Abstract
BACKGROUND AND PURPOSE: Controversy exists regarding the utility of antecedent venography in percutaneous vertebroplasty. Our purpose was to determine whether antecedent venography improves clinical outcomes and/or decreases extravertebral cement extravasation in these procedures.
METHODS: We retrospective reviewed outcomes of consecutive percutaneous vertebroplasty procedures performed at our institution to define two populations, each consisting of 24 patients treated at 42 vertebral levels. Group 1 included patients who underwent antecedent venography, and group 2 included patients treated without venography. Clinical outcomes were assessed with quantitative measurements of pain and mobility. Venograms and postprocedural radiographs were interpreted to evaluate the number of vertebrae with extravertebral cement extravasation, degree of extravasation at each level, and correlation between venography and vertebroplasty.
RESULTS: Pain improved in 19 of 20 group 1 patients, compared with 21 of 22 group 2 patients; mean postoperative pain levels were 1.3 and 1.8, respectively (P = .50), on a scale of 0 (no pain) to 10 (worst pain). All 11 group 1 patients with impaired preoperative mobility reported postoperative improvement, as did all 12 group 2 patients; mean levels of postoperative impaired mobility for groups 1 and 2 were 0.35 and 0.27, respectively (P = .43). Twenty-two of 42 vertebrae treated in group 1 demonstrated extravasation, compared with 28 of 42 in group 2 (P = .266); amounts of extravasation did not differ. Among 22 levels of extravasation in group 1, venograms in 14 showed correlative extravasation.
CONCLUSION: Antecedent venography does not significantly improve the effectiveness or safety of percutaneous vertebroplasty performed by qualified, experienced operators.
The spine is a highly vascularized tissue, one bathed in venous plexuses that allow direct communication between the intravertebral veins and the vena cava or azygos system. Also, the large basivertebral vein traverses the vertebral body to posteriorly egress into the epidural venous plexus. Because of the risk of extravertebral cement extravasation during percutaneous vertebroplasty, some physicians who perform the procedure to treat vertebral compression fractures advocate the use venography prior to cement injection to visualize these outflow tracts and assist with needle placement into the basivertebral vein (1). Venography allows direct imaging of potential venous outflow tracts from the vertebral body, and many practitioners believe that the use of this technique decreases the complications and increases the safety of percutaneous vertebroplasty (1, 2). It does, however, increase the patient’s exposure to radiation and potentially toxic contrast material; the technique increases the cost and length of the vertebroplasty procedure, and, when injected contrast agent fails to rapidly wash out from the vertebral body or adjacent disk space, it may hinder visualization for cement injection (3, 4).
The purpose of this study was to compare clinical and radiographic outcomes in a group of patients who underwent percutaneous vertebroplasty with antecedent venography and those in a group of patients who underwent vertebroplasty without venography to evaluate the relevance of pretreatment venography in percutaneous vertebroplasty.
Methods
Patient Selection
We retrospectively reviewed the outcomes of consecutive vertebroplasty procedures performed at our institution to define two patient populations, one patient population that underwent vertebroplasty with antecedent venography and another population that underwent vertebroplasty without antecedent venography. Chart review focused on preprocedural imaging, venographic findings, clinical outcomes, and the presence and degree of cement extravasation during vertebroplasty, where extravasation was defined as any cement that was located outside the confines of the vertebral body after vertebroplasty. Group 1 comprised 24 consecutive patients treated for osteoporotic compression fractures at 42 vertebral levels between August 2000 and June 2001; all of these levels were treated by using venography prior to cement injection. Group 2 comprised 24 consecutive patients who underwent vertebroplasty without pretreatment venography for the treatment of osteoporotic compression fractures at 42 vertebral levels between March 2001 and June 2001.
Preprocedural Workup
Jensen et al (1) and Maynard et al (2) have described screening and preprocedural evaluation in detail. Briefly, patients with subacute pain and corresponding fractures were considered appropriate candidates. Patients with fractures of uncertain age or atypical pain patterns were treated if their bone scans demonstrated increased activity (2).
