Abstract
Summary: Five cases of unilateral middle cerebral artery fenestration were observed during the prospective evaluation of 1466 consecutive cerebral angiograms (0.43% of 1170 patients) between January 1999 and July 2001. In each case, an early branching temporopolar artery was seen to arise from the inferior limb of the fenestrated segment. This finding suggests that early branching temporopolar arteries may participate in the formation of middle cerebral artery fenestration by interfering with the normal fetal development of the middle cerebral artery.
A fenestrated middle cerebral artery (MCA) is a rare anatomic variant, with reported angiographic and anatomic incidences of 0.17% and 1%, respectively (1, 2). Fenestration occurs when the lumen of an arterial segment is divided into two distinct but parallel channels. Mechanisms underlying the formation of arterial fenestration have been proposed for other typical locations. Incomplete fusion of parallel arterial segments accounts for the development of basilar artery fenestration, whereas the passage of a solid structure such as the hypoglossal nerve has been associated with distal vertebral artery fenestration (3). The mechanism leading to MCA fenestration, however, remains unclear. We report five cases of MCA fenestration documented by digital subtraction angiography. In each case, the temporopolar artery (TPA) was arising from the fenestrated segment. Based on these angiographic findings, we suggest that early branching TPA may participate in the formation of MCA fenestration by interfering with the normal fetal development of the MCA.
Case Reports
Five cases of MCA fenestration were incidentally documented at our institution (Neuroradiology Division, The Johns Hopkins Hospital, Baltimore, MD) between January 1999 and July 2001. A total of 1466 consecutive cerebral angiograms were obtained of 1170 patients during that period, either as diagnostic studies or as the initial diagnostic part of interventional procedures. All the angiograms were prospectively evaluated for arterial anatomic variations by one of the authors (P.G.). The frequency of MCA fenestration based on our series is of 0.43%. The five patients reported herein underwent bilateral selective studies of the carotid arteries, with fenestration being unilateral in each case (three times on the left side and twice on the right). No other anatomic variants were detected in these patients. The distances separating the internal carotid artery (ICA) bifurcation from the fenestrated segment and the origin of the TPA were evaluated by using the caliber of the cisternal segment of the ICA as a reference (4.1 mm according to the study presented by Gibo et al [4] in 1981). The angiographic findings of our five patients are summarized below.
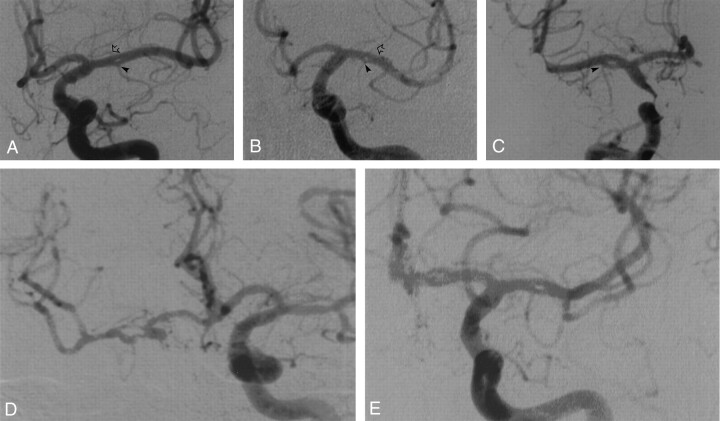
Case 1 involved a 75-year-old woman with vertebrobasilar insufficiency. Digital subtraction angiography documented fenestration of the M1 segment of the left MCA. A prominent lenticulostriate artery originated from the superior aspect of the fenestrated segment, and an early branching TPA arose from the inferior aspect, approximately 6.0 mm distal to the MCA origin (Fig 1A).
Fig 1.
Five angiographic observations of MCA fenestration. Anteroposterior digital subtraction angiograms. In each case, the fenestration is located in the M1 segment of the MCA.
A, Case 1. Fenestration of the left MCA in a 75-year-old woman. The arrowhead indicates an early branching TPA; open arrow, prominent lenticulostriate artery arising from superior aspect of fenestrated segment.
B, Case 2. Fenestration of the left MCA in a 72-year-old man. The arrowhead indicates an early branching TPA; open arrow, prominent lenticulostriate artery arising from superior aspect of fenestrated segment.
