Abstract
BACKGROUND AND PURPOSE: The patency of intracranial perforating arteries after stent placement is unknown despite the general clinical use of intracranial arterial stenting.
METHODS: We deployed stainless steel stents in the abdominal aorta across the lumbar artery in eight normal rabbits in which the diameters of the abdominal arterial vessels were similar to those of human intracranial arteries. We evaluated the patency via angiographic and scanning electron microscopic methods 3 months after stent placement. Histopathologic evaluation was also performed for one rabbit.
RESULTS: The lumbar arteries were patent, even when stent struts crossed the ostium, except in one rabbit in which intimal dissection occurred intraoperatively. The scanning electron microscopy showed that the regenerative endothelium had grown onto the strut at the ostium of the lumbar artery.
CONCLUSION: We confirmed the patency of the lumbar arteries in this study by using normal rabbits. Thus, intracranial stenting may not pose a risk of occluding perforating arteries of the same diameter of the lumbar artery, even if stent struts cover the ostium.
One major concern related to intracranial stenting is whether perforating arteries will become occluded after stent placement. The relationship between the abdominal aorta and the lumbar arteries in the rabbit is anatomically similar to that between the major intracranial arteries and the perforating arteries in humans with regard to diameter and angle of origin. In this study, we performed stent placement in the abdominal aorta across the ostium of the lumbar artery in normal rabbits and evaluated the status of the ostium 3 months after stent placement by angiographic, scanning electron microscopic, and histopathologic methods.
Methods
Surgical Procedure
Eight Japanese White rabbits weighing 2–3 kg were anesthetized by IV injection with pentobarbital (4 mg/kg). Each right femoral artery was exposed, and a 4F sheath was inserted by arteriotomy of the femoral artery. After measuring the diameter of the abdominal aorta using a Measurewire (Boston Scientific, Watertown, MA), we deployed the stainless steel stent (multi-link; Guidant Corporation, Temecula, CA) via a 0.014-in Transend Guidewire (Boston Scientific, Natick, MA) in the abdominal aorta. The stents were deployed such that they covered the ostium of the lumbar artery. Each stent was deployed to 6–8 atm for 15 seconds. After post-procedural angiography, the surgical procedure was finished. Aspirin (5 mg/kg) was administered transorally from 1 day before the surgery to 1 month after stent placement. Just before stent placement, heparin (100 U/kg) was intravenously administered. All animal care conformed to the institutional guidelines of Wakayama Medical College.
Follow-Up Angiography
Follow-up angiography was performed via the left femoral artery 1 week (n = 1) and 3 months (n = 7) after stent placement to evaluate patency of the lumbar artery.
Tissue Processing for Scanning Electron Microscopy
After follow-up angiography, each stented artery was harvested. Inferior vena cava exsanguination was performed with perfusion of 0.2-M phosphate-buffered saline with heparin via left ventricular puncture. The stented arteries were fixed with 0.1-M phosphate-buffered saline, including 1% paraformaldehyde and 1.25% glutaraldehyde. After rinsing these with 0.1-M phosphate-buffered saline, we gently opened the stented arteries with tungsten scissors so as not to damage the intraluminal surface and post-fixed these specimens with 1% OsO4 at 4°C for 1 hour. The stented arteries were then dehydrated with graded ethanol and t-butyl alcohol and freeze-dried. After coating with 20 nm of gold, we examined the patency and the degree of re-endothelialization at the ostia of the lumbar arteries.
Histopathologic Evaluation
One rabbit’s stented aorta was evaluated for the neointimal composition at the ostium of the lumbar artery by using immunohistopathologic methods (case 8). After exsanguination, the stented artery was harvested and then fixed with 10% neutral buffered formalin. The stented artery was carefully cut with a tungsten knife in 4–5-μm-thick sections. The cut sections were stained with hematoxylin and eosin to examine cell composition. The other sections were examined by immunohistochemical staining to identify the specific cell types with antibodies for endothelial cells (factor VIII) and smooth muscle cells (alpha-actin).
