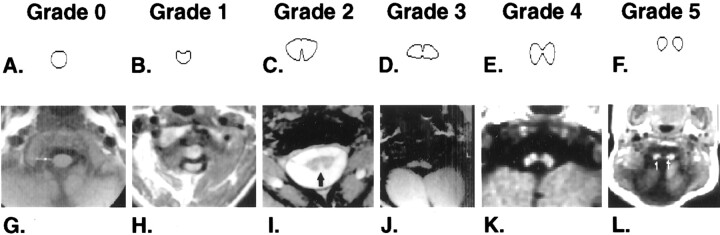
We reviewed the medical records of a 10-year period at the Children’s Hospital of Alabama and identified 23 patients with a diagnosis of KFS. All the available radiologic studies were reviewed. Of the patients with available radiologic studies, a total of 16 had undergone adequate axial cross-sectional imaging of the cervical spine. Thirteen had undergone MR imaging, two had undergone CT and/or myelography, and one had undergone unenhanced CT with adequate contrast to show cord morphology. One additional patient who had undergone MR imaging of the cervical spine was not included in this group because only sagittal imaging had been performed. A grading system of the severity of neuroschisis was developed on the basis of the appearance of clefting at the cervicomedullary junction on axial images (Table 1, Fig 2). The highest grade of clefting on any of the axial images was noted and the level assessed. Conventional images were also reviewed, and the number and levels of fused cervical vertebrae were also noted. All MR imaging had been performed with either a 0.5-T Diasonics system or a 1.5-T GE Signa system by using primarily T1-weighted standard spin-echo sequences for anatomic evaluation. All CT had been performed on a GE 9800 system.
TABLE 1:
Grading system for cervical cord neuroschisis
| Grade | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Axial imaging appearance | Normal cord | Deformity without cleft | Posterior cleft alone | Anterior cleft with or without posterior cleft | Bow tie configuration | Complete neuroschisis |
Fig 2.
Illustration of grading system for neuroschisis.
A-F, Line drawings of the grading system of neuroschisis.
G-L, Axial MR image examples of each grade chosen from patients in this study.
Of the 16 patients with adequate axial images available, 12 were successfully contacted and referred for neurologic assessment. The patient with only nonenhanced CT scans (grade 0) and three other patients (two with cord grade l and one with cord grade 4) who could not be contacted were not clinically assessed. General neurologic evaluation was performed in addition to a directed evaluation to check for the presence of gross mirror movements associated with voluntary active movements of each extremity. Correlation of cord morphology, mirror movements, and the extent of cervical fusion was performed.
Results
The original 23 patients identified ranged in age from 1 month to 26 years. Seventeen (74%) were female and six (26%) were male patients. Sixteen patients had undergone axial view imaging and, according to our grading system, four patients had normal cord appearance. Five had some form of extrinsic cord deformity related to bony canal stenosis or other mechanical factors without intrinsic cord clefting. One had isolated posterior clefting. This was the only patient who also had T2-weighted images, and no intrinsic signal abnormality of the cord was present. Two had anterior and posterior clefting. Two had bow-tie configuration, and one had complete diaschisis. The remaining patient had Chiari II malformation with vermian extension down to the body of C2, obscuring the posterior aspect of the cervical cord and preventing accurate assessment for neuroschisis. The location of maximum clefting used for grading ranged from the mid clivus to the body of C2 and grossly corresponded to the expected level of the cervicomedullary junction in all cases. The degree of osseous deformity at the craniocervical junction caused some variation in the assignment of the level of maximum clefting. The superior aspect of the cleft ranged from the mid clivus to the tip of the dens, and the inferior aspect ranged from the body of C2 to the mid-cervical block vertebral level, with the cleft length ranging from 2.0 to 5.0 cm (mean, 3.1 cm).
Of the 16 patients with cross-sectional images, four were not clinically assessed: three without neuroschisis and one with bow-tie configuration of neuroschisis. Of the 12 patients who were clinically assessed, the six patients without neuroschisis had no evidence of mirror movements. The patient with Chiari II malformation, which made cord evaluation difficult, did have mirror movements. The five remaining patients with documented neuroschisis were also shown to have mirror movements (Table 2).
