Abstract
Summary: Radiculomyelitic (silent) rabies and acute disseminated encephalomyelitis have similar clinical presentations but distinct management and prognostic implications. It is thus important to differentiate between the two antemortem. Because of their distinct pathologic abnormalities, MR imaging may be helpful in distinguishing between the two entities. We report a case in which MR imaging helped us to diagnose silent rabies antemortem, which was subsequently confirmed at autopsy.
Rabies is the most formidable encephalitis with unique clinical presentation almost always resulting in death. Radiculomyelitic rabies occurring after rabies postexposure prophylaxis after animal bite is often clinically difficult to differentiate from acute disseminated encephalomyelitis. Although MR imaging of the encephalitic form of rabies has been reported, this case further describes the MR imaging findings of the radiculomyelitic form and how it differs from the typical imaging presentation of acute disseminated encephalomyelitis.
Case Report
A 10-year-old boy presented with a history of fever that was present for 6 days, progressive weakness of both lower limbs for 4 days, and extreme agitation with behavioral changes for 2 days before admission. Although a history of hydrophobia was not forthcoming, the patient had been bitten by a stray dog 25 days previously on the left lower limb. Fifteen days after the bite, a course of Rabipur vaccine had been administered. The patient, however, had not received anti-rabies immunoglobulin. The dog was alive even 15 days after the bite. An examination performed at admission revealed that the child was in altered sensorium (Glasgow Coma Scale score, E3M4V2) that worsened during his hospital stay. There was symmetrical weakness of lower limbs with areflexia. There were no meningeal signs. The results of a fundus examination were normal at admission. Initially, clinical differential diagnoses of acute disseminated encephalomyelitis and silent rabies were considered. The weakness progressed to involve the upper limbs during a period of 48 hours. Four days after admission, the patient developed poor respiratory efforts, probably because of involvement of the brain stem. Manually administered intermittent positive pressure ventilation was started. Clinical investigations revealed normal hemogram, electrolytes, and chest radiograph. A CSF examination showed mild pleocytosis with lymphocytic predominance with normal biochemistry. Gram staining revealed no microorganisms, and the culture was sterile. The results of antigen detection tests (for meningococci, pneumococci, and Haemophilus influenzae) were negative. A corneal smear and skin biopsy did not show antigen on immunofluorescence. The results of nerve conduction velocity studies were normal.
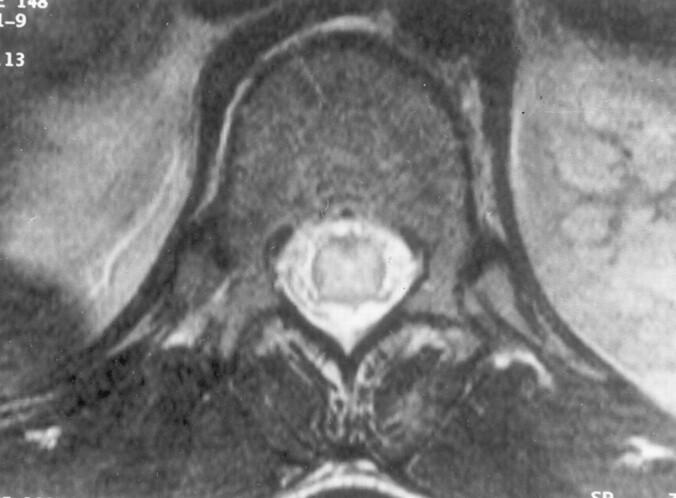
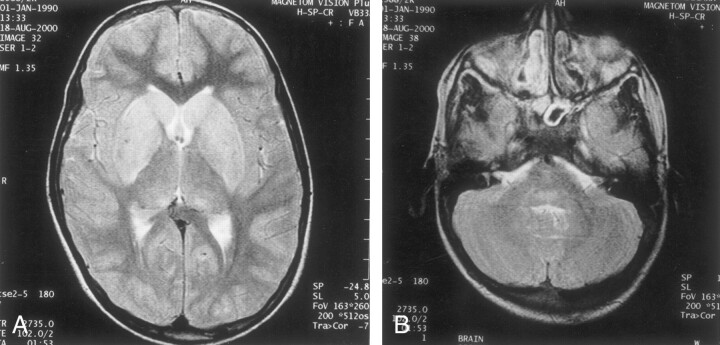
CT of the head was performed at admission, and the results were normal. MR imaging performed on the third day revealed diffuse T2-weighted hyperintensity involving central gray matter of the whole spinal cord (Fig 1), extending cranially to symmetrically involve bilateral thalami, hippocampi, basal ganglia, and the dorsal aspect of the brainstem (Fig 2). The white matter of the spinal cord and brain was spared with well-maintained gray matter–white matter distinction.