Results
Angiographic Findings
The angiographic results are summarized in the Table. The diameter of the aorta was measured with a measure wire. The deployed stent diameter was based on the manufacturer’s information. The diameters of lumbar arteries were measured by using the scale measure on the scanning electron microscopic images. They ranged from 473 to 802 μm (average diameter, 643 μm). The diameter of the lumbar artery for cases 3 and 8 were not measured because of dissection during stenting and use for histopathologic evaluation, respectively. Patent means that the ostium of the lumbar artery was still open without significant narrowing due to extension of the regenerated endothelium. The average diameter (n = 8) of the stented aorta was 2.9 mm, and the average stent-to-artery ratio was 1.17. There was one angiographic complication in which a stent was delivered without a guidewire, resulting in intimal dissection (case 3). We therefore excluded this case from our study. Lumbar arteries in the stented segments of all other rabbits were angiographically patent just after stent deployment. One rabbit (case 5) became paraparetic after stent placement despite confirmation of a patent lumbar artery. Follow-up angiography 1 week later also showed the patency of the lumbar artery. We therefore suspected that distal embolization might have caused a spinal cord infarction. Follow-up angiography performed 3 months after implantation in six rabbits showed the patency of the lumbar arteries without significant luminal narrowing (Fig 1).
TABLE.
Summary of cases
| Rabbit | Diameter of Aorta (mm) | Diameter of Lumbar Artery (μm) | Deployed Stent Diameter (mm) | Stent-to-Artery Ratio | Angiographic Complication | Patency of Lumbar Artery |
|
|---|---|---|---|---|---|---|---|
| Just After | 3 Months After | ||||||
| 1 | 2.8 | 672 | 3.08 | 1.10 | None | Patent | Patent |
| 2 | 3.1 | 473 | 3.52 | 1.14 | None | Patent | Patent |
| 3 | 2.8 | 3.33 | 1.19 | Dissection* | |||
| 4 | 2.3 | 802 | 3.32 | 1.44 | None | Patent | Patent |
| 5 | 2.6 | 729 | 3.32 | 1.28 | None† | Patent | Patent‡ |
| 6 | 3.2 | 559 | 3.52 | 1.10 | None | Patent | Patent |
| 7 | 3.2 | 620 | 3.52 | 1.10 | None | Patent | Patent |
| 8 | 3.3 | 3.52 | 1.07 | None | Patent | Patent | |
| Average | 2.9 | 643 | 3.39 | 1.17 | |||
In this case, the stent was delivered without a guidewire and caused intimal dissection.
This rabbit became paraparetic after stenting, although the lumbar artery was patent on the post-stenting angiogram.
Follow-up angiography of the rabbit was performed 1 week after stenting.
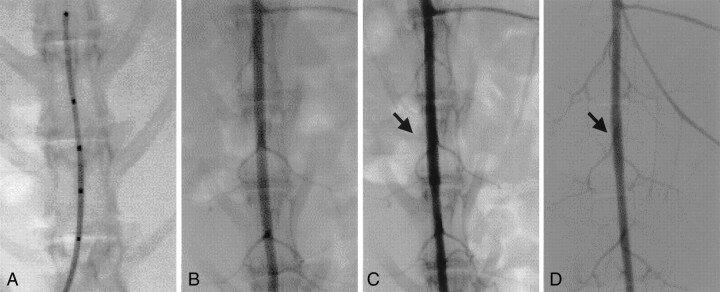
Fig 1.
Representative case (case 4).
A, Diameter of abdominal aorta is measured by using a measure wire.
B, Angiography is performed before stenting.
C, Just after stenting, angiography is performed to evaluate the patency of the lumbar artery (arrow).
D, Follow-up angiogram, obtained 3 months after stenting, confirms that the lumbar artery remains patent (arrow).
Scanning Electron Microscopic Findings
The pathomorphologic evaluation of the ostium of the lumbar artery and the status of the re-endothelialization were investigated by scanning electron microscopy after follow-up angiography. The scanning electron microscopic findings for the rabbit that had become paraparetic after stenting (case 5) revealed that the lumbar artery in the stent was patent without thrombus. The diameter of the lumbar artery was 729 μm, as measured by using a scale measure on the scanning electron microscopic image. The endothelial integrity was injured along the struts by stent expansion (Fig 2). The underlying smooth muscle fiber was also exposed in that part of the luminal surface, but we suspected that this damage was caused during postprocessing because the same observation was made with scanning electron microscopy performed at 3 months after stenting.
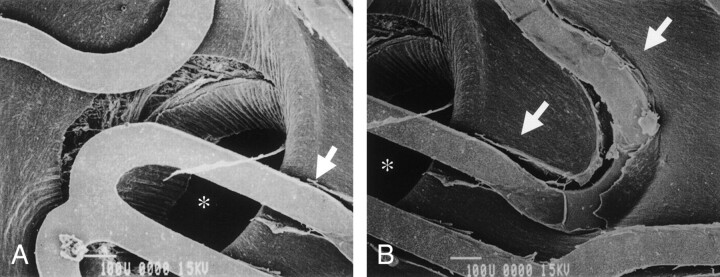
Fig 2.