TABLE 2:
Correlation of mirror image movements with cervical cord clefting
| Grade | 0 | 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|---|---|
| Axial imaging appearance | Normal cord | Deformity without cleft | Posterior cleft alone | Anterior cleft with or without posterior cleft | Bow tie configuration | Complete neuroschisis | Cord obscured | Total |
| MM (+) | 0 | 0 | 1 | 2 | 1 | 1 | 1* | 6 |
| MM (−) | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 6 |
| N/E | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 4 |
| Total | 4 | 5 | 1 | 2 | 2 | 1 | 1 | 16 |
Note.—MM indicates mirror movements; N/E, not clinically evaluated.
Chiari II malformation with posterior cord obscured by tonsillar peg.
Table 2 shows the correlation of mirror image movements with cervical cord clefting in patients with KFS. The results indicate some form of clefting in all except one patient with mirror movements and no clefting in patients without mirror movements. Four patients who underwent axial imaging were not clinically evaluated.
An analysis of the extent of cervical spine fusion and the presence of mirror movements was conducted. On the basis of conventional radiographic findings, patients were placed into three groups. Patients in the first group each had an isolated fusion of two cervical vertebrae, patients in the second group each had more than one isolated fusion, and patients in the third group each had at least three contiguous vertebrae involved. This revealed an increasing incidence of mirror movements with greater extent of cervical fusion (Table 3). A similar analysis also correlated the incidence of neuroschisis with more extensive cervical fusion (Table 4).
TABLE 3:
Correlation of conventional films with mirror movements
| Extent of Vertebral Fusion | Single Level of Fusion | >1 Level of Fusion | Extensive Fusion | Total |
|---|---|---|---|---|
| MM (+) | 0 | 2 | 4 | 6 |
| MM (−) | 2 | 2 | 2 | 6 |
| Total | 2 | 4 | 6 | 12 |
Note.—MM indicates mirror movements.
TABLE 4:
Correlation of conventional films with neuroschisis
| Extent of Vertebral Fusion | Single Level of Fusion | >1 Level of Fusion | Extensive Fusion | Total |
|---|---|---|---|---|
| NS (+) | 0 | 1 | 5 | 6 |
| NS (−) | 2 | 6 | 2 | 10 |
| Total | 2 | 7 | 7 | 16 |
Note.—NS indicates neuroschisis.
Table 3 shows the correlation of conventional films with mirror movements in patients with KFS. Mirror movements occurred only when fusion involved more than one vertebral level and, more commonly, when there was extensive vertebral fusion.
Table 4 shows correlation of conventional films with neuroschisis in patients with KFS syndrome. Neuroschisis had a higher incidence, with extensive vertebral fusion.
No mirror movements of the legs were observed, and none were induced with passive movement. Patients as young as 6 months had easily observed mirror movements of the arms and hands. The severity was never debilitating but frequently annoying to the patients. Clinical weakness due to the KFS anomaly at presentation was observed in only one patient. Neck pain with accelerated osteoarthritis was more common (three [13%] of 23 patients).
Almost every patient had some additional congenital abnormality. Sprengel deformity occurred most commonly (four [17%] of 23 patients). Associated CNS findings included occipital encephalocele, Chiari II malformation, Dandy-Walker cyst, semilobar holoprosencephaly, Duane syndrome, syringomyelia, nasofrontal dermoid, sensorineural hearing loss, and neurofibromatosis type 1. Other anomalies included cervical ribs, radial supranumary digit, tracheal and proximal bronchial stenosis, gastroesophageal reflux, sickle sacrum, cleft palate, and various renal anomalies.
Discussion
In 1912, Klippel and Feil (1) first described what is known today as KFS. In 1919, Feil (20) reported 13 additional cases, which he classified into three groups. Group 1 was the classic no neck form, with complete cervical fusion; group 2 had isolated fusions most commonly involving C2-C3 and/or C5-C6; and group 3 had separate thoracic and/or lumbar fusions in addition to cervical involvement. This classification has remained useful during the years, and the term KFS is generally regarded to include any form of congenital cervical vertebral fusion (9).