C, Case 3. Fenestration of the right MCA in a 39-year-old man. The arrowhead indicates an early branching TPA.
D, Case 4. Fenestration of the right MCA in an 82-year-old woman.
E, Case 5. Fenestration of the left MCA in a 50-year-old woman.
Case 2 involved a 72-year-old man who was being examined for subarachnoid hemorrhage. Digital subtraction angiography revealed fenestration of the M1 segment of the left MCA. A prominent lenticulostriate artery and an early branching TPA arose from the superior and inferior aspects, respectively, of the fenestrated segment. A 4.0-mm distance separated the TPA from the MCA origin (Fig 1B).
Case 3 involved a 39-year-old man who was undergoing a Wada test before surgical treatment for epilepsy. Fenestration of the M1 segment of the right MCA was observed. An early branching TPA arose from the inferior aspect of the fenestrated segment, approximately 5.5 mm distal to the MCA origin (Fig 1C).
Case 4 involved an 82-year-old woman who was being examined for recurrent transient ischemic attacks. Digital subtraction angiography showed an occluded right ICA, with left-to-right collateral flow via the anterior communicating artery. The right MCA showed very proximal M1 fenestration. An early branching TPA arose from the inferior limb of the fenestrated segment, approximately 4.1 mm distal to the MCA origin, and a large lenticulostriate artery arose from the upper limb (Fig 1D).
Case 5 involved a 50-year-old woman with suspected vasculitis. Digital subtraction angiography documented fenestration of the M1 segment of the left MCA. An early branching TPA was arising from the lower limb of the fenestrated segment, approximately 5.2 mm distal to the MCA origin, and a large lenticulostriate artery was arising from the distal part of its upper limb (Fig 1E).
Discussion
We report the incidental angiographic observations of five cases of unilateral fenestration located on the proximal portion of the MCA (M1 segment). Contrary to fenestration located in other arterial segments, such as basilar, vertebral, and anterior spinal arteries, MCA fenestration has rarely been reported and the mechanism underlying its formation remains uncertain. Reported incidences of MCA fenestration vary slightly with the method of investigation from which they are derived (eg, angiographic, surgical, or necropsic studies). The incidence of angiographically documented MCA fenestration has been estimated to be 0.26% by Ito et al (5), whereas the nine cases observed by Sanders et al (2) in a large angiographic series (5190 studies) resulted in a percentage of 0.17%. Crompton (6) detected one case of MCA fenestration while evaluating 347 MCA by means of direct surgical observation (0.28%), and Umansky et al (1) reported a frequency of 1% in a necropsy study. It seems understandable that a morphology characteristic such as a cerebral artery fenestration is more reliably detected by an investigation technique based on direct anatomic specimen observation rather than by indirect radiologic demonstration or by direct observation of a limited surgical field. The frequency of 1% reported by Umansky et al should therefore be considered as the most representative of the real incidence of MCA fenestration. Despite the relatively small size of our sample (1170 patients), the incidence in our series (0.43%) is consistent with previously reported results. The slightly higher detection rate found in our study compared with previous angiographic series is certainly related to recent technical developments, such as the image-processing abilities offered by digital subtraction angiography.
The distance separating the origin of the TPA from the ICA bifurcation has been evaluated by Umansky et al (7). The mean values reported were 7.5 mm for the right side and 7.4 mm for the left side. In our five cases of MCA fenestration, the TPA arose from the fenestrated segment itself and the distance between its origin and the ICA bifurcation was estimated to be 4.0, 4.1, 5.2, 5.5, and 6 mm, respectively (average, 5.0 mm). These findings indicate that in each case, fenestration was associated with an early branching TPA. Although no mention of an association between early TPA branching and MCA fenestration was found in the literature, such a finding was documented in a few published reports. In a surgical case of MCA fenestration described by Umansky et al (1), the TPA originated from the distal aspect of the fenestrated segment but no evaluation of the distance separating this artery from the ICA bifurcation was provided. A figure presented by Sanders et al (2) shows an artery arising from MCA fenestration. It is unclear whether this artery, which is not mentioned in the text, corresponds to a TPA or to an anterior temporal artery, but both situations would correspond to an early branching vessel.