Case 5. Scanning electron microscopic findings obtained 1 week after stenting for the rabbit that became paraparetic after stenting reveal that the lumbar artery (asterisk) in the stent is patent without thrombus. The diameter of the lumbar artery is 729 μm. Endothelial integrity is injured along the struts by stent expansion (arrows), and underlying smooth muscle fiber is exposed in part of the luminal surface because of post-processing (original magnification ×100).
In all other rabbits, scanning electron microscopy performed 3 months after implantation showed the patency of lumbar arteries and regenerated endothelial cells covering the stent struts in contact with the aorta. These endothelial cells appeared to have been growing onto the struts crossing the ostium of the lumbar artery, although the covering was incomplete at this time point (Fig 3). The extension of regenerated endothelial cells encroaching on the ostia of lumbar arteries was also observed in several cases (Fig 3A), but the degree of narrowing due to this extension was not significant at this point. The diameters of lumbar arteries, measured by using a scale measure on the scanning electron microscopic image, ranged from 473 to 802 μm (average diameter, 643 μm) (Table). The smaller branches could not be evaluated because no stent struts crossed the ostia of these branches.
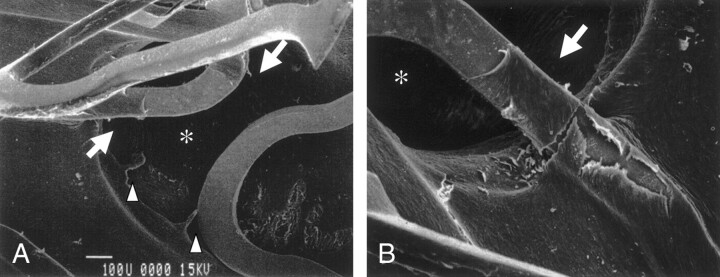
Fig 3.
Representative case (case 4). Scanning electron microscopic findings obtained 3 months after stent placement reveal an open ostium of the lumbar artery (asterisk) and also show that regenerated endothelial-like cells are covered with stent strut in contact with the aorta and extend to the struts crossing the ostium of the lumbar artery (arrows). In this case, the extension of the regenerated endothelial cells into the ostia of lumbar arteries was observed (arrow), although the degree of narrowing due to this extension was not significant.
A, Original magnification ×80.
B, Original magnification ×150.
Histopathologic Findings
Macroscopic analysis of the stented arteries revealed that the stent struts that were in contact with the aortic wall were covered by a thin membranous layer, and the ostia of the lumbar arteries were widely open, regardless of whether a stent strut crossed the lumbar artery. Microscopic analysis of sections stained with hematoxylin and eosin revealed stretching of the media of the aorta by the strut and reactive smooth muscle cell proliferation around the strut. This cellular component was confirmed to be smooth muscle cells by immunohistochemical staining for alpha-actin. The luminal surface of the stented artery was lined with a monolayer of cells resembling regenerative endothelial cells, although immunohistochemical staining for factor VIII failed to show any immunoreactivity. Cells resembling regenerative endothelium were also found on the strut across the ostium (Fig 4).
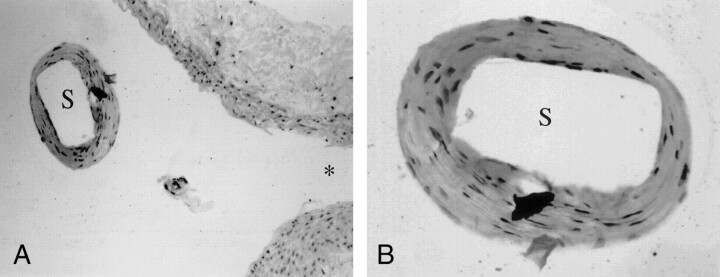
Fig 4.
Microscopic findings stained with hematoxylin and eosin reveal that the luminal surface of the struts (S) across the ostium of the lumbar artery (asterisk) is lined with a monolayer of cells resembling regenerative endothelial cells.
A, Original magnification ×200.
B, Original magnification ×400.
Discussion
Several cases of successful intracranial stenting have recently been reported with the advent of flexible coronary stents (1–9). According to these reports, intracranial stenting may be safe and useful for the treatment of wide-necked aneurysms and for elastic recoil or dissection after percutaneous transluminal angioplasty for atherosclerotic lesions. However, stenting of intracranial atherosclerotic lesions has not yet been widely adopted, partially because of many unresolved questions. One concern is whether stent placement may lead to occlusion of perforating arteries originating from the stented vessel, although no cases of symptomatic occlusions of perforating arteries after intracranial stenting have been reported in the clinical literature (2).
