Abstract
Summary: CNS infection occurring after therapeutic angiography is rare. We present a case report of a brain abscess complicating endovascular coiling of an intracranial aneurysm. We recommend the use of prophylactic antibiotics, especially when performing therapeutic CNS angiography.
Endovascular coiling of intracranial aneurysms has been used increasingly during the past few years. It provides an alternative to surgical clipping, especially for patients who are poor surgical candidates. Although less invasive than craniotomy, there have been reports of complications, such as aneurysm rupture and cerebral infarction. CNS infection is a rare complication of intra-cranial endovascular procedures. A review of the literature revealed only two reported cases occurring after embolization of cerebral arteriovenous malformations (1, 2). We report the case of a patient who developed a peri-aneurysm abscess after undergoing an uneventful aneurysm coiling procedure.
Case Report
The patient was a right-handed 70-year-old woman who was referred to our institution with an incidentally diagnosed, unruptured large right posterior communicating artery aneurysm measuring 2.2 × 2.2 × 2.7 cm. This was initially seen on a CT scan of the brain that was obtained to evaluate a chronic frontotemporal headache even though there appeared to be no indication by history to suggest previous aneurysmal hemorrhage. The patient denied any other associated neurologic symptoms. Her history was significant for hypertension, diabetes mellitus, hypercholesteremia, and several pack-years’ history of cigarette smoking. An examination revealed no neurologic deficit. Because of the patient’s age and medical condition, surgical clipping of the aneurysm was not recommended. Instead, the patient was given the option of endovascular coiling of the aneurysm.
The aneurysm was coiled with 51 Guglielmi detachable coils of variable configurations and dimensions during a single session. The procedure lasted approximately 12 hours, and no prophylactic antibiotic was administered. Immediately after the procedure, the patient suffered a focal neurologic deficit that was likely ischemic in nature, expressed as a transient mild left-sided pronator drift that resolved after 24 hours. The patient was discharged 2 days after the procedure.
One month after discharge, the patient was rehospitalized for acute alteration in her sensorium. She had developed gradually worsening left-sided body and facial weakness and several episodes of falling during a 2-week period. During that time, she had also complained of occasional headaches, double vision, swallowing difficulties, and speech problems. An examination revealed no fever, but the patient was lethargic and aroused only with painful stimuli. She had mild left hemiparesis, withdrawing that side in response to pain only, and inconsistently followed commands. She also displayed a partial right cranial nerve III palsy with left facial weakness sparing the frontalis muscle (central). She had no nuchal rigidity. Peripheral WBC count was 14,100/mm3, and CSF studies showed the following: yellow and hazy appearance; RBC count, 155/mm3; WBC count, 445/mm3 (56% neutrophils, 32% lymphocytes); glucose, 49 mg/dL (serum glucose level, 248 mg/dL); and protein, 241 mg/dL. Findings from gram stains and cultures of the peripheral blood and CSF were negative, but a urinary tract infection was present. A CT scan of the brain, even though it had significant artifact from the coils, showed edema involving the right caudate and thalamus, causing some mass effect on the adjacent lateral ventricle. Further evaluation with MR imaging and MR angiography revealed prominent ring-enhancing areas adjacent to the aneurysm and extending into the right side of the pons, midbrain, and basal ganglia with surrounding edema (Fig 1). MR angiography showed that the aneurysm was nearly totally thrombosed. These imaging findings and the CSF results were thought to be consistent with an abscess. Because of the high risks of aspirating such an abscess (deep and hemorrhage potential), it was elected to treat this empirically without having cultures to confirm infection and help identify the bacterial species.