There are many conditions recognized as being associated with KFS (6–9, 21–32), including mirror movements, which were first described by Bauman (2) in 1932 and confirmed by Mitchell (33) in 1934. After those publications, little regarding mirror movements was published in the literature until 1967, when Baird et al (34) reported 10 of 13 patients with KFS who had electromyographic evidence of involuntary contralateral mirrored movements of the abductor pollicis brevis muscle with voluntary ipsilateral movement. EMG evidence of similar mirror movements was found in only one of 13 age- and sex-matched controls. Today, we recognize that mirror movements tend to affect the hands, although the entire upper extremity and, rarely, even the legs can be involved. They tend to affect homologous specific muscles, although isolated reports of index finger flexion causing contralateral triceps movement and arm motion affecting the opposite arm and both legs have been presented. The movements tend to worsen with fatigue, show varying degrees of suppressibility, and can range from subclinical (detected only by EMG) to incapacitating. Often, generalized increased tone in the affected limbs occurs during mirroring, which may be related to efforts to suppress the movements. Cases in which the severity of mirroring changes with variation in neck flexion and also of mirror movements induced with passive motion of the opposite extremity have been reported (3–5, 14, 19, 35, 36).
Mirror movements have been classified into three general categories: a physiologic form often present at birth, which rapidly disappears with myelination and neurologic maturation, not thought to persist beyond 10 years; a hereditary form, predominantly autosomal dominant, although sporadic and recessive forms have been reported (usually milder but otherwise clinically indistinguishable from other forms); and a pathologic form, associated with various entities (Table 5) of which KFS is the most common (11, 12, 15, 37).
TABLE 5:
Entities associated with mirror movements
| Agenesis of the corpus callosum |
| Basilar invagination of the skull |
| Spina bifida occulta |
| Friedrich’s ataxia |
| Kallmann’s syndrome |
| Usher’s syndrome |
| Phenylketonuria |
| Congenital hemiparesis |
| Diabetes insipidus |
| Mental retardation |
| Schizophrenia |
| Extrapyramidal system disease |
| CNS insult (tumor, CVA, SAH, trauma) |
A brief review of the anatomy (38) and embryology (39) of the developing spinal cord and corticospinal tracts is pertinent to the discussion of the proposed mechanisms of mirror movements. From 20 to 30 days’ gestation, pairs of condensing paraxial mesoderm form in cranial-to-caudal sequence adjacent to the closing neural tube, induced by the ventrally located notochord. Eventually, 42–44 paired somites are formed. As the brain is formed, descending corticospinal tract fibers arising in nuclear layer V of the cortex (predominantly in the precentral motor cortex but also in the premotor area, the postcentral gyrus, and the adjacent parietal cortex) descend to form the massive pyramids, which carry approximately one million fibers at birth. At the junction of the medulla and the cervical spinal cord, the corticospinal tract undergoes incomplete decussation, dividing into three tracts (Fig 3). The crossed fibers form the large lateral corticospinal tract (90%), while uncrossed descending fibers form the small anterior corticospinal tract (8%) and the relatively minute anterolateral corticospinal tract (2%). The crossed lateral corticospinal tract in the posterior part of the lateral funiculus enters spinal gray matter at the intermediate zone and distributes to laminae IV, V, VI, and VII of the anterior spinal gray matter. The uncrossed anterior corticospinal tract, oval in cross-section, adjacent to the anterior median fissure, and distinguishable mainly in cervical segments, crosses at the upper cervical spinal levels in the anterior white commissure and mostly terminates in lamina VII and the centromedial parts of the anterior horn. The anterolateral corticospinal tract remains uncrossed and terminates in the base of the posterior horn, intermediate gray, and central parts of the anterior horn. An understanding of the origins, terminations, and relative sizes of these tracts is helpful in understanding theories proposed to explain mirror movements.
