Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to determine the location of the pituitary bright spot in patients with pituitary macroadenomas before surgery.
METHODS: A total of 54 patients with pituitary macroadenomas were retrospectively included in this study. Nonenhanced spin-echo T1-weighted MR images were reviewed to identify the location of the high-intensity-signal posterior pituitary lobe that indicates antidiuretic hormone (ADH) storage. Images were acquired with a 1.5-T machine by using 3-mm-thick contiguous sections in two or three orthogonal planes and a 300 × 512 matrix.
RESULTS: The bright spot corresponding to ADH storage was identified in 44 (81%) patients. Two groups of patients were defined by the height of the macroadenoma: Group A patients (n = 27) had pituitary macroadenomas less than 20 mm in height, and group B (n = 27) had macroadenomas 20 mm or larger. In group A, the bright spot was identified in 25 patients (93%); it was located in the sella in 24 of these cases (96%). In group B, the bright spot was identified in 19 patients (70%); it was in an ectopic location in 14 of these cases (74%).
CONCLUSION: MR imaging can be used to depict the pituitary bright spot in most patients with pituitary macroadenomas before surgery. The bright spot is usually identified at its expected location within the sella in patients with pituitary macroadenomas less than 20 mm in height, whereas an ectopic location is common when pituitary macroadenomas are larger more than 20 mm.
MR imaging can easily depict the anterior and posterior lobes of the pituitary gland in healthy adult volunteers (1). The anterior pituitary appears isointense relative to the pons, whereas the posterior pituitary, the neurohypophysis, has hyperintense signal on T1-weighted images (1, 2). Numerous hypotheses were advanced in the past 2 decades in an attempt to explain the origin of this spontaneous hyperintense signal (3–10). Even if an adequate biophysical explanation has not been widely accepted, the hyperintense signal of the posterior pituitary on T1-weighted images is relatively agreed to reflect the location of antidiuretic hormone (ADH) storage, whether that signal is due to vasopressin, neurophysin, the phospholipid vesicular membrane, or some combination thereof.
The variations of the posterior pituitary gland in pathologic conditions of the anterior lobe have been only rarely described in the literature (7, 11). Nevertheless, it is of great importance because diabetes insipidus (DI), a pathologic condition associated with polyuria, polydipsia, and low urinary osmolality resulting from low ADH release from the pituitary posterior lobe into the blood, is one of the most common postoperative complications of transsphenoidal surgery; it is reported to occur in 17–72% of cases (12–14). Although postoperative DI is transient in most cases, in a large series of 1572 patients (13), as many as 24% of patients required specific therapy. In a 1987 MR study (7), the pituitary bright spot that corresponds to ADH storage was detected in only 43% of the patients with pituitary macroadenomas, including 9% in whom it was displaced. Preoperative knowledge of the ADH storage location in patients with pituitary macroadenomas may be valuable for the neurosurgeons, because, it often is not seen during transsphenoidal surgery (12). The purpose of this study was to assess the accuracy of MR imaging in the preoperative localization of the pituitary bright spot in patients with pituitary macroadenomas and to determine whether patients with an ectopic location are at higher risk of DI.
Methods
Images in a total of 54 patients (36 men, 18 women; mean age, 44 years; range, 19–86 years) with pituitary macroadenomas were retrospectively reviewed. Their macroadenomas included 28 that secreted prolactin; 13, growth hormone; and eight, follicle-stimulating hormone (FSH) and luteinizing hormone (LH); five were nonfunctioning macroadenomas. Preoperatively, no clinical or biologic evidence of diabetes insipidus was present in any patient.
Because the posterior pituitary lobe is intrinsically hyperintense on T1-weighted images (1), the so-called bright spot, only nonenhanced spin-echo T1-weighted images were reviewed in this study. All of the acquisitions were obtained with a standard head coil with a 1.5-T scanner by using the following parameters: TR/TE/NEX, 500/14/3; field of view, 24 cm; matrix, 300 × 512; and section thickness, 3 mm. A total of 35 patients underwent imaging in the coronal and sagittal planes; five, in the coronal and axial planes; one, in the sagittal and axial planes; and 13, in all three planes.