Fig 1.
Axial T2-weighted image of the dorsal spine reveals hyperintensity of central gray matter, with sparing of the white matter.
Fig 2.
Images show cranial extension to symmetrically involve bilateral thalami, hippocampi, basal ganglia, and the dorsal aspect of the brainstem.
A, T2-weighted MR image of the brain, obtained at the level of basal ganglia, reveals hyperintensity of the caudate head and lentiform nucleus.
B, T2-weighted MR image of the brain, obtained at the level of the pons, reveals hyperintensity of the posterior portion of the pons.
The IV administration of methylprednisolone (30 mg/kg) was started on day 2 after admission, in view of clinical suspicion of acute disseminated encephalomyelitis. The administration of steroids was discontinued once MR imaging findings of exclusive gray matter involvement were shown. On day 6 after admission, the patient developed dilated pupils that were unresponsive to light with absent doll’s eye movement. EEG performed at this time showed diffuse slowing, with no epileptiform discharges. Life support was withdrawn on day 10 of the hospital stay, after consultation with the patient’s family.
Gross Pathologic Findings at Autopsy
The brain was moderately edematous. No external abnormality was noted. A pathologic examination of brain slices showed that there was no definite abnormality, except for slight blurring of cortical gray matter in some foci. The brainstem and cerebellum appeared relatively congested. The spinal cord showed no gross abnormality.
Microscopic Pathologic Findings
Lymphocytic infiltration of the meninges had occurred. Significant changes were noted in the spinal cord, medulla, and pons. These included marked neuronal loss, microglial hyperplasia, neuronophagia, and microglial nodule formation. The hippocampus also showed similar changes. Many Negri bodies were seen in this area. Perivascular lymphocytic cuffing was more marked in the brain stem and spinal cord (Fig 3). White matter was spared. Dorsal root ganglia and the nerve roots showed extensive lymphocytic infiltrates. Immunostain was strongly positive for rabies virus antigen (Fig 3, inset).
Fig 3.
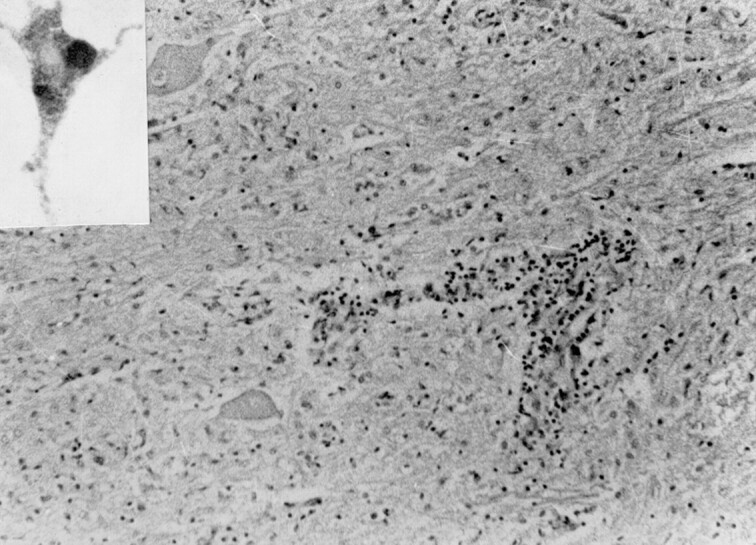
Perivascular lymphocytic infiltrate and microglial hyperplasia in the cervical cord (hematoxylin and eosin, original magnification ×140). Inset shows positive immunostaining using monoclonal anti-rabies antibodies.
Discussion
Silent or radiculomyelitic rabies presenting with ascending paralysis like Guillian-Barré syndrome, constitutes 20% of cases with pathologic abnormalities involving spinal cord or dorsal ganglion cells (1). It is especially evident after postexposure prophylaxis (2). Rabies in its classical form presents as brain stem encephalitis. Cases presenting as paralytic illness, especially in the setting of postexposure prophylaxis, which per se may result in acute disseminated encephalomyelitis, can pose a diagnostic dilemma.
Rabies has an affinity for bulbar and limbic neurons and specifically involves the brainstem. Pathologically, the changes are those of a polioencephalomyelitis with inflammatory change occurring predominantly in the gray matter (3). The pathognomonic histologic features of rabies are the Negri bodies, although they are present in only 50–80% of cases proven by virus isolation methods (4, 5). In cases of silent rabies, the most striking pathologic changes are seen in the spinal cord (1). There may be lymphocytic leptomeningeal infiltration and, within the cord, neuronal degeneration in the anterior horn with inflammatory cell infiltration (6). In cases of silent rabies, the brainstem and cerebrum may also have inflammatory cell infiltration and, in cases with clinical bulbar involvement, neuronophagia and microglial nodules may occur (1).