In experimental work, several studies have investigated the patency of side branches from the stented arteries after stent placement. Wakhloo et al (10) reported that the cervical branches originating from the canine vertebral arteries within the stented segments remained angiographically patent 9 months after self-expanding nitinol stent placement. Whitbread et al (11) assessed the patency of the renal artery 6 months after placing a Wallstent across the orifice of the renal artery in six pigs. In their study, no renal arteries were occluded based on follow-up aortograms, and scanning electron microscopy of the stented aorta showed partial endothelialization of the stent but the renal artery origins remained widely patent. Malina et al (12) also showed that the renal arteries remained patent without any significant decrease in the renal blood flow and renal dysfunction after placing Gianturco and Palmaz stents. Birch et al (13) compared the effects of crossing renal artery ostia with three various stents (Wallstent, Palmaz, and Memotherm). In all cases that used the Wallstent (nine cases), all ostia were patent despite that the most organized collagen matrix on the strut was in contact with the aorta. However, in one of nine cases that used the Palmaz stent and in 12 of 13 cases that used the Memotherm stent, the neointima caused partial ostial occlusion. The authors suggested that the tissue reaction to the stent was dependent on the shape and composition of the struts and that the fine rounded stainless steel struts excited less reaction.
We studied the patency of lumbar arteries after stenting using normal rabbits to investigate whether perforating arteries, smaller than the renal artery in diameter, would be occluded after intracranial stenting. ACS/GUIDANT RX Multi-link stents, made of a laser-cut stainless steel, were used in this study. The characteristics of this stent include good conformability due to the very thin flat strut and the composition of the strut. Rabbits were used in this study because the relationship between major intracranial arteries and perforating arteries originating from these arteries in humans approximated that of the abdominal aorta and lumbar arteries in the rabbit with respect to diameter and angle of origin. In previous reports regarding microsurgical anatomy, the diameters of the middle cerebral, vertebral, and basilar arteries ranged from 2.4 to 4.6 mm (average, 3.9 mm), from 2.0 to 5.7 mm (average, 3.5 mm), and from 3.0 to 5.5 mm (average, 3.9 mm), respectively (14, 15). The diameter of the perforating arteries ranged from 100 to 600 μm in the vertebral artery, from 200 to 500 μm in the basilar artery, and from 120 to 1250 μm in the middle cerebral artery. In our series, the average diameter of abdominal aorta and lumbar artery originating from the abdominal aorta were 2.9 mm and 643 μm, respectively.
Our results showed the patency of lumbar arteries without significant luminal narrowing and flow reduction on the follow-up angiograms of all rabbits. This was confirmed by scanning electron microscopy performed 3 months after stenting, which showed that the regenerative endothelium had grown along the struts crossing the ostium of lumbar artery. However, there were still bare portions of the struts crossing the ostium. Endothelialization in these portions might lag behind the portion in contact with the luminal wall in cases of complete re-endothelialization because of blood flow. It is unclear which kind of cellular response may happen in this bare portion over a longer period, although there are several likely possibilities. These bare portions may remain “bare.” Endothelial cells may completely encompass the stent struts without ostial occlusion. Proliferative smooth muscle cells and regenerative endothelium may cover the ostium completely. The fate of the bare portion will be determined by the degree of the ostial coverage by the stent struts.
Regarding tissue response after stent placement, Schatz et al (16, 17) suggested that stents were covered initially by a thin layer of thrombus and fibrin that was later replaced by neointimal muscular proliferation and that thrombosis was essential for healing but must be controlled. Grewe et al (18) also reported the same tissue response. Schatz (17) suggested that the successful vascular stent should depend on minimal thrombosis and rapid endothelialization and that stent-induced thrombus formation could be avoided successfully with anticoagulant and antiplatelet agents. The shape and composition of the stent strut might be important factors for ostial occlusion. Antiplatelet therapy after stent placement might also play an important role in preventing ostial occlusion. We performed antiplatelet therapy consistent with previous reports for 1 month after stenting for this study (19, 20), but we might need to continue longer term antiplatelet therapy to lead to complete endothelialization around struts crossing the ostium with minimal muscular proliferation. This longer term antiplatelet therapy also might be necessary to prevent the extension of regenerative endothelial cells encroaching on the ostia of lumbar arteries, although significant narrowing and restriction of the ostia were not observed 3 months after stenting.
Conclusion
We confirmed patency of the lumbar arteries branching from the stented abdominal aorta 3 months after stent placement in normal rabbits. These results suggest that intracranial perforating arteries, 643 μm in average diameter, might also remain patent for the first 3 months after implantation. However, a longer term study of more than 3 months, with atherosclerotic models, is required to extend these results.
