Abstract
Summary: We describe the case of a 40-year-old man with spontaneous intracranial hypotension who presented with cervical radiculopathy associated with epidural venous engorgement. Epidural venous engorgement can occur secondary to intracranial hypotension and manifests intracranially as pachymeningeal venous engorgement. In the cervical spine, two cases of epidural venous engorgement due to intracranial hypotension have been reported in the literature, and neither patient presented with symptoms related to nerve compression. Epidural venous engorgement should be considered in the differential diagnosis of an enhancing epidural mass in the cervical spine. Diagnostic clues include sparing of the anterior midline and posterior aspects of the epidural space and, if present, pulsation artifact.
Intracranial hypotension typically presents with postural headaches, often with one or more of the following symptoms: nausea, vomiting, dizziness, diplopia, photophobia, hearing impairment, neck pain, and blurred vision (1). It is most commonly due to postoperative or spontaneous dural tears, but may also be seen following lumbar puncture or with overdraining CSF shunts (1). The major abnormal intracranial MR findings associated with intracranial hypotension include diffuse pachymeningeal enhancement, subdural fluid collections, and evidence of brain descent (1–3). In the spine, MR imaging may reveal an extraarachnoid CSF collection, dilated asymmetric veins, dural enhancement, and occasionally the site of CSF leakage (1, 4–6). We report a case of spontaneous intracranial hypotension and associated radicular symptoms due to epidural venous engorgement.
Case Report
A 40-year-old man presented with intermittent, primarily left-sided headaches that radiated down the left side of his neck. Within a month of presentation, he underwent brain MR imaging, the findings of which were reportedly normal. Several weeks after the onset of persistent headaches, he had difficulty raising his right arm, with some right-sided neck and shoulder pain without arm pain or loss of sensation. He noted that the headaches worsened when he bent over or rose from a seated position. He also experienced the sensation of left ear obstruction with these positional changes.
Physical examination revealed obvious atrophy of the right deltoid muscle and right latissimus dorsi, with prominent winging of the right scapula, particularly with forward arm extension. There was weakness of the latissimus dorsi, deltoid, and supra- and infraspinatus muscles and milder weakness of the triceps. Otherwise, his examination findings, including deep tendon reflexes, were normal. MR evaluation of his cervical spine was performed at the onset of his upper extremity findings and revealed a bilateral symmetric epidural mass. We were unable to obtain these images as they were acquired at an outside hospital. Subsequent MR studies showed interval enlargement and contraction of this epidural mass with associated fluctuations of his extremity weakness and headaches, which suggested the possibility of a vascular lesion in the cervical spine. Spinal angiography was then performed, but the findings were normal.
Several months later, a repeat brain MR examination (performed outside our institution and irretrievable) reportedly demonstrated marked pachymeningeal enhancement primarily around the frontal convexities and along the tentorium, without evidence of downward displacement of the brain. In addition, symmetrically dilated vascular structures were again seen anterior to the cervical cord without cord compression (Figs 1 and 2). MR venography of the neck confirmed that these structures were engorged veins (Fig 3). The intracranial findings suggested intracranial hypotension as a cause of the cervical venous engorgement. Lumbar puncture was subsequently performed, and the opening pressure was too low to measure. CSF was obtained only after the patient assumed a reverse Trendelenberg position. CSF analysis was normal. Then, 0.54 mCi of indium-111 diethylenetriaminepentaacetic acid (DTPA) was administered intrathecally, and imaging was performed immediately after injection and at 4 hours (Fig 4) and 24 hours after injection. On immediate and delayed images, an extraarachnoid accumulation of the radionuclide was seen in the lumbar spine and extended to the left side of the spinal canal, consistent with CSF leakage from a dural tear. Soon after this procedure, the patient’s symptoms and physical examination findings resolved gradually and spontaneously. A follow-up MR examination performed 6 months later showed prominent improvement in the epidural venous engorgement (Fig 5).