Semple vaccine, which is still used in parts of Asia and Africa, prepared from phenol-treated infected neural tissue from a variety of animal species, has a high incidence of postvaccinial neurologic complications (approximately 1:200–1600) (7). With the introduction of more purified forms of vaccine, the incidence of these neurologic complications has been reduced to fewer than 1:8000 with transverse myelitis, enchephalomyelitis, optic neuritis, and polyradiculitis being the more common complications (1). Microscopic changes include small perivascular demyelinating lesions, which coalesce to form large multicentric lesions scattered throughout the gray matter and white matter of the spinal cord, cerebrum, and optic nerves, and mild lymphocytic leptomeningitis (8).
Neuroimaging of these patients had revealed normal cerebral CT and MR imaging results early in the disease; cerebral edema causing loss of gray:white matter distinction may occur later (9, 10). Low-matter–density changes in the basal ganglia, brainstem, and medial temporal lobes has been reported resulting from increased water concentration in brain tissue and degradation of membrane phospholipid constituents (9, 11). In view of the distinct pathologic abnormalities of acute disseminated encephalomyelitis and silent rabies, we can postulate that MR imaging can reliably distinguish between these two close clinical differentials. The MR imaging of this patient showed excellent correlation between pathologic findings and imaging findings. The images exquisitely showed the predominant gray matter involvement of brain and spinal cord, which is a hallmark of rabies and an important differentiating point from acute disseminated encephalomyelitis (3). The white matter, on the other hand, was totally spared in contrast to acute disseminated encephalomyelitis, with which white matter is predominantly involved (8). The hyperintensity of the gray matter of the cord on the T2-weighted image corresponds to inflammatory cell infiltration. The lesions were continuous and symmetrical, involving central gray matter of the entire cord, unlike other demyelinating conditions, which are expected to be patchy and discontinuous, involving predominantly the white matter. The high signal intensity on the T2-weighted image in the bilateral hippocampi, thalami, basal ganglia, and the dorsal aspect of brainstem reflect the pathologic studies of rabies that have shown maximal concentration of Negri bodies and anti-rabies antigen as revealed by immunohistochemistry (6). This single case report highlights the potential usefulness of MR imaging in a clinical scenario of ascending paralysis.
References
- 1.Chopra JS, Banerjee AK, Murthy JM, Pal SR. Paralytic rabies: a clinico-pathological study. Brain 1980;103:789–802 [DOI] [PubMed] [Google Scholar]
- 2.Bhaba SK, Bharucha NE, Bharucha EP. Viral infections. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD, eds. Neurology in Clinical Practice. 2nd ed. Boston, Mass: Butterworth-Heinemann;1996. :1267–1269
- 3.Whitley RJ, Middlebrooks M. Rabies. In: Scheld WM, Whitley RJ, Durack DT, eds. Infections of the Central Nervous System. New York, NY: Raven Press;1991. :127–144
- 4.Dupont JR, Earle KM. Human rabies encephalitis: a study of forty-nine fatal cases with a review of the literature. Neurology 1965;15:1023–1034 [DOI] [PubMed] [Google Scholar]
- 5.Koprowski H. The mouse inoculation test. In: Laboratory Techniques in Rabies. 3rd ed. Geneva, Switzerland: World Health Organization;1973. :85–93 [PubMed]
- 6.Jogai S, Radotra BD, Banerjee AK. Immunohistochemical study of human rabies. Neuropathology 2000;20:193–203 [DOI] [PubMed] [Google Scholar]
- 7.Tangchai P, Vejjajiva A. Pathology of the peripheral nervous system in human rabies. Brain 1971;94:299–306 [DOI] [PubMed] [Google Scholar]
- 8.Alvord EC Jr. Disseminated encephalomyelitis: its variations in form and their relationships to other diseases of the nervous system. In: Vinken PJ, Bruyn GW, Klawans HI, eds. Handbook of Clinical Neurology: Demyelinating Diseases. Vol 47. Amsterdam, the Netherlands: Elsevier,1985. :467–502
- 9.Roine RO, Hillbom M, Valle M, et al. Fatal encephalitis caused by a bat-borne rabies-related virus: clinical findings. Brain 1988;111:1505–1516 [DOI] [PubMed] [Google Scholar]
- 10.Sing TM, Soo MY. Imaging findings in rabies. Australas Radiol 1996;40:338–341 [DOI] [PubMed] [Google Scholar]
- 11.Pleasure SJ, Fischbein NJ. Correlation of clinical and neuroimaging findings in a case of rabies encephalitis. Arch Neurol 2000;57:1765–1769 [DOI] [PubMed] [Google Scholar]