Two independent observers (Y.N., J.-F.B.) carefully assessed the presence, location, and shape of the bright spot. The presence of a pituitary bright spot, believed to correspond to ADH storage, was recorded if it was visible along the hypothalamohypophyseal tract. Bright spots in which chemical-shift artifact was seen were excluded, because they could have represented a lipoma, which is the main differential diagnosis of an ectopic posterior pituitary gland (15, 16). The location was called eutopic when the bright spot was seen in its normal anatomic location immediately anterior to the dorsum sella, and it was considered ectopic when it was outside the sella. In cases of interobserver disagreement regarding the visualization of a pituitary bright spot, the opinion of a third reader (F.B.) was considered decisive.
Among the 54 patients who underwent preoperative imaging, 28 underwent surgery at other institutions, and their postoperative data were not accessible. Thus, we reviewed the files of 26 patients who underwent surgery at our institution for clinical and biologic evidence of postoperative DI, defined as a 2-h urine excretion rate of more than 2500 mL/d and a specific gravity of less than 1.005 g/L (13). The preoperative locations of the bright spots in patients with DI were separately reviewed to evaluate whether the location was an indicator of a higher risk of this complication.
Results
An area of hyperintense signal that corresponds to ADH storage was seen on T1-weighted images in 44 (81%) of 54 patients with pituitary macroadenomas. Remarkably, the high-signal-intensity area was clearly seen and easily differentiated from the dorsum sella in all 19 patients with axial images. Because we saw obvious variations in the depictions, locations, and shapes of the bright spot, we arbitrary defined two groups of patients based on the height of the macroadenoma. Group A (n = 27) included macroadenomas less than 20 mm in height, and group B (n = 27) included those of this height limit or larger. In group A, the bright spot was seen in 25 (93%) of 27 cases. It was visualized at its expected location (eutopic), immediately anterior to the cortex of the dorsum sella in 24 (96%) of 25 cases, with a rounded or flattened shape (Fig 1). In only one case (4%), was the bright spot ectopic; it was observed at the infundibulum (Fig 2). In group B, the hyperintense signal corresponding to the site of ADH storage was visualized in 19 (70%) of 27 cases. It was seen in the sella in only five (26%) of 19 cases and was therefore ectopic in the large majority of the cases (14 [74%] of 19 cases); in two cases (11%) it was eutopic and ectopic at the same time. The shape was either round or flat (Fig 3), or it had a striking figure 3 appearance when the ADH storage area was ectopic and eutopic at the same time (Fig 4).
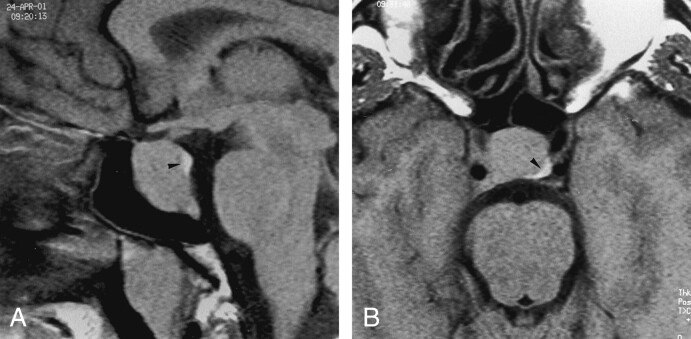
Fig 1.
T1-weighted images (500/14/3) in a 37-year-old man with a 16-mm growth hormone (GH)–secreting macroadenoma with an infrasellar extension (group A) show the eutopic bright spot (arrowhead) within the sella, immediately anterior to the dorsum sella and mildly displaced on the left side. No postoperative data were available.
A, Sagittal image.
B, Axial image.
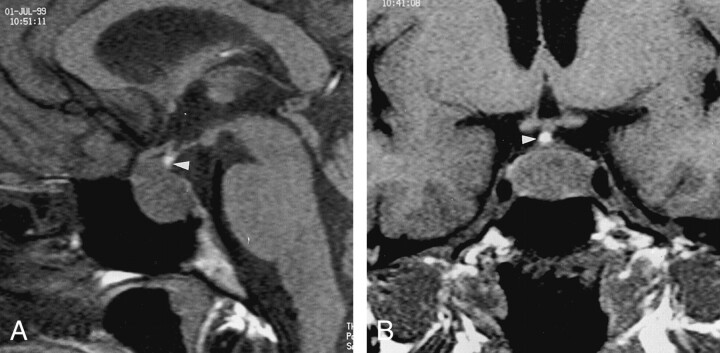
Fig 2.