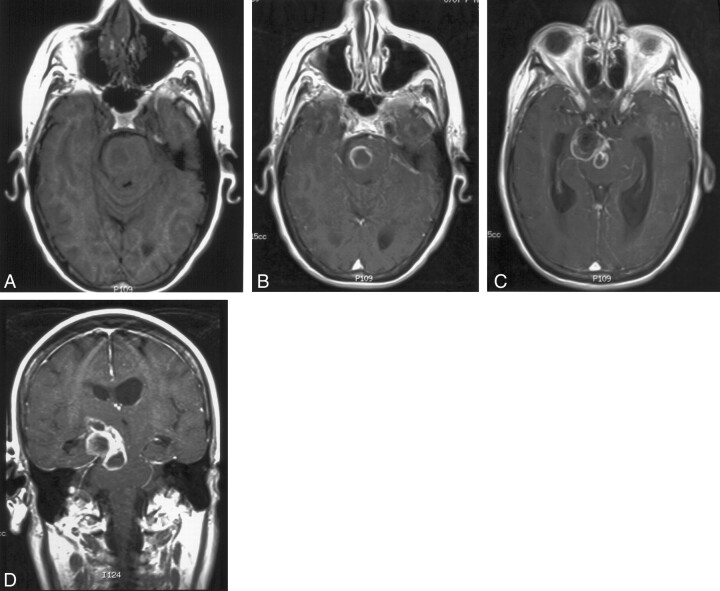
Fig 1.
MR images of the brain of a 70-year-old woman show a thrombosed aneurysm after endovascular coiling, with adjacent ring-enhancing lesion representing an abscess involving the brainstem and the right basal ganglia region.
A, Axial nonenhanced T1-weighted MR image (400/8/2 [TR/TE/NEX]), obtained through the pons, shows a focal low-intensity area.
B, Axial contrast-enhanced T1-weighted MR image (500/8/2), obtained through the pons, shows a ring-enhancing lesion.
C, Axial contrast-enhanced T1-weighted MR image (500/8/2), obtained through the midbrain, shows a ring-enhancing lesion adjacent to a thrombosed right posterior communicating artery aneurysm.
D, Coronal contrast-enhanced T1-weighted MR image (400/8/2), obtained through the brainstem, shows an oblong curved ring-enhancing lesion wrapping over a thrombosed right posterior communicating artery aneurysm.
The patient was treated with broad spectrum IV administered antibiotics (cefotaxime, 2 g every 6 hours; vancomycin, 1 g every 6 hours; metronidazole, 500 mg every 6 hours; and fluconazole, 400 mg daily) for 6 weeks. Follow-up MR imaging was performed approximately 2 weeks after completion of the antibiotic course. This revealed near total resolution of the ring-enhancing lesion and edema (Fig 2).
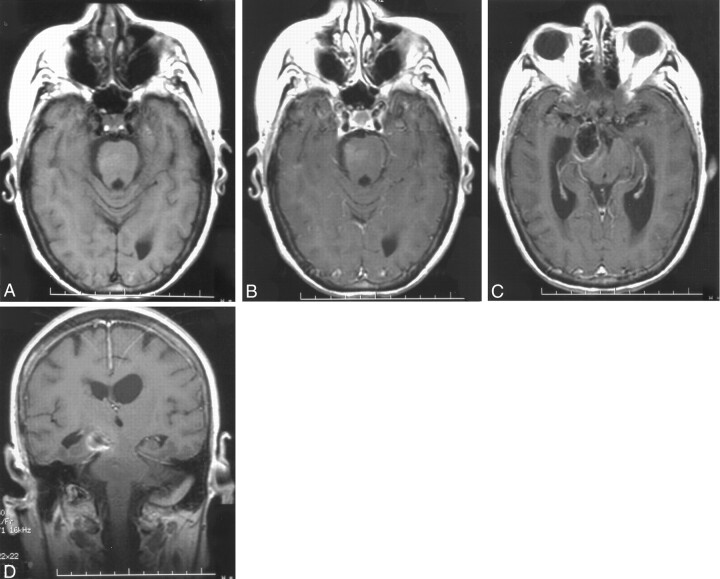
Fig 2.
MR images of the brain, obtained approximately 2 weeks after completion of the 6-week antibiotic course, show interval near total resolution of edema and enhancement.
A, Axial nonenhanced T1-weighted MR image (500/11/2), obtained through the pons, shows no focal low-intensity area.
B, Axial contrast-enhanced T1-weighted MR image (500/11/2), obtained through the pons, shows that the ring-enhancing lesion has resolved.