Fig 3.
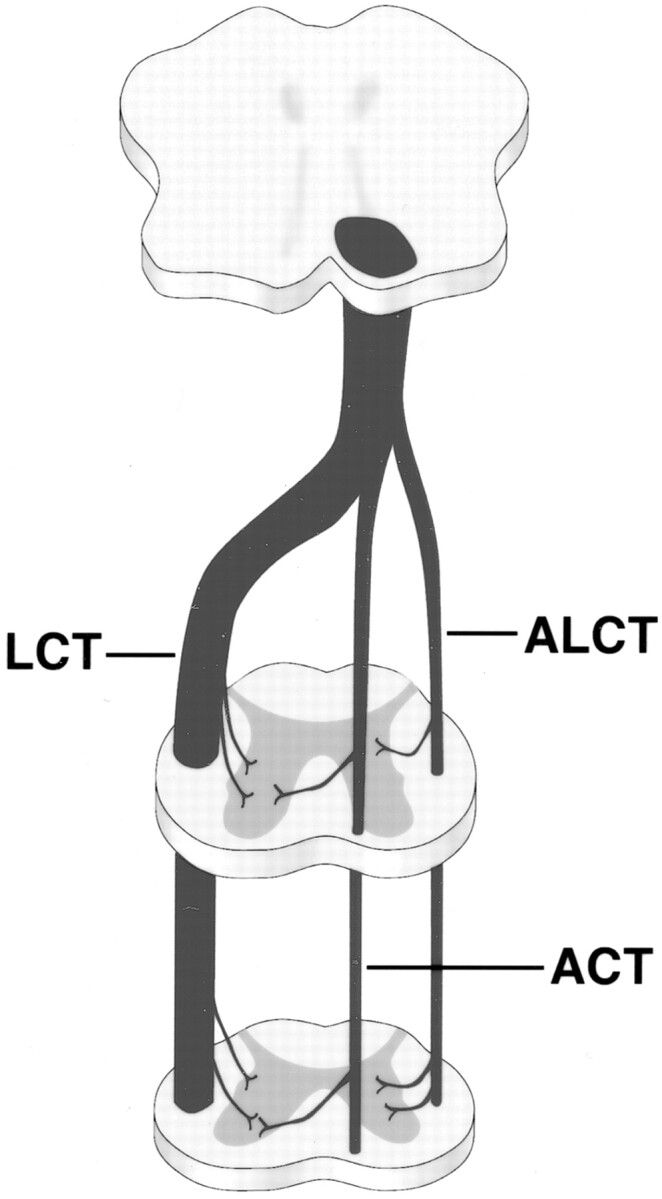
Diagrammatic representation shows normal pathways of descending corticospinal tracts, including crossed lateral corticospinal tract (LCT), uncrossed anterior corticospinal tract (ACT), and anterolateral corticospinal tract (ALCT).
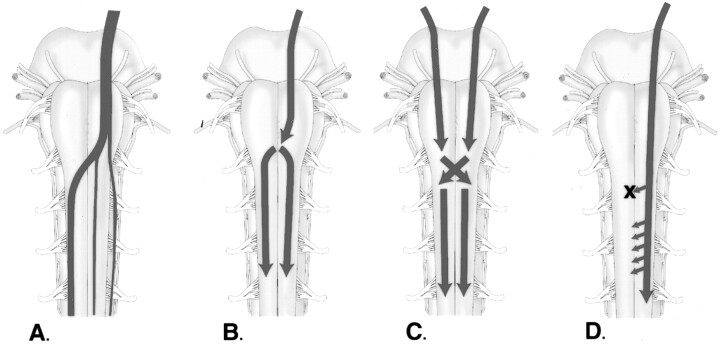
Various causes of mirror movements have been proposed (Fig 4). Gardner (3) discussed physiological cross talk via false synapses (enlapses), which can be produced in the lab by mechanical pressure on a nerve (similar to the mechanism for hemifacial spasm) and proposed similar effects of hindbrain herniation on the pyramidal decussation (Fig 7B). Schott and Wyke (4) proposed deficiency in the pyramidal decussation, requiring development of alternate less specific pathways, as a cause (Fig 4C). Mirror movements in patients with dysgenetic or absent corpus callosum raised theories of deficient contralateral cortical inhibition (Fig 4D). Farmer et al (36) performed a detailed study using EMG on a patient with mirror movements not associated with KFS and concluded, based on timing, that the impulse for the movements arose from a single source and therefore indicated abnormal fibers with bilateral branching. van der Linden and Bruggeman (11) proposed an aberrant reorganization of a fast conducting uncrossed or double crossed corticospinal system, challenging the double branching theory presented by Farmer et al, based on the lack of bilateral facilitated responses in their study. Since that time, it has not been determined whether the conflict in the data was related to flaws in the studies or differing mechanisms of mirror movements among patients within the studies.