T1-weighted images (500/14/3) in a 30-year-old woman with a 15-mm FSH- and LH-secreting macroadenoma (group A) illustrates a round, ectopic, bright spot (arrowhead) at the infundibulum. No postoperative DI occurred.
A, Sagittal image.
B, Coronal image.
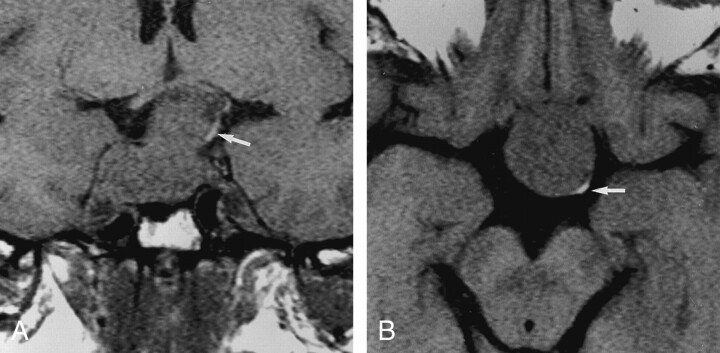
Fig 3.
T1-weighted images (500/14/3) in a 34-year-old man with a 35-mm nonsecreting macroadenoma show a flattened ectopic posterior lobe (arrow), largely displaced from the midline. No postoperative diabetes insipidus occurred.
A, Coronal image.
B, Axial image.
Fig 4.
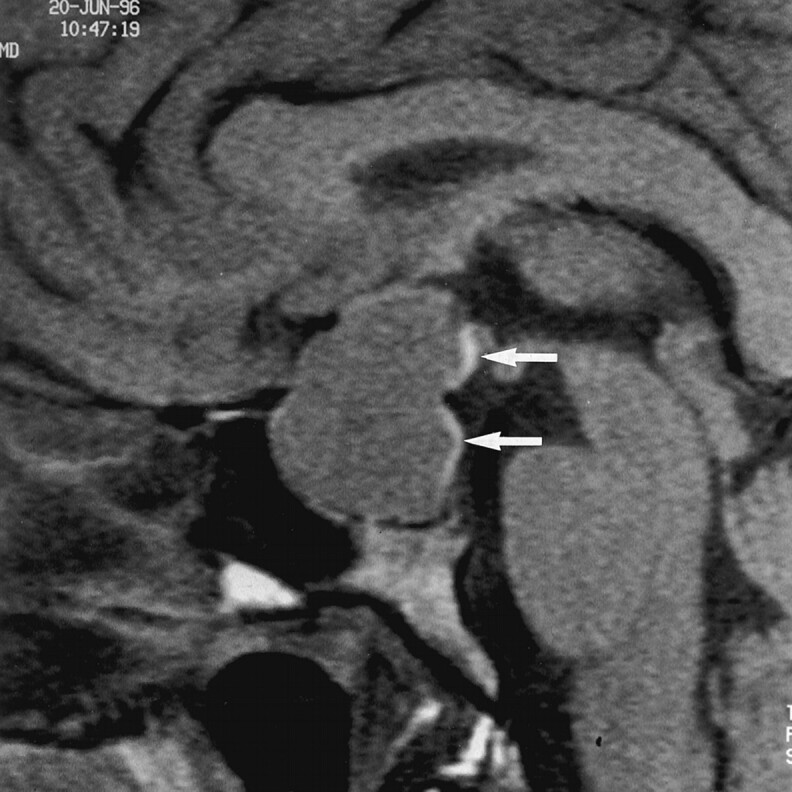
Sagittal T1-weighted image (500/14/3) in a 33-year-old woman with a 26-mm GH-secreting macroadenoma. No postoperative DI occurred. The figure 3 appearance of the high-signal-intensity area (arrows), which corresponds to ADH storage, is considered eutopic and ectopic at the same time. This shape is probably due to the compression of the hypothalamohypophyseal tract filled with ADH vesicles by the sellar diaphragm.
Among the 26 patients who underwent surgery at our institution, 12 were in group A, and 14 were in group B. Preoperatively, the bright spots were eutopic in 14 patients, ectopic in seven, both eutopic and ectopic in one, and not seen in four. Postoperatively, 10 (38%) of 26 patients had DI: Five cases were transient, and five were considered definitive because the patients still needed appropriate therapy after a mean follow-up of 19 months (range, 6–45 months). Definitive DI occurred in two (14%) of 14 patients with a preoperative eutopic bright spot and in one (14%) of seven patients with an ectopic bright spot. Another case occurred in a patient in whom the bright spot was eutopic and ectopic at the same time, and the last definitive DI case occurred in a patient in whom the bright spot was not seen preoperatively.