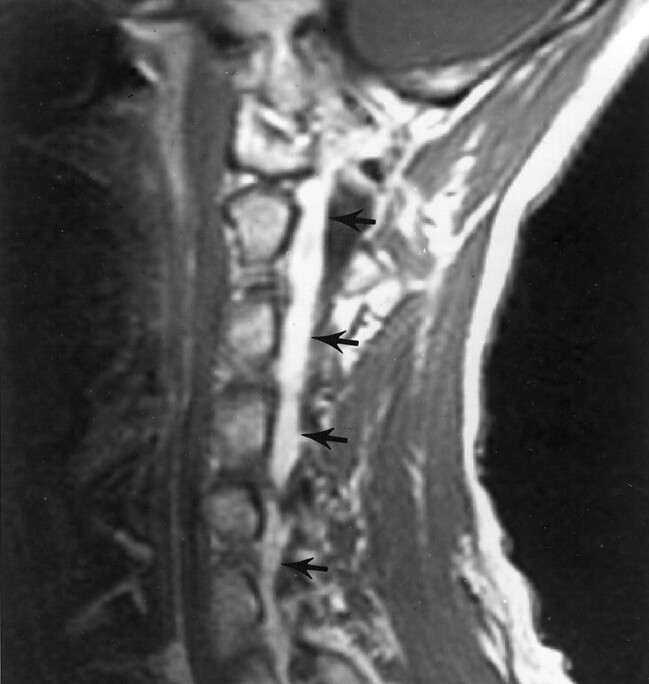
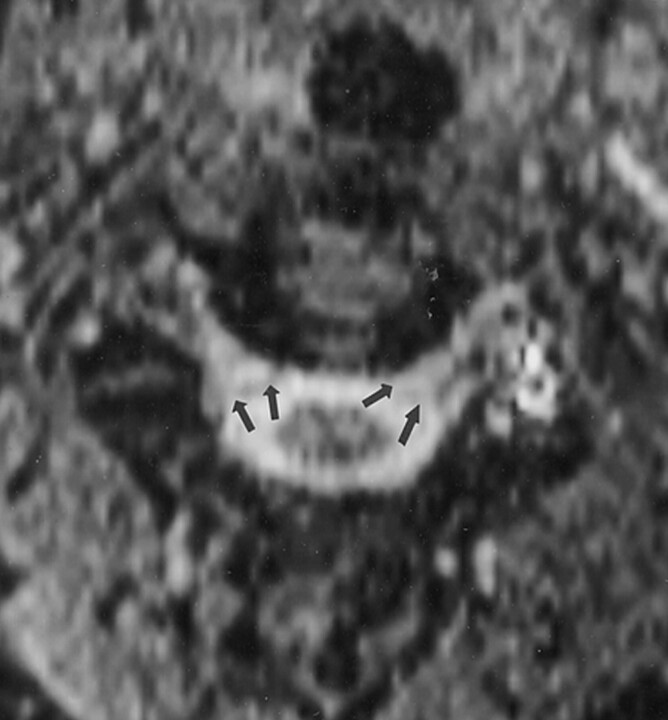
Fig 1.
Contrast-enhanced sagittal T1-weighted (500/9/2 [TR/TE/NEX]) MR image shows the extent of venous engorgement in the cervical epidural space (arrows).
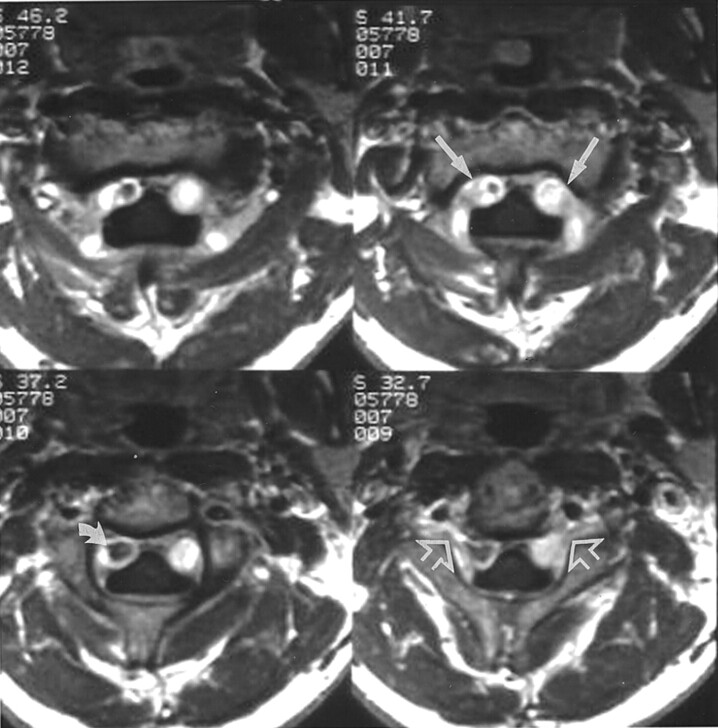
Fig 2.