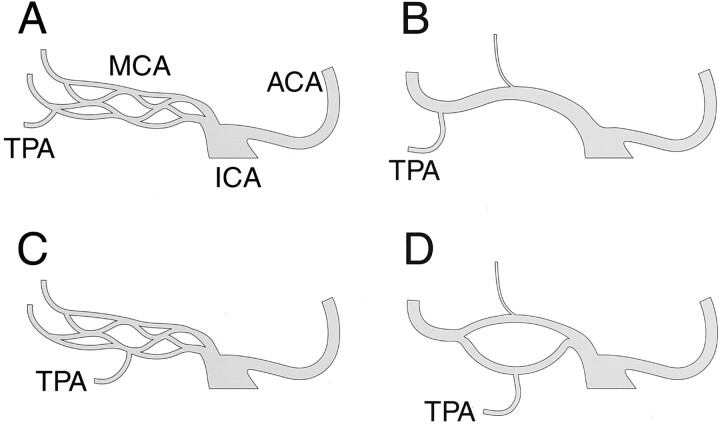
During the normal development of the cranial arterial system, the distal primitive ICA initially divides into a large branch, the future anterior choroidal artery, and numerous small arterial twigs that will subsequently constitute the primitive anterior cerebral arteries and MCA (8) (Fig 2A and 2B). It is reasonable to think that MCA fenestration represents the partial persistence of more than one of these arterial twigs normally coalescing into the definitive MCA. Our five observations show an association between early branching TPA and the formation of MCA fenestration. These angiographic findings can be used to suggest that the formation of MCA fenestration may be facilitated by the presence of an early branching TPA. The early branching TPA could either interfere with the coalescence of primitive arterial twigs into a common proximal MCA (M1 segment) or keep patent collateral channels that would normally involute (Fig 2C and D). A prominent lenticulostriate artery originating from the upper limb of the fenestrated segment was noted in four of the five cases and could represent an additional predisposing factor for the formation of MCA fenestration.
Fig 2.
Hypothetic mechanism underlying MCA fenestration formation. Anteroposterior schematic representations of the ICA bifurcation into anterior cerebral arteries (ACA) and MCA.
A, Normal MCA development, fetal stage. At this stage, the MCA is constituted by multiple arterial twigs arising from the distal ICA.
B, Normal MCA development, adult configuration. The multiple MCA twigs have been replaced by a single proximal MCA trunk (M1 segment).
C, Fenestrated MCA development, fetal stage. The fetal stage is similar to the normal development illustrated in A. An early branching TPA is shown, which might predispose to the formation of fenestration, either by precluding the fusion of several primitive twigs into a single trunk or by interfering with the regression of some of the twigs.
D, Fenestrated MCA development, adult configuration. Fenestration of the M1 segment is shown, with an early branching TPA arising from the inferior aspect of the fenestrated segment, as observed in our five angiographic cases.
References
- 1.Umansky F, Dujovny M, Ausman JI, Diaz FG, Mirchandani HG. Anomalies and variations of the middle cerebral artery: a microanatomical study. Neurosurgery 1988;22:1023–1027 [DOI] [PubMed] [Google Scholar]
- 2.Sanders WP, Sorek PA, Mehta BA. Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. AJNR Am J Neuroradiol 1993;14:675–680 [PMC free article] [PubMed] [Google Scholar]
- 3.Hassler O. Morphological studies of the large cerebral arteries. Acta Psychiatr Neurol Scand 1961;36([suppl 154]):37–42 [PubMed] [Google Scholar]
- 4.Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. Neurosurgery 1981;55:560–574 [DOI] [PubMed] [Google Scholar]
- 5.Ito J, Maeda H, Inoue K, Onishi Y. Fenestration of the middle cerebral artery. Neuroradiology 1977;13:37–39 [DOI] [PubMed] [Google Scholar]
- 6.Crompton MR. The pathology of ruptured middle cerebral artery aneurysms with special to the differences between the sexes. Lancet 1962;2:421–425 [DOI] [PubMed] [Google Scholar]
- 7.Umansky F, Juarez SM, Dujovny M, Ausman JI, Diaz FG, Gomes F, Mirchandani HG, Ray WJ. Microsurgical anatomy of the proximal segments of the middle cerebral artery. J Neurosurg 1984;61:458–467 [DOI] [PubMed] [Google Scholar]
- 8.Padget DH. The development of the cranial arteries in the human embryo. Contr Embryol Carneg Instn 1948;32:205–261 [Google Scholar]