Discussion
ADH, also called vasopressin, is a hormone synthesized in the supraoptic and paraventricular nuclei of the thalamus. It is enveloped together with a carrier protein called neurophysin in neurosecretory granules and transported downward through the axons to the stalk and the posterior pituitary lobe, where it is stored. ADH is released by means of exocytosis into the blood stream when it is needed. The posterior pituitary is, therefore, the distal reservoir for the storage of ADH in the posterior portion of the pituitary fossa. Since the very beginnings of the MR imaging era, this anatomic region has been the subject of numerous publications, as well as the source of discrepancies, because it has a surprisingly appearance with an unusual hyperintense signal on T1-weighted images (3–10). Correlating anatomy and MR images, Mark et al (3) were the first to suggest that this high-intensity-signal area could correspond to a fat pad located in the posterolateral recesses of the sella; however, they later rejected this theory by using a fat-suppressed MR technique (9). Fujisawa et al (4) then demonstrated that this hyperintense signal was absent in patients with central DI and speculated that it may, therefore, reflect the functional integrity of the posterior lobe. The authors hypothesized that neurosecretory granules containing ADH could be the cause of the short T1 value in the posterior pituitary (1, 4, 17). Hence, two theories emerged, and they are still the subjects of controversy. One proposed that the hyperintense signal arises from the vasopressin–neurophysin II–copeptin complex (6), whereas the other suggests that it results from the phospholipids that form the membrane surrounding the neurosecretory granule (8). Even if the posterior pituitary high-intensity puzzle is not biophysically solved, that this hyperintense signal reflects ADH storage is widely accepted.
In normal conditions, the bright signal of the posterior pituitary lobe, which is often located in a small depression of the dorsum sella, is clearly differentiated from the anterior lobe on dedicated thin-section axial CT or MR images (1, 18). However, the visibility of the bright spot on T1-weighted MR images ranges widely, from 52% to 100%, in the literature (1, 7, 19). Furthermore, few articles have discussed the visibility of the pituitary posterior lobe in the setting of sellar or parasellar tumors, and, to our knowledge, none have done so exclusively with a series of a single tumor type (7, 11). These factors may be relevant, however, because pituitary adenomas are often resected with transsphenoidal surgery (20), which may result in the postoperative complication of DI in 17–72% of patients (12–14). Fortunately, the large majority of these reported cases of DI are transient, probably because of the ability of the hypothalamohypophyseal axis to regenerate or reorganize after an injury, when the hypothalamic cell bodies have not been completely destroyed (21). However, even if this complication is transient in most cases, the polyuria-polydipsia syndrome resulting from DI is the cause of serious discomfort that requires hormone replacement treatment; occasionally, it is a permanent deficit. Therefore, relationships between the anterior and posterior pituitary lobes are relevant in cases of macroadenomas. In a series of 44 patients with pituitary macroadenomas, Colombo et al (7) found that only 43% had a detectable bright spot on T1-weighted images. This low frequency was presumably explained in this 1987 article with the marked compression of the posterior lobes caused by macroadenomas. The bright spot was identified almost twice as frequently in our series (81%), and this finding is probably explained by the use of a better technique with a high-resolution matrix (300 × 512) and contiguous 3-mm-thick sections, especially in the axial plane. The use of such sections certainly allowed us to clearly detect very thin areas of hyperintense signal that correspond to ADH storage, even when they were largely displaced from the midline (Fig 3), when they would probably be missed on sagittal images. Indeed, the bright spot that is indicative of the ADH storage was found in every patient who underwent imaging in the axial plane with our technique. Our results support those of Fujisawa et al (1), who observed the hyperintense signal of the posterior lobe in all 60 healthy patients who underwent imaging with parameters that included the axial plane.