Contrast-enhanced axial T1-weighted (400/8/2) MR images at the level of the C2 vertebral body show intense bilateral enhancement (straight solid arrows) in the anterolateral cervical epidural space, consistent with the dilated epidural venous plexus. Note prominent flow void (curved arrow) in the enhancing right plexus and extension of dilated venous structures (open arrows) into the intervertebral foramina.
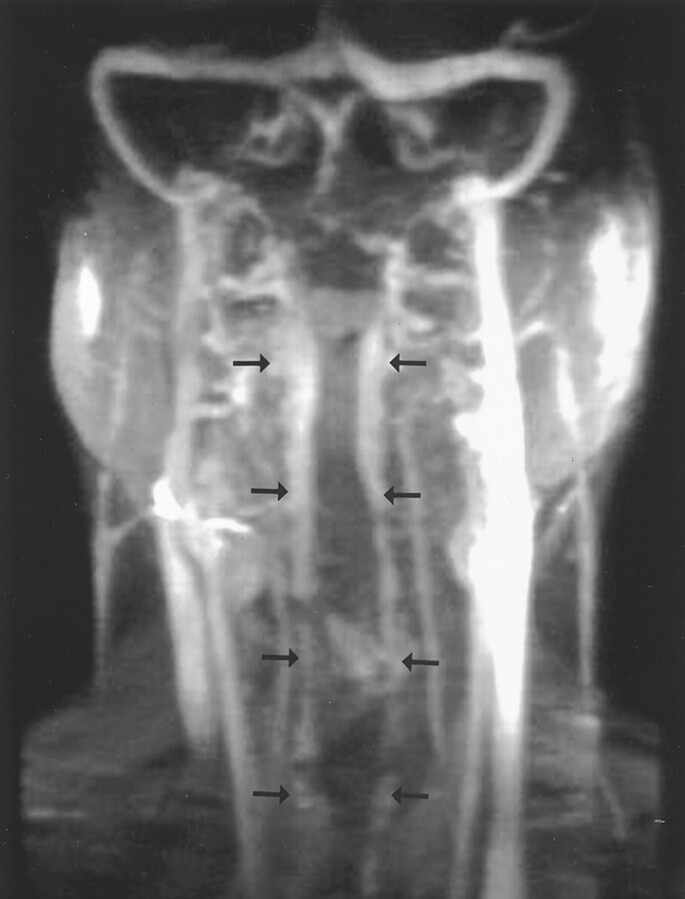
Fig 3.
MR venogram (coronal reconstruction of maximum intensity projection) demonstrates cervical epidural venous engorgement from the most superior portion of the cervical spine to the thoracic inlet bilaterally (arrows).
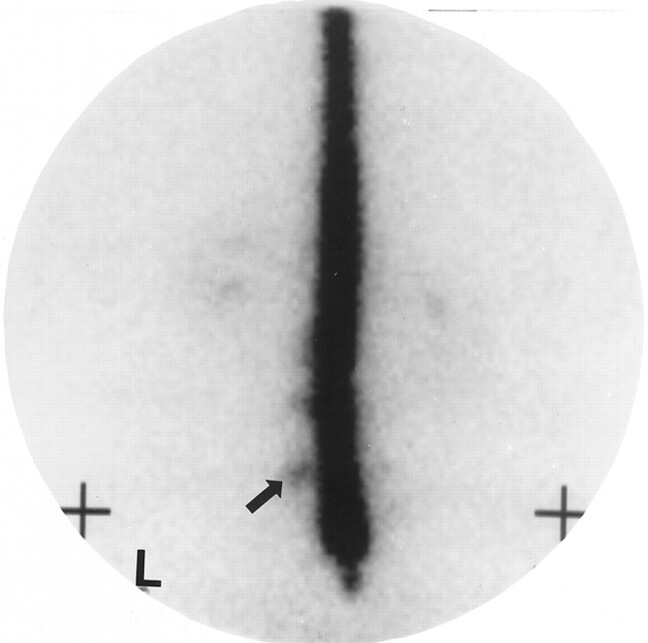
Fig 4.