Fig 4.
Proposed mechanisms of mirror movements.
A, Normal pathways.
B, Proposed mechanisms of mirror movements include bilateral signals originating at the pyramidal decussation, either by cross talk or by double branching fibers.
C, Deficiency at the decussation, resulting in development of accessory or double branched pathways inferiorly within the spinal cord.
D, Deficient contralateral cortical inhibition, resulting in the generation of bilateral cortical signals.
Although neuropathologic studies and modern imaging of patients with KFS have been very limited to date, many sources have shown neuroanatomic abnormalities in these patients. Soon after the initial report was presented by Avery and Rentfro (13), Gunderson and Solitare (5), in 1968, presented a report with identical findings of nearly complete cervical diaschisis and no shown pyramidal decussation. They also noted that the “lower spinal cord was more nearly normal except that the anterior columns were enlarged and the posterior and lateral columns were diminished in size.” The juxtaposition of these two findings within the report suggests a relationship. One logical possibility would be abnormal reorganization of corticospinal tracts, with prominence of the uncrossed anterior corticospinal tracts at the expense of the lateral corticospinal tracts. No mention was made of neurologic evaluation. Whittle and Besser (8), in addition to others (40–42), have also reported neuroschisis and/or mirror movements in association with KFS without correlation made between them.
Thus far, it has not been determined whether only one or more than one mechanism exists for mirror movements. Developmentally, completely independent unilateral movements require sophisticated control mechanisms. In pathologic states, mirror movements may be delayed in resolution as the child matures or may even reappear after a CNS insult. Thus, in certain cases, mirror movements may merely reflect incomplete CNS development or subsequent loss of normal control pathways. Worsening with neck flexion, as noted by Schott and Wyke (4) and in the report by Notermans et al (37) of abnormal arm EMG signals produced in a patient by neck extension suggests a mechanical component. Although it has been documented that as many as one of three patients with Chiari II can have some form of neuroschisis (49), the possibility of cross talk caused by hindbrain herniation proposed by Gardner (3) remains. The rarely reported passively inducible mirror movements would likely require an explanation and possibly a different mechanism. It would at least seem plausible, however, that developmental cervico-medullary neuroschisis could alter the path of descending corticospinal fibers, the vast majority of which cross in this region, necessitating recruitment of unbranched pathways and possibly inducing dual branching at more inferior sites. Recruitment of the anterior corticospinal tract in particular, which is poorly developed in the inferior cord, might in theory also explain the propensity toward upper extremity involvement.
Johnson (10), a neurosurgeon, proposed classifying patients with KFS into three subgroups to help stratify the risk of developing complications that could otherwise be treated or minimized by early neurosurgical intervention. Group 1 had a mechanically unstable pattern of fusion, and group 3 had significant spinal stenosis. Group 2 was a heterogeneous group, with hindbrain and/or cord abnormalities, including syrinx, myelodysplasia, and diastematomyelia. This group was considered the most difficult to identify clinically and, therefore, the most prone to develop long-term complications that were otherwise potentially preventable. Clues to this group included webbed neck, decreased swallowing reflexes, and cyclic regurgitation. We propose that mirror movements may also indicate increased incidence of neuroschisis and/or other structural abnormality of the cord that might have clinical significance in this group (43–46).