Procedural Technique and Materials in Group 1
Patients were placed in the prone position on the fluoroscopy table, and the vertebral levels to be treated were marked under fluoroscopic guidance. The area was prepared and draped in a sterile manner, and the skin over the vertebral body was anesthetized with 0.25% bupivacaine down to the level of the periosteum. An 11-gauge bone biopsy needle was placed by using a transpediculate approach under fluoroscopic guidance, with the tip of the needle in the midline, as depicted on the anteroposterior (AP) view, and in the anterior one third of the vertebral body on the lateral projection. In some cases, the contralateral pedicle was traversed, and a second injection was performed. Contralateral injections were performed at the discretion of the operator, usually in cases in which the cement failed to sufficiently traverse to the contralateral hemivertebra.
Venography.—Venography was performed at the discretion of the operators (M.E.J., W.F.M., D.F.K.). The technique was as follows: after needle placement, a short extension tube was attached to the needle hub, and 2–5 mL of diluted contrast medium (Omnipaque 300; Nycomed, Princeton, NJ) was mixed with saline in a 50:50 ratio and injected. Biplane digital subtraction angiography was performed at a rate of 2 frames per second during the injection of contrast material (Figs 1A, 2A).
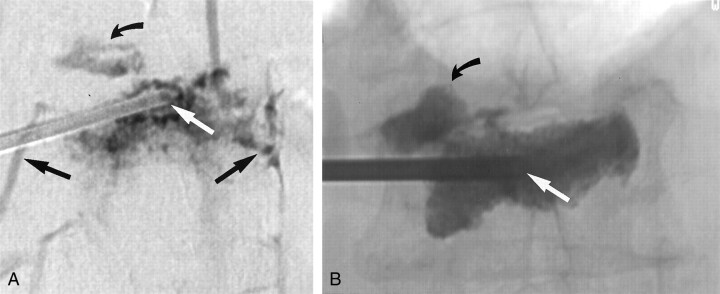
Fig 1.
Images in a 77-year-old woman with an L1 vertebral body fracture.
A, AP digital subtraction venogram shows the tip of an 11-gauge needle (straight white arrow) at the midline of the vertebral body. Multiple routes of contrast material egress are present, including routes through the superior endplate (curved black arrow) and bilateral paravertebral veins (straight black arrows).
B, AP plain radiograph obtained after vertebroplasty shows that the tip of the needle remains at the midline (white arrow). The needle position has not been altered because direct or rapid venous filling during venography was not observed. Cement fills most of the vertebral body, and it has also extravasated into the superior disk space (black arrow), in the exact same pattern as that predicted by using the venogram in A.
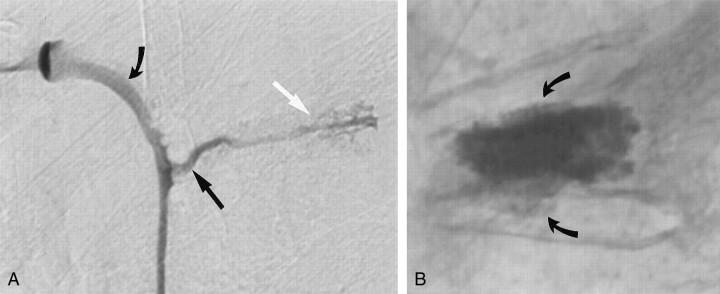
Fig 2.
Images in a 83-year-old woman with a T6 vertebral body fracture.
A, Lateral digital subtraction venogram shows the tip of the needle (straight white arrow) in the midportion of the vertebral body. Contrast material exits rapidly via a prevertebral vein (straight black arrow) and empties into the hemiazygos vein (curved black arrow). The rapid venous filling warranted an increase in the viscosity of the cement to minimize potential complications.
B, Lateral plain radiograph obtained after vertebroplasty shows that cement fills most of the vertebral body and that is has extravasated into both the superior and inferior endplates (arrows). No evidence for prevertebral cement extravasation is present.