Indium-111 DTPA cisternogram (anterior view) obtained approximately 4 hours after intrathecal injection shows extraarachnoid accumulation of the radionuclide (arrow) at the lumbar level. L indicates left.
Fig 5.
Axial gradient-echo T2-weighted (40/15/1, flip angle 5°) MR image at the C2-C3 level obtained 6 months after symptoms spontaneously resolved shows improvement in the epidural venous engorgement bilaterally (arrows).
Discussion
Intracranial hypotension is a result of low CSF volume caused by either spontaneous or postoperative leakage. The syndrome has been reported to occur after head trauma, a tear in a spinal nerve root sheath, perineural cyst, or spinal arachnoid diverticulum (1). Iatrogenic causes include lumbar puncture or overdraining ventricular or spinal shunts (1, 4). Spontaneous intracranial hypotension is thought to result from rupture of a spinal arachnoid membrane that allows CSF passage into the subdural or epidural space (3, 7, 8). It is typically not attributable to a major traumatic event or prior diagnostic or therapeutic intervention; however, intracranial hyppotension may be associated with a history of minor trauma such as sports activities or severe coughing (1, 3).
In all cases of intracranial hypotension reported in the literature, the patients presented with headaches (1). These headaches are typically generalized and pulsating, and often resolve with recumbency (1). Other clinical features may include nausea or emesis, diplopia, neck pain, disturbance in hearing, vertigo, photophobia, and visual deficits (1, 3). CSF pressures have reportedly been low, normal, or even high. CSF examination may be normal, or may reveal xanthocromia, lymphocytic pleocytosis, or increased protein possibly secondary to dural venous engorgement (1, 3).
With few exceptions, intracranial MR imaging of intracranial hypotension reveals diffuse pachymeningeal enhancement (1, 2, 7–9). Subdural fluid collections and brain descent, as measured by inferior displacement of the iter relative to the incisural line, may also be seen (1–3). Pachymeningeal enhancement is characteristically thick, smooth, and uninterrupted (1–3). The enhancement is thought to result from accumulation of gadolinium-based contrast material in engorged dural veins and in the interstitium of the dura (8). Mokri et al (1) reported subdural fluid collections that were mostly bilateral and without mass effect in 69% of patients with intracranial hypotension. The authors also described imaging evidence of brain or brain-stem descent in 62% of patients in their series. Ventricular size in subjects with intracranial hypotension is usually small (1), but can reverse after treatment of the CSF leakage.
In the relatively few reports in the literature of spinal imaging findings of intracranial hypotension, MR images showed extradural fluid collections and pachymeningeal enhancement (1, 4–6). MR images may also depict the site of CSF leakage in the spine (1). Herein, we described a case of intracranial hypotension with symmetric bulky epidural enhancement seen along the anterolateral borders of the cervical spine. This enhancement was compatible with engorged epidural venous structures based on their characteristic location in the anterolateral spinal canal and sparing of the midline (Fig 1). Renowden et al (6) described a single case of intracranial hypotension with epidural venous engorgement in the region of the cauda equina, and Rabin et al (4) described another case of intracranial hypotension with prominent flow voids within the ventral epidural space near the midline of the upper thoracic spine adjacent to a ventral extradural fluid collection.