References
- 1.Higashida RT, Smith W, Gress D, et al. Intravascular stent and coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944–949 [DOI] [PubMed] [Google Scholar]
- 2.Lanzino G, Fessler RD, Miletich RS, Guterman LR, Hopkins LN. Angioplasty and stenting of basilar artery stenosis: technical case report. Neurosurgery 1999;45:404–408 [DOI] [PubMed] [Google Scholar]
- 3.Malek AM, Higashida RT, Halbach VV, Phatouros CC, Meyers PM, Dowd CF. Tandem intracranial stent deployment for treatment of an iatrogenic, flow-limiting, basilar artery dissection: technical case report. Neurosurgery 1999;45:919–924 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MB, Pride GL, Graybeal DF, Purdy PD. Percutaneous transluminal angioplasty and stenting of midbasilar stenoses: three technical case reports and literature review. Neurosurgery 1999;45:925–931 [DOI] [PubMed] [Google Scholar]
- 5.Gomez CR, Misra VK, Liu MW, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke 2000;31:95–99 [DOI] [PubMed] [Google Scholar]
- 6.Mori T, Kazita K, Chokyu K, Mima T, Mori K. Short-term arteriographic and clinical outcome after cerebral angioplasty and stenting for intracranial vertebrobasilar and carotid atherosclerotic occlusive disease. AJNR Am J Neuroradiol 2000;21:249–254 [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez CR, Misra VK, Campbell MS, Soto RD. Elective stenting of symptomatic middle cerebral artery stenosis. AJNR Am J Neuroradiol 2000;21:971–973 [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen PA, Perl J II, Barr JD, Markarian GZ, et al. Stent-assisted angioplasty of intracranial vertebrobasilar atherosclerosis: an initial experience. J Neurosurg 2000;92:771–778 [DOI] [PubMed] [Google Scholar]
- 9.Morris PP, Martin EM, Regan J, Braden G. Intracranial development of coronary stents for symptomatic atherosclerotic disease. AJNR Am J Neuroradiol 1999;20:1688–1694 [PMC free article] [PubMed] [Google Scholar]
- 10.Wakhloo AK, Tio FO, Lieber BB, Schellhammer F, Graf M, Hopkins LN. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol 1995;16:1043–1051 [PMC free article] [PubMed] [Google Scholar]
- 11.Whitbread T, Birch P, Rogers S, Beard JD, Gaines PA. The effect of placing an aortic Wallstent across the renal artery origins in an animal model. Eur J Endvasc Surg 1997;13:154–158 [DOI] [PubMed] [Google Scholar]
- 12.Malina M, Lindh M, Ivancev K, Frennby B, Lindblad B, Brunkwall J. The effect of endovascular aortic stents placed across the renal arteries. Eur J Endovasc Surg 1997;13:207–213 [DOI] [PubMed] [Google Scholar]
- 13.Birch PC, Start RD, Whitbread T, Palmer I, Gaines PA, Beard JD. The effects of crossing porcine renal artery ostia with various endovascular stents. Eur J Endovasc Surg 1999;17:185–190 [DOI] [PubMed] [Google Scholar]
- 14.Marinkovic SV, Kovacevic MS, Marinkovic JM. Perforating branches of the middle cerebral artery: microsurgical anatomy of their extracerebral segments. J Neurosurg 1985;63:266–271 [DOI] [PubMed] [Google Scholar]
- 15.Gibo H, Carver CC, Rhoton AL, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg 1981;54:151–169 [DOI] [PubMed] [Google Scholar]
- 16.Schatz R, Palmaz J, Tio FO, Garcia F, Garcia O, Reuter SR. Balloon-expandable intracoronary stents in the adult dog. Circulation 1987;76:450–457 [DOI] [PubMed] [Google Scholar]
- 17.Schatz RA. A view of vascular stents. Circulation 1989;79:445–457 [DOI] [PubMed] [Google Scholar]
- 18.Grewe PH, Deneke T, Machraoui A, Barmeyer J, Muller KM. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol 2000;35:157–163 [DOI] [PubMed] [Google Scholar]
- 19.Rogers C, Edelman ER. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation 1995;91:2995–3001 [DOI] [PubMed] [Google Scholar]
- 20.Rogers C, Parikh S, Seifert P, Edelman ER. Endogenous cell seeding: remnant endothelium after stenting enhances vascular repair. Circulation 1996;94:2909–2914 [DOI] [PubMed] [Google Scholar]