Cement Preparation and Injection.—The cement was prepared by combining polymethylmethacrylate (PMMA) powder; liquid PMMA monomer; tobramycin powder, for infection control; and barium sulfate, for opacification; as previously described (1). The cement was mixed until it was viscous, at which time it was injected under fluoroscopic guidance into the vertebral body. The injection of cement was terminated when it began to fill the posterior one fourth of the vertebral body or when extravertebral extravasation occurred. In most cases, a single pedicle was injected. After the procedure, the patient was observed during the recovery period until his or her neurologic and physiologic status returned to its baseline level.
Procedural Technique and Materials in Group 2
The procedure was identical to that performed for group 1, above, except that venography was not performed prior to cement injection. Needle placement, with a transpediculate approach, was monitored with fluoroscopy, and adequate positioning entailed locating the tip of the needle in the midline on the AP view and in the anterior one third of the vertebral body on the lateral projection. The injection of cement was monitored with continuous fluoroscopy, and it was terminated when cement began to fill the posterior one fourth of the vertebral body or when extravertebral extravasation occurred.
Assessment
Clinical Outcomes.—Patients were assessed prior to the procedure regarding degree of pain and limitation of mobility. Patients were then reexamined on postoperative day 1 and again between 1 week and 1 month after surgery. Procedural notes were also reviewed to evaluate procedural complications, including hemodynamic and respiratory changes.
Pain.—Pain was assessed by using an ordinal scale of 0–10, with which the patients were asked to rate their pain. On this scale, 0 represented no pain, and 10 represented the worst pain they had ever had. We defined a positive clinical response as an improvement of three points or more on the quantitative scale. We assessed the number of patients in each group who achieved a pain-free status, which was represented as 0 on the quantitative scale. We calculated mean levels postoperative pain for both groups.
Mobility.—Mobility was assessed by using a five-point scale as follows: 0 indicated that the patient was walking without assistance; 1, walking with assistance; 2, wheelchair bound; 3, restricted to sitting in bed; and 4, restricted to lying flat in bed. A positive clinical response was defined as a postoperative improvement of one point or better on this scale. We calculated mean levels of postoperative mobility for both groups.
Cement Extravasation.—Both AP and lateral postprocedural radiographs that demonstrated cement localization were obtained for each of the 48 patients at all 84 vertebral levels (Figs 1B, 2B). A qualified observer (D.F.K.) who was blinded to whether antecedent venography had been performed interpreted these images. In addition, procedural notes were reviewed for any comments regarding cement extravasation. Radiographs were assessed for the presence of cement extravasation beyond the vertebral body. Sites of extravasation were divided into the epidural venous plexus, lateral paravertebral veins, prevertebral venous plexus, and intervertebral disk space. The extent of extravasation was then measured in each of these locations by using the diameter of an 11-gauge bone biopsy needle, which was approximately 3 mm, as a reference value. A single blinded observer (D.F.K.) performed the measurements; one measurement was obtained for each vertebral level that demonstrated extravasation. Values were obtained by directly measuring extravasation distances on the plain radiograph with a ruler. Extravasation into epidural, paravertebral, and prevertebral veins was measured in units of length, while disk space extravasation was measured in units of volume. Because of overlying cement within the vertebral bodies, identifying three extravasation measurements (ie, height, anteroposterior width, lateral width) was impossible, except with disk space extravasation. The two groups were compared with regard to both the number of vertebral levels that demonstrated extravasation and the extent of extravasation in each of the aforementioned categories.
Venography Correlation.—For the 24 patients in group 1, a qualified observer (J.R.G.) retrospectively interpreted the venograms. Procedural notes were also reviewed to document the venographic results and to correlation them with the observer’s findings. Venographic results were interpreted with regard to the route of extravasation of contrast material after the vertebral injection. These routes were divided into the epidural venous plexus, lateral paravertebral veins, prevertebral venous plexus, and intervertebral disk space. Routes of egress were then compared with cement extravasation patterns to assess the correlation between venographic and cement extravasation results.
Statistical Analysis
Clinical Outcome.—The Wilcoxon rank sum test was used to evaluate the statistical significance of differences between the two groups in terms of postoperative levels of pain and mobility. This approach was used in each of the patients to preserve the operant form of the data.