Imaging considerations of KFS include multiple modalities. Screening of the cervicomedullary spinal cord in these patients can be accomplished accurately by performing MR imaging. Sagittal and axial view T1-weighted images were emphasized in our studies, but techniques such as MR myelography (47, 48), which produces a myelographic effect, may provide even more detail of the pathologic anatomy. In many cases, unenhanced CT can provide information regarding osseous detail as well as gross cord morphology and would less likely require sedation. In addition, conventional images of the cervical spine are helpful screening tools in the early identification of KFS in patients with mirror movements who are otherwise asymptomatic, and flexion/extension views are essential in evaluating for instability in these patients (6, 7, 10).
Our study shows a strong association between the presence of cervicomedullary neuroschisis and mirror movements in patients with KFS. There was also correlation between the extent of cervical vertebral fusion and the incidence of both neuroschisis and mirror movements. Our study included a female predominance in the incidence of KFS (17 [74%] of 23 patients), despite no overall sex predilection previously documented in the literature. Ulmer et al (42) evaluated 24 patients with KFS by using MR imaging and/or postmyelography CT. Fourteen (58%) of the patients were female, and five (21%) showed some form of neuroschisis. That article is currently the only other study evaluating the incidence of neuroschisis in patients with KFS. Because of the retrospective nature of our study and the tertiary referral base of our institution, the quantity and severity of neuropathologic abnormalities in cases of KFS (six [38%] of 16 patients with some form of neuroschisis) may be overestimated by our data. Prospective studies with a larger number of patients would better delineate the relationship between sex and KFS as well as the association between mirror movements and cervico-medullary neuroschisis within this group.
In conclusion, there is a strong association of mirror movements and cervicomedullary neuroschisis in patients with KFS. The association between neuroschisis and mirror movements has plausible neuropathologic explanations based on the current literature. It is proposed that the screening of patients with pathologic mirror movements may not only help identify clinically unsuspected KFS but may also help stratify risk within this patient population and help identify those patients who might benefit from early neurosurgical intervention.
References
- 1.Klippel M, Feil A. Un cas d’absence des vetebres cervicales avec cage thoracique remontant jusqu’a base du craine. N Iconog de la Salpetriere 1912;25:223–250 [Google Scholar]
- 2.Bauman GI. Klippel-Feil syndrome. JAMA 1932;98:129–132 [Google Scholar]
- 3.Gardner WJ. Klippel-Feil syndrome, iniencephalus, anencephalus, hindbrain hernia and mirror movements: overdistension of the neural tube. Childs Brain 1979;5:361–379 [DOI] [PubMed] [Google Scholar]
- 4.Schott GD, Wyke MA. Congenital mirror movements. J Neurol Neurosurg Psychiatry 1981;44:586–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunderson CH, Solitare GB. Mirror movements in patients with the Klippel-Feil syndrome: neuropathologic observations. Arch Neurol 1968;18:675–679 [DOI] [PubMed] [Google Scholar]
- 6.Morrison SG, Perry LW, Scott LP III. Congenital brevicollis (Klippel-Feil syndrome) and cardiovascular anomalies. Am J Dis Child 1968;115:614–620 [DOI] [PubMed] [Google Scholar]