In hypophysectomized rats in which the hypothalamohypophyseal tract is interrupted, a tissue that structurally and functionally resembles the posterior lobe develops superior to the created gap (21). This newly reorganized posterior pituitary is commonly defined as an ectopic posterior pituitary. With in vivo MR imaging in humans, several authors (7, 11, 16, 17, 22) showed that an ectopic posterior lobe can develop at the proximal stump of a transected, compressed, or obstructed pituitary stalk and that it compensates for the dysfunction of the genuine posterior lobe. el Gammal et al (11) retrospectively reported their experience in 13 patients with sellar tumors, some of whom underwent postoperative imaging and others, preoperative imaging, in whom subsequent high-signal-intensity areas were found near the median eminence, corresponding to ectopic posterior pituitary regeneration. The authors indirectly supported the results of Colombo et al (7), who found four cases (9%) with ectopic nodular bright spots in their series of patients with pituitary macroadenomas. However, no accurate description of those compressive tumors was given in these articles, particularly, information about their size or height. We found that, when pituitary macroadenomas were smaller than 20 mm in height, the bright spot, when visualized, was seen in almost all cases (96%) in its usual location immediately anterior to the dorsum sella. We demonstrated that this storage was visible outside the sella, in total or in part, in 74% of macroadenomas larger than 20 mm. This finding suggests that large macroadenomas are more compressive in the hypothalamohypophyseal tract and that they prevent ADH vesicles from descending to its usual location. In addition, two of our patients with large pituitary macroadenomas had a pituitary bright spot inside and outside the sella at the same time; to our knowledge, this is the first time this finding has been reported. In these two cases, the ADH storage area was compressed and spanned the hypothalamohypophyseal tract (Fig 4). Therefore, the bright spot appeared here with a striking figure 3 shape, which probably resulted from a hypothalamohypophyseal tract filled with ADH vesicles compressed by the pituitary macroadenoma and sellar diaphragm. Our results illustrate that MR imaging can enable in vivo visualization of the reorganization of ADH storage above or behind a large intra- or suprasellar mass that obstructs the hypothalamohypophyseal tract.
Little is known about the potential relationship between the preoperative site of ADH storage and the occurrence of postoperative DI. In our series, two (14%) of 14 patients with eutopic bright spot and one (14%) of seven with an ectopic bright spot had definitive DI. Although those percentages may appear high, they are still in the wide 0.5–15% range in a recent national American survey (12). We acknowledge that this study was retrospective and based on small cohorts, with values that are statistically insignificant. We still believe that, when the bright spot corresponding to ADH storage is superiorly displaced (thus closer to the hypothalamic nuclei and farther from the surgical approach from the sphenoid sinus), it may be less vulnerable, and the resultant occurrence of DI may be lower. However, this intuitive thought must be verified with further, larger studies. In addition, because the stalk and posterior lobe often are not seen during pituitary macroadenoma surgery (a fibrous layer may largely displace, flatten, or cover it) and because postoperative DI may occur as a consequence of stalk manipulations (12), further prospective studies are necessary to evaluate if preoperative knowledge of the location of the pituitary bright spot in patients with pituitary macroadenomas could help neurosurgeons to preserve the hypothalamohypophyseal tract; thereby, the risk of postoperative DI may be decreased.
Conclusion
The high-signal-intensity area on T1-weighted images that corresponds to ADH storage can be clearly identified with MR imaging before surgery in most patients with pituitary macroadenomas. In macroadenomas less than 20 mm in height, the pituitary bright spot is seen at its expected location immediately anterior to the dorsum sella in almost all cases. In macroadenomas larger than 20 mm, is the bright spot is ectopic in the large majority of patients, presumably because of a greater mass effect.
Acknowledgments
The authors thank the technical team of the Department of Neuroradiology of Besançon for their contributions, Michel Gaudron for his remarkable photographic work, and Dr Steven G. Bramlet and Dr David H. Miller for their help in reviewing the manuscript for this article.
Dr Fabrice Bonneville was supported by grants from the French Society of Radiology and the French Society of Neuroradiology.
Presented as an oral paper at the ASNR 39th annual meeting, Boston, MA, April 2001.