Cement Extravasation.—The Pearson χ2 test with Yates continuity correction was used to evaluate differences between groups 1 and 2 in the number of vertebral levels (total, 42) that demonstrated extravertebral cement extravasation. The Fisher exact test was used to evaluate qualitative differences between the two groups (ie, to compare percentages within subgroups) in extravertebral extravasation into the epidural venous plexus, lateral paravertebral veins, prevertebral venous plexus, and intervertebral disk space. The two-sided Student t test was used to evaluate quantitative differences between the two groups in regard to extravertebral extravasation into the epidural venous plexus, lateral paravertebral veins, prevertebral venous plexus, and intervertebral disk space.
Results
Patient Population
We enrolled a total of 48 patients (35 women, 13 men) in the study. The stratification of the groups is shown in Table 1.
TABLE 1:
Demographic stratification
| Criterion | Group 1 | Group 2 |
|---|---|---|
| No. of patients | 24 | 24 |
| Men | 5 | 8 |
| Women | 19 | 16 |
| Patient age (y) | 74 (52–92) | 73 (47–87) |
| Level | 42 | 42 |
| Mid thoracic, T5–T8 | 7 | 10 |
| Lower thoracic, T9–T12 | 8 | 13 |
| Upper lumbar, L1–L3 | 17 | 13 |
| Lower lumbar, L4–L5 | 10 | 6 |
| Compression (%) | 30.4 (10–70) | 33.6 (5–90) |
| Amount of PMMA (mL) | 4.65 | 3.09 |
| Approach | ||
| Unipediculate | 27 | 37 |
| Bipediculate | 15 | 5 |
Note.—Data in parentheses are ranges.
We limited our study groups to patients who were treated for osteoporotic compression fractures, excluding four patients (11 vertebral levels) with fractures secondary to primary or metastatic neoplastic lesions. In two patients who were included in the study, malignancies had been diagnosed at the time of treatment, but findings from vertebral biopsy performed prior to cement injection revealed an absence of neoplastic cells. Also, we excluded one procedure that entailed the repeat treatment of a vertebral level because the previously placed cement hindered our ability to visualize vertebral filling and extravasation. Etiologies for osteoporosis in the groups included menopause, chronic steroid use, and idiopathic causes.
In group 1, 15 (36%) of 42 vertebral levels were injected by using a bipediculate approach, with a mean cement volume of 4.65 mL ± 2.08 per level. In group 2, 5 (12%) of 42 vertebral levels were injected with cement by using a bipediculate approach, with a mean cement volume of 3.09 mL ± 1.69 per level.
Clinical Outcome
Our findings demonstrated no statistically significant difference between the two groups in regard to pain relief or an improvement in mobility after vertebroplasty. Tables 2 and 3 summarize our results.
TABLE 2:
Number of patients with clinical improvement at follow-up
| Patients | Group 1 | Group 2 |
|---|---|---|
| With pain improvement | 19 of 20 (95) | 21 of 22 (95) |
| Without pain | 14 of 20 (70) | 14 of 22 (64) |
| With preoperative impaired mobility | 11 of 20 (55) | 12 of 22 (55) |
| With mobility improvement | 11 of 11 (100) | 12 of 12 (100) |
Note.—Follow-up data were available in 20 (83%) of 24 patients in group 1 and in 22 (92%) of 24 patients in group 2. Data in parentheses are percentages.
TABLE 3:
Postoperative clinical outcomes
| Postoperative Outcome | Mean Points |
P Value | |
|---|---|---|---|
| Group 1 | Group 2 | ||
| Pain* | 1.3 | 1.8 | .50 |
| Impaired mobility† | 0.35 | 0.27 | .43 |
Pain was assessed by using an ordinal scale of 0–10, on which 0 represented no pain, and 10 represented the worst pain the patient had ever had.
Mobility was assessed by using a five-point scale as follows: 0 indicated that the patient was walking without assistance; 1, walking with assistance; 2, wheelchair bound; 3, restricted to sitting in bed; and 4, restricted to lying flat in bed.