Enlarged cervical epidural veins are often seen in conjunction with vascular lesions such as arteriovenous malformations, fistulas, and varicose veins. Cervical epidural venous engorgement may also occur when the internal jugular veins are compromised because of tumor or infection. In this situation, the epidural veins compensate for the jugular vein blood flow, and consequently, they become enlarged (10). Only two reports exist in the literature of engorgement of the cervical epidural venous plexus associated with intracranial hypotension (11, 12), and in both cases the patients were without radicular symptoms. Clarot et al (11) reported a case of intracranial hypotension with a positive Romberg sign, though no relationship to epidural venous engorgement was demonstrated, particularly given the anterior location of the epidural process, and there was no evidence of cord involvement. Another report in the literature described a patient presenting with severe neck pain on flexion 1 week after lumbar puncture (12). An MR examination showed what appeared to be a cervical anterior epidural mass. An epidural abscess was suspected, and the patient was treated with antibiotics. Her symptoms did not resolve, however, and at surgery the epidural mass was revealed to be enlargement of the epidural venous plexus. This patient also did not have radicular symptoms.
In a series of 26 subjects with intracranial hypotension, Mokri et al (1) reported two (8%) of the 26 had upper extremity pain with possible radicular features. Spinal MR imaging was reported specifically with respect to these two cases. These spinal MR studies showed only extraarachnoid fluid collections, meningeal enhancement, or no abnormality at all. Schabel et al (13) described a case of bilateral arm pain secondary to lumbar puncture. The authors hypothesized that intracranial hypotension was the cause for arm pain from central traction causing irritation of a cervical nerve. However, no intracranial or spinal imaging was performed in this case to confirm this diagnosis or exclude epidural venous engorgement.
Dickman et al (14) described a case of myelopathy developing from epidural varicose veins compressing the cord at the cervicothorocic level, which demonstrated the potential symptomatic effects of venous pressure on nervous structures. Caruso et al (10) described a patient with giant cervical epidural veins and secondary right-sided radicular pain after a craniotomy for head trauma. Although the authors take note of their patient’s enlarged cervical epidural veins and secondary radicular pain, they do not identify intracranial hypotension as the cause of radicular pain. In light of our case and that reported by Caruso et al, we hypothesize that upper extremity pain and possible radicular features of intracranial hypotension are the result of the compressive effects of enlarged venous structures. It is not clear, however, why only the right side was affected by radicular symptoms in both our patient and the patient described by Caruso et al (10).
Diffuse pachymeningeal enhancement is thought to reflect the Monro-Kellie rule (3, 8), which describes the inverse relationship of CSF volume and intracranial blood volume within the rigid confines of the skull. If the CSF volume decreases, the intracranial pressure is maintained by increased blood volume, particularly in the more capacious venous system. This reflex mechanism protects nervous tissue by maintaining a constant buffer (ie, blood or CSF) subjacent to its bony covering. Though this principle was described for intracranial processes and helps to explain the reason for intracranial pachymeningeal enhancement, it can also be applied to the bony spinal canal. Explaining pachymeningeal enhancement in the spine becomes more difficult because the hypervascular outer dural layer covering the brain does not extend to cover the spine, and the single layer of dura that does cover the spine is relatively avascular (15). Perhaps reports of pachymeningeal enhancement along the spinal canal in intracranial hypotension more accurately reflect prominent epidural venous engorgement.
Treatment of intracranial hypotension varies, depending on its origin and type. If intracranial hypotension is the result of a shunt procedure or surgery, then treatment is usually surgical. Spontaneous intracranial hypotension is often treated first with conservative management, and if this is not effective, an epidural blood patch is used. Sometimes, as was the case with our patient, symptoms can improve or resolve without treatment, which is most likely due to the spontaneous healing of the dural tear (1).
Epidural venous engorgement should be suspected in the setting of intracranial hypotension. Although the engorged venous plexus can resemble a mass, certain imaging features should be considered to avoid misdiagnosis. The epidural venous plexus is symmetrically located in the anterolateral aspect of the cervical canal, sparing the posterior and anteromedial aspects. The midline septum, if seen, remains intact, preventing extension across the midline. Venous structures may also be seen to expand and contract on serial images. Flow artifact may also be seen on MR images. Some pathologic entities that may have a similar appearance include lymphoma or granulomatous disease; however, these processes typically do not stay within the anatomic boundaries described above and do not spontaneously diminish in size. Epidural venous engorgement can also be seen in conjunction with a spinal vascular malformation or with expanded collateral venous pathways, and these causes should be excluded before the diagnosis of intracranial hypotension is made. Because of the infrequency of intracranial hypotension, we believe that epidural venous engorgement should be considered when it is accompanied by radicular symptoms.