References
- 1.Fujisawa I, Asato R, Nishimura K, et al. Anterior and posterior lobes of the pituitary gland: assessment by 1.5 T MR imaging. J Comput Assist Tomogr 1987;11:214–220 [DOI] [PubMed] [Google Scholar]
- 2.Nishimura K, Fujisawa I, Togashi K, et al. Posterior lobe of the pituitary: identification by lack of chemical shift artifact in MR imaging. J Comput Assist Tomogr 1986;10:899–902 [PubMed] [Google Scholar]
- 3.Mark L, Pech P, Daniels D, Charles C, Williams A, Haughton V. The pituitary fossa: a correlative anatomic and MR study. Radiology 1984;153:453–457 [DOI] [PubMed] [Google Scholar]
- 4.Fujisawa I, Nishimura K, Asato R, et al. Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr 1987;11:221–225 [DOI] [PubMed] [Google Scholar]
- 5.Fujisawa I. Hyperintense signal on MR images of the pituitary gland. AJNR Am J Neuroradiol 1991;12:581–583 [PMC free article] [PubMed] [Google Scholar]
- 6.Kurokawa H, Fujisawa I, Nakano Y, et al. Posterior lobe of the pituitary gland: correlation between signal intensity on T1-weighted MR images and vasopressin concentration. Radiology 1998;207:79–83 [DOI] [PubMed] [Google Scholar]
- 7.Colombo N, Berry I, Kucharczyk J, et al. Posterior pituitary gland: appearance on MR images in normal and pathologic states. Radiology 1987;165:481–485 [DOI] [PubMed] [Google Scholar]
- 8.Kucharczyk W, Lenkinski RE, Kucharczyk J, Henkelman RM. The effect of phospholipid vesicles on the NMR relaxation of water: an explanation for the MR appearance of the neurohypophysis? AJNR Am J Neuroradiol 1990;11:693–700 [PMC free article] [PubMed] [Google Scholar]
- 9.Mark LP, Haughton VM, Hendrix LE, et al. High-intensity signals within the posterior pituitary fossa: a study with fat-suppression MR techniques. AJNR Am J Neuroradiol 1991;12:529–532 [PMC free article] [PubMed] [Google Scholar]
- 10.Oot R, New PF, Buonanno FS, et al. MR imaging of pituitary adenomas using a prototype resistive magnet: preliminary assessment. AJNR Am J Neuroradiol 1984;5:131–137 [PMC free article] [PubMed] [Google Scholar]
- 11.el Gammal T, Brooks BS, Hoffman WH. MR imaging of the ectopic bright signal of posterior pituitary regeneration. AJNR Am J Neuroradiol 1989;10:323–328 [PMC free article] [PubMed] [Google Scholar]
- 12.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 1997;40:225–236 [DOI] [PubMed] [Google Scholar]
- 13.Hensen J, Henig A, Fahlbuscht R, Meyer M, Boehnert M, Buchfelder M. Prevalence, predictors and patterns of postoperative polyuria and hyponatremia in the immediate course after transphenoidal surgery for pituitary adenomas. Clin Endocrinol 1999;50:431–439 [DOI] [PubMed] [Google Scholar]
- 14.Massoud AF, Powell M, Williams RA, Hindmarsh PC, Brook CG. Transsphenoidal surgery for pituitary tumours. Arch Dis Child 1997;76:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benshoff ER, Katz BH. Ectopia of the posterior pituitary gland as a normal variant: assessment with MR imaging. AJNR Am J Neuroradiol 1990;11:709–712 [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahams JJ. Ectopia of the posterior pituitary gland or lipoma? [letter]. AJNR Am J Neuroradiol 1991;12:579–581 [PMC free article] [PubMed] [Google Scholar]
- 17.Fujisawa I, Kikuchi K, Nishimura K, et al. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology 1987;165:487–489 [DOI] [PubMed] [Google Scholar]
- 18.Bonneville JF, Cattin F, Portha C, Cuenin E, Clere P, Bartholomot B. Computed tomographic demonstration of the posterior pituitary. AJR Am J Roentgenol 1986;146:263–266 [DOI] [PubMed] [Google Scholar]
- 19.Brooks BS, el Gammal T, Allison JD, Hoffman WH. Frequency and variation of the posterior pituitary bright signal on MR images. AJNR Am J Neuroradiol 1989;10:943–948 [PMC free article] [PubMed] [Google Scholar]
- 20.Visot A. Neurosurgery and pituitary tumors: surgical indications and outcome. Presse Med 2001;30:401–404 [PubMed] [Google Scholar]
- 21.Billenstien DC, Leveque TF. The reorganization of the neurohypophyseal stalk following hypophysectomy in the rat. Endocrinology 1955;56:704–717 [DOI] [PubMed] [Google Scholar]
- 22.Kuroiwa T, Okabe Y, Hasuo K, Yasumon K, A Mizushima, K Masuda. MR imaging of pituitary dwarfism. AJNR Am J Neuroradiol 1990;12:161–164 [PMC free article] [PubMed] [Google Scholar]