- 7.Gunderson CH, Greenspan RH, Glaser GH, Lubs HA. The Klippel-Feil syndrome: genetic and clinical reevaluation of cervical fusion. Medicine (Baltimore) 1967;46:491–512 [DOI] [PubMed] [Google Scholar]
- 8.Whittle IR, Besser M. Congenital neural abnormalities presenting with mirror movements in a patient with Klippel-Feil syndrome: case report. J Neurosurg 1983;59:891–894 [DOI] [PubMed] [Google Scholar]
- 9.Erskine CA. An analysis of the Klippel-Feil syndrome. Arch Pathol 1946;41:269–281 [PubMed] [Google Scholar]
- 10.Johnson MC. Klippel-Feil syndrome revisited: diagnostic pitfalls impacting neurosurgical management. Childs Nerv Syst 1992;8:322–325 [DOI] [PubMed] [Google Scholar]
- 11.van der Linden C, Bruggeman R. Bilateral small-hand-muscle motor evoked responses in a patient with congenital mirror movements. Electromyogr Clin Neurophysiol 1991;31:361–364 [PubMed] [Google Scholar]
- 12.Rasmussen P. Persistent mirror movements: a clinical study of 17 children, adolescents, and young adults. Dev Med Child Neurol 1993;35:699–707 [DOI] [PubMed] [Google Scholar]
- 13.Avery LW, Rentfro CC. Klippel-Feil syndrome. Arch Neurol Psychiatry 1936;36:1068–1076 [Google Scholar]
- 14.Matthews PB, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. J Physiol 1990;428:561–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forget R, Boghen D, Attig E, Lamarre Y. Electromyographic studies of congenital mirror movements. Neurology 1986;36:1316–1322 [DOI] [PubMed] [Google Scholar]
- 16.Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology 1991;41:1505–1510 [DOI] [PubMed] [Google Scholar]
- 17.Briton TC, Meyer B-U, Benecke R. Central motor pathways in patients with mirror movements. J Neurol Neurosurg Psychiatry 1991;54:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 1993;116:1223–1247 [DOI] [PubMed] [Google Scholar]
- 19.Regli F, Filippa G, Wiesendanger M. Hereditary mirror movements. Arch Neurol 1967;16:620–623 [DOI] [PubMed] [Google Scholar]
- 20.Feil A. L’absence et la diminution des vertebres cervicales (etude clinique et pathogenique): le syndrome de reduction numerique cervicale. Paris, Thesis,1919
- 21.Whiting DM, Chou SM, Lanzieri CF, Kalfas IH, Hardy RW. Cervical neuroenteric cyst associated with Klippel-Feil syndrome: a case report and review of the literature. Clin Neuropathol 1991;10:285–290 [PubMed] [Google Scholar]
- 22.McRae DL. Craniovertebral junction. In: Newton TH, Potts DG, eds. Radiology of the Skull and Brain. Vol 1. St Louis, Mo: Mosby;1971. :260–274
- 23.Poznanski AK, Stern AM, Gall JC. Skeletal anomalies in genetically determined congenital heart disease. Radiol Clin North Am 1971;9:435–458 [PubMed] [Google Scholar]
- 24.Ross CA, Curnes JT, Greenwood RS. Recurrent vertebrobasilar embolism in an infant with Klippel-Feil anomaly. Pediatr Neurol 1987;3:181–183 [DOI] [PubMed] [Google Scholar]
- 25.Widgerow AD. Klippel-Feil anomaly, cleft palate, and bifid tongue. Ann Plast Surg 1990;25:216–222 [DOI] [PubMed] [Google Scholar]
- 26.Rock JP, Spickler EM. Anomalous rib presenting as cervical myelopathy: a previously unreported variant of Klippel-Feil syndrome. J Neurosurg 1991;75:465–467 [DOI] [PubMed] [Google Scholar]
- 27.Vaquero J, Herrero J, Cabezudo J, Leunda G. Klippel-Feil syndrome with epidural fibroblastoma in the area of vertebral fusion. Arch Neurol 1982;39:318–319 [DOI] [PubMed] [Google Scholar]
- 28.Palant DI, Carter BL. Klippel-Feil syndrome and deafness. Am J Dis Child 1972;123:218–221 [DOI] [PubMed] [Google Scholar]