Follow-Up.—We successfully obtained follow-up data in 42 (88%) patients. In group 1, those who underwent vertebroplasty with antecedent venography, 20 (83%) of 24 patients complied with follow-up at 1 month. In group 2, those who underwent vertebroplasty without antecedent venography, 22 (92%) of 24 complied with follow-up at 1 month.
Pain.—Mean pain improvements were 8.1 points and 7.2 points for groups 1 and 2, respectively. An improvement of three points or more was achieved In 19 (95%) of 20 group 1 patients, compared with 21 (95%) of 22 group 2 patients. In addition, 14 (70%) of group 1 patients had a postoperative pain-free status, compared with 14 (64%) group 2 patients. The mean levels of postoperative pain for groups 1 and 2 were 1.3 ± 2.31 and 1.8 ± 2.74, respectively (P = .50).
Mobility.—Of the 20 group 1 patients that complied with follow-up, 11 (55%) reported some degree of preoperative limited mobility, compared with 12 (55%) of 22 group 2 patients. All 11 (100%) group 1 patients with a preprocedural mobility limitation reported at least 1 level of improvement in mobility (range, 1–4 points; mean, 2.36 points) within 1 month, as did all 12 (100%) group 2 patients with a preprocedural mobility limitation (range, 1–4 points; mean, 1.92 points). The mean levels of postoperative impaired mobility for groups 1 and 2 were 0.35 ± 0 0.67 and 0.27 ± 0.77, respectively (P = .43).
Cement Extravasation
Twenty-two (52%) of 42 vertebral levels treated in group 1 demonstrated extravasation, compared with 28 (67%) of 42 levels in group 2 (P = .266) (Tables 4 and 5). As shown in Table 4, differences in the rates of extravasation between groups 1 and 2 were not statistically significant in any of the extravasation subgroups measured. As shown in Table 5, the mean distances or volume of cement extravasation in each subgroup were also similar between groups 1 and 2.
TABLE 4:
Number of vertebral bodies with extravasation by compartment
| Compartment | Group 1 | Group 2 | P Value |
|---|---|---|---|
| Epidural | 7 | 10 | .33 |
| Paravertebral | 7 | 7 | >.99 |
| Prevertebral | 3 | 4 | .34 |
| Intervertebral disk space | 9 | 13 | .4 |
TABLE 5:
Cement extravasation by compartment
| Compartment | Group 1 | Group 2 | P Value |
|---|---|---|---|
| Epidural (mm) | 4.14 | 2.90 | .23 |
| Paravertebral (mm) | 4.43 | 5.86 | .58 |
| Prevertebral (mm) | 4.67 | 8.75 | .44 |
| Intervertebral disk space (mm3) | 617 | 272 | .26 |
Venographic Correlation
All 42 venograms in group 1 demonstrated at least one route of contrast extravasation. Cement extravasation occurred in 22 (52%) of these 42 vertebral bodies. Among these 22 cases of cement extravasation, venograms in 14 (64%) showed a correlative extravasation pattern. The correlation was excellent for pre- and paravertebral extravasation, in which 10 (100%) of 10 cement extravasations were predicted with venography. In nine cases os cement extravasation into the endplate, corresponding venograms depicted such extravasation in three (33%). In seven cases of epidural cement extravasation, corresponding venograms depicted such extravasation in four (57%). The number of individual compartments that demonstrated extravasation (n = 26) did not equal the number of cases of extravasation (n = 22) because several cases had multiple avenues of egress.
Procedural Complications
Of the 84 levels treated in this study, we documented only one procedural complication, which occurred in a patient that had undergone antecedent venography. During needle placement, the operator perforated the thecal sac, causing a cerebrospinal fluid leak. The patient had a postprocedural headache and left-sided pain. She was instructed to remain in the supine position, and analgesics were administered. Both the headache and pain resolved completely over a few days, with no permanent sequelae.
No patient in either group had any clinically apparent complications as a result of cement deposition. Specifically, no evidence of spinal cord compression or pulmonary embolism was present. Cardiovascular and respiratory parameters, including oxygen saturation, blood pressure, and heart rate, remained within normal limits throughout each procedure in every patient in both groups. These findings correlate with those of Kaufmann et al (unpublished data, 2001), who found no clinically important alterations in such parameters as a result of PMMA injection.
