Abstract
Summary: Changes in the brainstem were demonstrated with MR imaging in a patient with mycosis fungoides. Previous reports of CNS involvement in this rare disease have not had similar findings.
We report a case with changes in the midbrain and pons in a patient in whom cranial nerve signs developed 2 years after the initial diagnosis of mycosis fungoides (MF), in the absence of systemic involvement elsewhere. Pathologic examination revealed tumoral invasion of numerous vessels but no infiltrative neoplastic process. The area of abnormal signal intensity in the brainstem in this case is indicative of ischemia rather than tumoral involvement.
MF is a form of cutaneous T-cell lymphoma that usually occurs in patients older than 50 years. MF is a rare disease, occurring with an incidence of 4.2 cases per million population in the United States (1). MF infrequently involves the CNS. At autopsy, 10% is the rate quoted; however, clinical manifestations are even less frequent than this (2). Involvement of the CNS is observed an average of 3–5 years from initial diagnosis (3), and it typically occurs in patients with advanced infiltration in other organs. In the patient in this report, MF had been diagnosed 2 years before neurologic manifestations appeared; MR images showed brainstem abnormalities. Her skin manifestations were fairly well controlled after local radiation therapy and interferon administration, and she did not have systemic manifestations of the disease.
Case Report
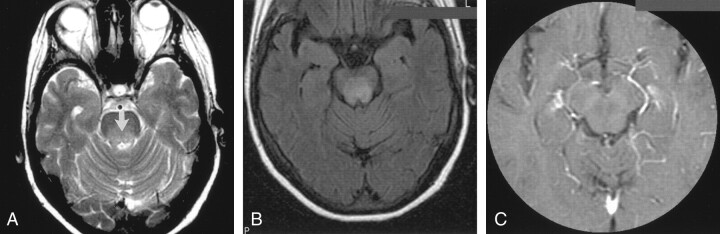
The patient presented at the age of 61 years in the summer of 1998, with some erythematous lesions involving the skin over her back. Biopsy findings were interpreted as compatible with a cutaneous T-cell lymphoma (CD 4+, CD 30–). She was treated with topical carmustine (BCNU) chemotherapy, to which the disease responded well. In November 1999, she had a mass that involved the left pinna. She received local radiation therapy with 9-MeV electrons and a custom wax bolus of a dose of 15 Gy in five daily fractions over 1 week. Interferon was started at a does of 3 million units 3 times weekly. She did well after that until June 2000 when she presented with a 2-week history of occipital headaches, bilateral cranial nerve VI deficits, and decreased visual acuity in the left eye. She was found to have pallor of the left optic disc and mild posterior vitreitis. MR images showed normal morphology of the cerebellum, brainstem, and cerebral hemispheres. Diffuse hyperintensity was depicted on images obtained with proton density–weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences; this involved the posterior half of the pons and cerebral peduncles (Fig 1A and B). No enhancement was seen after the injection of gadolinium-based contrast agent (Fig 1C). Decadron was started; initially, the patient did well. However, in August 2000, the patient was readmitted because her condition deteriorated. Findings on follow-up MR images, obtained 8 weeks after the first images, were unchanged. The possibility of paraneoplastic encephalopathy limited to the brainstem was entertained.
Fig 1.
Axial MR images of the brain in a 61-year-old woman with a 2-year history of cutaneous T-cell lymphoma (MF) and recent-onset CNS symptoms.
A, T2-weighted image (3000/98/1) obtained through the pons and superior cerebellar peduncles shows a diffuse area of hyperintensity (arrow) in the posterior half of the pons.
B, FLAIR image obtained through the cerebral peduncles at the level of the third cranial nerves shows an extensive area of hyperintensity in the tegmentum. The border between the lesion and the spared substantia nigra is sharp.
C, Contrast-enhanced image (450/20/2) shows a lack of uptake in the lesion.
Thereafter, her condition deteriorated rapidly; the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) was unresponsive to treatment with fluid restriction, hypotension, and hypothermia. Her condition was not considered stable enough for the patient to undergo biopsy, and she was treated with radiation therapy to the brain. A dose of 30 Gy was given in 10 daily fractions over 2 weeks. The patient’s symptoms improved during treatment, but no objective improvement in her condition was noted, and she died on the day after her treatment was completed.
Autopsy was performed. Multiple sections of the brain stem did not have any infiltrative neoplastic process. However, numerous vessels were filled with blast cells, which stained with T-cell markers; the most likely diagnosis was thought to be intravascular lymphoma. Evidence of a small area of ischemic damage in the frontal cortex was noted.
Discussion
A few previous scattered reports have described involvement of the CNS in cases of MF when histologic transformation has occurred from the typical T-cell to large anaplastic large cell lymphoma. Rapid and fatal progression of the disease usually indicate such transformation (3, 4). Only one report (5), however, has described a patient in whom peripheral neurologic symptoms developed without evidence of systemic disease.