References
- 1.Mokri B, Piepgras DG, Miller GM. Syndrome of orthostatic headaches and diffuse pachymeningeal gadolinium enhancement. Mayo Clin Proc 1997;72:400–413 [DOI] [PubMed] [Google Scholar]
- 2.Pannullo SC, Reich JB, Krol G, Deck MD, Posner JB. MRI changes in intracranial hypotension. Neurology 1993;43:919–926 [DOI] [PubMed] [Google Scholar]
- 3.Christoforidis GA, Mehta BA, Landi JL, Czarnecki EJ, Piaskowski RA. Spontaneous intracranial hypotension: report of four cases and review of the literature. Neuroradiology 1998;40:636–643 [DOI] [PubMed] [Google Scholar]
- 4.Rabin BM, Roychowdhury S, Meyer JR, Cohen BA, LaPat KD, Russell EJ. Spontaneous intracranial hypotension: spinal MR findings. AJNR Am J Neuroradiol 1998;19:1034–1039 [PMC free article] [PubMed] [Google Scholar]
- 5.Moayeri NN, Henson JW, Schaefer PW, Zervas NT. Spinal dural enhancement on magnetic resonance imaging associated with spontaneous intracranial hypotension: report of three cases and review of the literature. J Neurosurg 1998;88:912–918 [DOI] [PubMed] [Google Scholar]
- 6.Renowden SA, Gregory R, Hyman N, Hilton-Jones D. Spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry 1995;59:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokri B, Parisi JE, Scheithauer BW, Piepgras DG, Miller GM. Meningeal biopsy in intracranial hypotension: meningeal enhancement on MRI. Neurology 1995;45:1801–1807 [DOI] [PubMed] [Google Scholar]
- 8.Fishman RA, Dillon WP. Dural enhancement and cerebral displacement secondary to intracranial hypotension. Neurology 1993;43:609–611 [DOI] [PubMed] [Google Scholar]
- 9.Schievink WI, Tourje J. Intracranial hypotension without meningeal enhancement on magnetic resonance imaging: case report. J Neurosurg 2000;92:475–477 [DOI] [PubMed] [Google Scholar]
- 10.Caruso RD, Smith MV, Chang JK, Wasenko JJ, Rosenbaum AE. Giant cervical epidural veins after craniectomy for head trauma. AJNR Am J Neuroradiol 1998;19:903–906 [PMC free article] [PubMed] [Google Scholar]
- 11.Clarot F, Callonnec F, Douvrin F, et al. Giant cervical epidural veins after lumbar puncture in a case of intracranial hypotension. AJNR Am J Neuroradiol 2000. :21:787–789 [PMC free article] [PubMed] [Google Scholar]
- 12.Shinaver CN, Caldemeyer KS. Engorged anterior epidural venous plexus mimics an anterior epidural mass. J Neuroimaging 1998;8:242–244 [DOI] [PubMed] [Google Scholar]
- 13.Schabel JE, Wang ED, Glass PS. Arm pain as an unusual presentation of postdural puncture intracranial hypotension. Anesth Analg 2000;91:910–912 [DOI] [PubMed] [Google Scholar]
- 14.Dickman CA, Zabramski JM, Sonntag VK, Coons S. Myelopathy due to epidural varicose veins of the cervicothoracic junction: case report. J Neurosurg 1988;69:940–941 [DOI] [PubMed] [Google Scholar]
- 15.Meltzer CC, Fukui MB, Kanal E, Smirniotopoulos JG. MR imaging of the meninges, I: normal anatomic features and nonneoplastic disease. Radiology 1996;201:297–308 [DOI] [PubMed] [Google Scholar]