Discussion
Percutaneous vertebroplasty has been introduced as an effective, minimally invasive procedure for the treatment of vertebral compression fractures (1–10). The initial developers of the technique proposed the use of venography to enhance the safety of cement deposition, and these developers published much of the existing literature on the basis of their early experience (1). Our group, however, has become comfortable in performing vertebroplasty without venography, and we considered it relevant to demonstrate the lack of benefit with venography when experienced operators perform percutaneous vertebroplasty. In our study, we attempted to clarify the relevance of venography by presenting, to our knowledge, the first objective comparison of vertebroplasty performed with antecedent venography and vertebroplasty performed without antecedent venography. We hoped that such a study would aid operators in understanding the relationship between the risks and the benefits of performing this procedure.
The purpose of antecedent venography in percutaneous vertebroplasty is to optimize the safety of the latter procedure. However, we included clinical outcomes in this study because we believed that the safety vertebroplasty without venography is moot if its effectiveness is suboptimal compared with that of procedures performed with venography. To our knowledge, no group has compared the clinical effectiveness of the two approaches, and our aim was to demonstrate that the omission of venography before vertebroplasty sacrifices neither effectiveness nor safety.
Our results demonstrate that venography does not significantly improve the effectiveness or safety of percutaneous vertebroplasty procedures performed by qualified, experienced operators. We did not find any statistically significant difference between patients undergoing vertebroplasty alone and those undergoing antecedent venography and vertebroplasty in terms of either pain relief or improvement in mobility after the procedure. Furthermore, we did not find any statistically significant difference between the two groups in either the fraction of vertebral levels that demonstrated cement extravasation or the extent of extravasation at each level. Finally, venograms depicted cement extravasation in only 64% the cases in which it was present. Neither group had any procedural complications associated with cement deposition or extravasation. These findings show that vertebroplasty can be safely performed with or without antecedent venography.
Our approach to percutaneous vertebroplasty changed between 2000 and 2001 because of an evolving belief that antecedent venography had little or no value in the procedure. This study was initiated as means to validate that hypothesis. Three operators perform vertebroplasty procedures performed at our institution; each had performed approximately 150 procedures prior to the study period. The 48 cases included in the study, therefore, increased our experience by approximately a 10%. During the study period, no other parameters of the procedure were altered or modified, and no new practitioners joined the group. No systematic differences between the practitioners were present, because each was a highly qualified, experienced operator.
In our 8 years of experience with vertebroplasty, rapid venous filling during antecedent venography occurred on several occasions. However, such a finding is less worrisome than the rare occurrence of direct filling of a vein with no filling of the trabecular space. Historically, we altered our procedure in several ways on the basis of these abnormal findings. On two occasions, we used Gelfoam pledgets to inhibit extravasation. On one occasion, we successfully repositioned the needle and continued with the procedure. On another occasion, we filled the vein with cement, allowed it to harden, and then used a new needle to inject cement. Most commonly, however, we alter the consistency of the PMMA to increase its viscosity. This approach sufficed in all of the patients in this study in whom venograms demonstrated such findings.
To our knowledge, no group has evaluated the utility of venography. Jensen et al (1) and Martin et al (5) advocate the use of antecedent venography to decrease complications associated with needle placement within the basivertebral venous plexus and to delineate the route of cement egress. However, some authors (7) disagree with this perspective, stating that the different flow characteristics of contrast material and cement hamper the predictive value of venography. Others (3, 5) argue that antecedent venography hinders visualization during cement injection.
Extravertebral extravasation appears to be an inevitable occurrence in a substantial proportion of patients who undergo vertebroplasty, but symptomatic sequelae of this phenomenon are rare. Chiras et al (3) report cement-induced nerve root compression in a patient who underwent vertebroplasty alone. Martin et al report a similar complication in a patient who underwent pretreatment venography. Padovani et al (8) report the occurrence of a postprocedural symptomatic pulmonary embolism, which was successfully treated with anticoagulation, in a patient who underwent vertebroplasty alone. Others (4) have reported similar complications. Conversely, Jensen et al (1) report occurrences of asymptomatic pulmonary embolism in patients who underwent antecedent venography. Although contrast material–induced renal failure and allergic reactions are theoretical risks in venography, to our knowledge, no examples of these exist in the current literature.