- 29.Derkay CS, Grundfast KM, McCullough DC. Sudden onset of velopharyngeal insufficiency in Klippel-Feil syndrome. Ear Nose Throat J 1990;69:548–552 [PubMed] [Google Scholar]
- 30.Zimbler S, Belkin S. Birth defects involving the spine. Orthop Clin North Am 1976;7:303–314 [PubMed] [Google Scholar]
- 31.Delashaw JB, Park TS, Wayne MC, Vollmer DG. Cervical meningocele and associated spinal anomalies. Childs Nerv Syst 1987;3:165–169 [DOI] [PubMed] [Google Scholar]
- 32.Rosen CL, Novotny EJ, D’Andrea LA, Petty EM. Klippel-Feil sequence and sleep-disordered breathing in two children. Am Rev Respir Dis 1993;147:202–204 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell H. Klippel-Feil syndrome (congenital webbed neck). Arch Dis Child 1934;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baird PA, Robinson GC, Buckler WS. Klippel-Feil syndrome: a study of mirror movement detected by electromyography. Am J Dis Child 1967;113:546–551 [DOI] [PubMed] [Google Scholar]
- 35.Ford FR. Mirror movements. In: Diseases of the Nervous System in Infancy, Childhood and Adolescence. 6th ed. Springfield, Mass: Charles C Thomas;1973. :224–225
- 36.Farmer SF, Ingram DA, Stephens JA. Mirror movements studies in a patient with Klippel-Feil syndrome. J Physiol 1990;428:467–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notermans S, Go K, Boonstra S. EMG studies of associated movements in a patient with Klippel-Feil syndrome. Psychiatr Neurol Neurochir 1970;73:257–266 [PubMed] [Google Scholar]
- 38.Carpenter MB, Sutin J. Development and histogenesis of the nervous system. In: Human Neuroanatomy. 8th ed. Baltimore, Md: Williams & Wilkins;1983. :61–84
- 39.Moore KL. The developing human. In: Clinical Oriented Embryology. Philadelphia, Pa: W.B. Saunders Company;1982. :53–69
- 40.Wolf AL, Tubman DE, Seljeskog EL. Diastematomyelia of the cervical spinal cord with tethering in an adult. Neurosurgery 1987;21:94–98 [DOI] [PubMed] [Google Scholar]
- 41.Mahmoud ON, Larson DA, Maxwell RE, Chou SN. Neuroschisis of the cervical spinal cord in a patient with Klippel-Feil syndrome. Neurosurgery 1987;20:629–631 [DOI] [PubMed] [Google Scholar]
- 42.Ulmer JL, Elster AD, Ginsberg LE, Williams DW III. Klippel-Feil syndrome: CT and MR of acquired and congenital abnormalities of cervical spine and cord. J Comput Assist Tomogr 1993;17:215–224 [PubMed] [Google Scholar]
- 43.Cochrane DD, Haslam RH, Myles ST. Cervical neuroschisis and meningocoele manqué in type I (no neck) Klippel-Feil syndrome. Pediatr Neurosurg 1990. –91;16:174–178 [DOI] [PubMed] [Google Scholar]
- 44.Ritterbusch JF, McGinty LD, Spar J, Orrison WW. Magnetic resonance imaging for stenosis and subluxation in Klippel-Feil syndrome. Spine 1991;16(suppl 10):S539–S541 [DOI] [PubMed] [Google Scholar]
- 45.Nagib MG, Maxwell, RE, Chou SN. Klippel-Feil syndrome in children: clinical features and management. Childs Nerv Syst 1985;1:255–263 [DOI] [PubMed] [Google Scholar]
- 46.Nagib MD, Maxwell RE, Chou SN. Identification and management of high-risk patients with Klippel-Feil syndrome. J Neurosurg 1984;61:523–530 [DOI] [PubMed] [Google Scholar]
- 47.el Gammal T, Brooks BS. MR cisternography: initial experience in 41 cases. AJNR Am Neuroradiol 1994;15:1647–1656 [PMC free article] [PubMed] [Google Scholar]
- 48.el Gammal T, Brooks BS, Freedy RM, Crews CE. MR myelography: imaging findings. AJR Am J Roentgenol 1995;164:173–177 [DOI] [PubMed] [Google Scholar]
- 49.MacKenzie NG, Emery JL. Deformities of the cervical cord in children with neurospinal dysraphism. Dev Med Child Neurol 1971;13:(suppl)25:58–67 [Google Scholar]