MF usually evolves slowly over many years. At presentation, the disease is usually limited to the skin, with lesions that resemble eczema or psoriasis. Later, tumors develop in the skin, and involvement of lymph nodes is observed. Finally, visceral involvement occurs; this is often subclinical. The prognosis depends on the stage of the disease (6). CNS involvement is unusual; it was observed in 1.6% of 187 patients with cutaneous T-cell lymphoma in one series (7). Howlett et al (8) and Zonenshayn et al (4) reported cases of brain parenchymal metastasis. These lesions were contrast-enhancing masses that were proved to be tumoral at histologic analysis. These patients also had widespread lymph node and visceral involvement. Chua et al (9) reported eighth cranial nerve deficits that caused deafness in a patient in whom CT and MR imaging findings were negative but in whom CSF findings revealed lymphomatous leptomeningeal disease. Hallahan et al (3) found that, on average, CNS involvement occurred at 5.3 years (range, 6 months to 19 years) after the initial diagnosis of MF in nine patients. Three of these patients underwent CT scanning of the brain, but only one had positive findings for an enhancing mass; findings in the other two were normal. Of the six autopsies performed in this series, four showed meningeal involvement. One had tumor involving the brainstem. Visceral infiltration was widespread in all cases.
Our patient differs from those of previous case reports in several important ways. Systemic visceral involvement had not occurred when neurologic symptoms developed. The lesions depicted on MR images were not space occupying and did not show contrast enhancement. CSF findings were negative for MF. At autopsy, the lesion in the brainstem consisted of ischemic changes related to the numerous vessels filled with cells that stained with T-cell markers. This feature is identical to the occlusion of vessels by lymphoid infiltrate described in angiocentric lymphoma (AL), the term used in the Revised European American Lymphoma (REAL) classification (10). Among the peripheral T-cell neoplasms, AL refers to processes that are localized in extranodal sites, infrequently the skin; these may occur for years prior to diagnosis (11). AL tends to invade and destroy vessels, often resulting in coagulative necrosis. Although the use of intrathecal and/or intraventricular chemotherapy—in combination with brain irradiation—has been proposed for the treatment of CNS MF, it would likely not have been useful in this case.
Conclusion
The anatomic-pathologic findings in the case consisted of ischemic changes in the brainstem due to vascular infiltration by tumoral cells rather than neoplastic invasion of the parenchyma. The diffuse area of hyperintensity on MR images without mass effect or contrast material uptake can be explained on this basis.
References
- 1.Weinstock MA, Horm JW. Mycosis fungoides in the United States: increasing incidence and descriptive epidemiology. J Am Med Assoc 1988;260:42–46 [PubMed] [Google Scholar]
- 2.Epstein E, Levin D, Croft D, Lutzer M. Mycosis fungoides: survival, prognostic features, response to therapy, and autopsy findings. Medicine 1972;51:61–72 [PubMed] [Google Scholar]
- 3.Hallahan D, Griem M, Griem S, Duda E, Baron J. Mycosis fungoides involving the central nervous system.J Clin Oncol 1986;4:1638–1644 [DOI] [PubMed] [Google Scholar]
- 4.Zonenshayn M, Sharma S, Hymes K, Knopp EA, Golfinos JG, Zagzag D. Mycosis fungoides metastasizing to the brain parenchyma: case report. Neurosurgery 1998;42:933–937 [DOI] [PubMed] [Google Scholar]
- 5.Peris K, Fargnoli MC, Berardelli A, Crecco M, Tomaselli R, Chimenti S. Peripheral nervous system involvement in a patient with large T-cell lymphoma arising from a pre-existing mycosis fungoides. Br J Dermatol 1998;139:299–301 [DOI] [PubMed] [Google Scholar]
- 6.Weinstock MA, Reynes JF. The changing survival of patients with mycosis fungoides: a population-based assessment of trends in the United States. Cancer 1999;85:208–212 [PubMed] [Google Scholar]
- 7.Kaufman DK, Habermann TM, Kurtin PJ, O’Neill BP. Neurological complications of peripheral and cutaneous T-cell lymphomas. Am Neurol 1994;36:625–629 [DOI] [PubMed] [Google Scholar]
- 8.Howlett DC, Malcom PN, Wong WL, Pembakian H, Smith NP Ayers AB. Case report: symptomatic intracranial involvement by mycosis fungoides. Clin Oncol 1995;7:395–396 [DOI] [PubMed] [Google Scholar]
- 9.Chua SL, Seymour JF, Prince HM. Deafness from eighth cranial nerve involvement in a patient with large-cell transformation of mycosis fungoides. Eur J Haematol 2000;64:340–343 [DOI] [PubMed] [Google Scholar]
- 10.Grew JP. Hematologic malignancies. In: Lee GR, ed. Wintrobe’s Clinical Hematology. 10th ed. Baltimore, Md: Williams and Wilkins; 1999:2466
- 11.Mederos LJ, Jafle ES. Pathology of non-Hodgkin’s lymphomas and Hodgkin’s disease. In: Wiernik PH, ed. Neoplastic Diseases of the Blood. 3rd ed. New York, NY: Churchill Livingstone; 1995:781