While the contributions of venography to radiation exposure, as well as the length and cost of the procedure, are small, they do exist. Although precise measurements of radiation exposures were unavailable, the additional exposure was related to biplane digital subtraction angiography, which was performed at a rate of 2 frames per second for 5–10 seconds with the unilateral transpediculate approach. The exposure was doubled with the bipediculate approaches. The procedure was lengthened by approximately 5 minutes in uncomplicated cases, and the contrast material increased the cost of the procedure. Such factors should be considered in the decision to use antecedent venography.
Although our study represents an objective investigation of the effectiveness and safety of antecedent venography in percutaneous vertebroplasty, it has several limitations. First, its retrospective nature lacks the randomization of a prospective clinical trial, and the sample size may lack the power necessary to demonstrate a real difference in complications. Second, various aspects may have impaired the accuracy of the measurements of extravertebral extravasation. The limited ability to assess the extent and contour of compression on AP and lateral plain radiographs made the identification and quantification of extravasation difficult. The use of reference distances, such as the diameter of a bone biopsy needle, introduced assumptions that may have skewed measurements, as does the use of geometric estimations of nongeometric extravasation patterns. Third, group 1 patients underwent bipediculate cement injection more frequently than group 2 patients. Extravasation might have been more likely during the second injection procedure because the first injection may have obscured the needle tip. Also, slightly greater volumes of cement were used in group 1 compared with those in group 2; these volumes also might have increased the risk of extravasation. Finally, perhaps the most clinically relevant limitation of this study arose from the fact that highly experience operators performed all of the procedures. Ideally, such individuals should perform all percutaneous vertebroplasty procedures; however, in practice, the level of experience varies dramatically. In those adept at performing vertebroplasty, venography may be a superfluous procedure. Conversely, venography may be extremely beneficial, or even necessary, with less experienced operators. With these operators, one might expect more complications associated with cement injection, and thus, venography may have a more pronounced role in providing visual guidance and improving safety.
The results of this study evince the need for further evaluation of the utility of pretreatment venography in vertebroplasty procedures. Prospective randomized controlled trials may assist in clarifying the uncertainties that continue to surround the use of this technique with regard to its usefulness in predicting the route of cement egress and in curtailing clinically or radiographically evident cement extravasation. Additionally, long-term studies may aid in delineating the clinical importance of asymptomatic extravertebral cement deposition. Finally, these findings indicate the need to evaluate venography with regard to its use by less experienced operators.
Conclusion
The use of antecedent venography does not significantly improve the effectiveness or safety of percutaneous vertebroplasty procedures performed by qualified, experienced operators.
References
- 1.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897–1094 [PMC free article] [PubMed] [Google Scholar]
- 2.Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol 2000;21:1807–1812 [PMC free article] [PubMed] [Google Scholar]
- 3.Chiras J, Depriester C, Weill A, Sola-Martinez MT, Deramond H. Vertebroplasties percutaneous. J Neuroradiol 1997;24:45–59 [PubMed] [Google Scholar]
- 4.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology 2000;39:1410–1414 [DOI] [PubMed] [Google Scholar]
- 5.Martin JB, Jean B, Sugui K, et al. Vertebroplasty: clinical experience and follow-up results. Bone 1999;25:11S–15S [DOI] [PubMed] [Google Scholar]
- 6.Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology 1996;99:241–247 [DOI] [PubMed] [Google Scholar]
- 7.Mathis JM, Barr JD, Belkoff SM, Barr MS, Jensen ME, Deramond H. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–381 [PMC free article] [PubMed] [Google Scholar]
- 8.Padovani B, Kasriel O, Brunner P, Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:375–377 [PMC free article] [PubMed] [Google Scholar]
- 9.Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994;15:83–86 [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LD. Fractures of the osteoporotic spine. Orthop Clin North Am 1990;21:143–150 [PubMed] [Google Scholar]