C, Axial contrast-enhanced T1-weighted MR image (500/11/2), obtained through the midbrain, shows resolution of the ring-enhancing lesion adjacent to a thrombosed right posterior communicating artery aneurysm.
D, Coronal contrast-enhanced T1-weighted MR image (450/11/2), obtained through the brainstem, shows resolution of the ring-enhancing lesion adjacent to a thrombosed right posterior communicating artery aneurysm.
Discussion
Bacteremia and even septicemia have been well documented to occur after diagnostic and therapeutic angiography (3, 4). One study reported a bacteremia occurrence rate of 32% after therapeutic angiography procedures lasting >2 hours (5). Pyogenic abscesses after therapeutic angiography have been reported to occur in the liver, spleen, lungs, retroperitoneal space, and perineum. Infective aortitis has also been reported (6–12). Two cases of CNS abscesses have been reported previously after endovascular treatment of arteriovenous malformations (1, 2).
The cause of infection and source of contamination are unclear. In our case, infection was likely caused by improper handling of the catheters; this has been reported to be the source in 83% of postoperative infections (13). The large number of coils used in our case certainly increased the chances of infection with repeated handling of the catheters. Another factor is the length of the procedure, as has been shown in surgical procedures. The presence of a urinary tract infection at presentation adds to the uncertainty of the source of infection; a source unrelated to the procedure is still possible.
In the normal brain, an optimally functioning blood-brain barrier provides resistance to infection (14). Cases of brain abscesses have been described after damage to this barrier, as in postischemic abscesses (15, 16). With a high incidence of bacteremia during therapeutic angiography, the risk of infection is heightened if the procedure causes any ischemia (14–16). This possibility exists in our case, in which the patient suffered a transient neurologic deficit. Whether the deficit resulted solely from the mass effect of having placed so many coils into the aneurysm or from a transient ischemic event is difficult to prove.
In this case, antibiotics were not used during the procedure, and this raises the interesting question regarding the routine use of antibiotic prophylaxis with all endovascular procedures. Perhaps this should be considered, especially if foreign materials are being placed and/or the procedures carry high risk for ischemic complications.
References
- 1.Mourier KL, Bellec C, Lot G, et al. Pyogenic parenchymatous and nidus infection after embolization of an arteriovenous malformation: an unusual complication: case report. Acta Neurochir (Wien) 1993;122:130–133 [DOI] [PubMed] [Google Scholar]
- 2.Zurin AA, Ushikoshi S, Houkin K, Kikuchi Y, Abe H, Saitoh H. Cerebral abscess as an unusual complication of coil embolization in a dural arteriovenous fistula: case report. J Neurosurg 1997;87:109–112 [DOI] [PubMed] [Google Scholar]
- 3.Shawker TH, Kluge RM, Ayella RJ. Bacteremia associated with angiography. JAMA 1974;229:1090–1092 [PubMed] [Google Scholar]
- 4.Meyer P, Reizine D, Merland JJ, Guerin JM. Nosocomial septicemia and therapeutic angiography: apropos of 4 cases [in French]. Ann Radiol (Paris) 1988;31:34–36 [PubMed] [Google Scholar]
- 5.Meyer P, Reizine D, Aymard A, et al. Septic complications in interventional radiography: evaluation of risk and preventive measures: preliminary studies. J Intervent Radiol 1988;3:73–75 [Google Scholar]
- 6.Chen C, Tsang YM, Hsueh PR, et al. Bacterial infectious association with hepatic arteriography and transarterial embolization for hepatocellular carcinoma: a prospective study. Clin Infect Dis 1999;29:161–166 [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Naddaf S, Abujudeh HH, Atay S, Abdel-Dayem HM. Ga-67 imaging of perisplenic abscess after splenic embolization. Clin Nucl Med 1998;23:394–395 [DOI] [PubMed] [Google Scholar]
- 8.Cubiella J, Sans M, Llovet JM, et al. Pulmonary abscess as a complication of transarterial embolization of multinodular hepatocellular carcinoma. Am J Gastroenterol 1997;92:1942–1943 [PubMed] [Google Scholar]
- 9.Koga F, Goto S, Suzuki S. Retroperitoneal abscess formation accompanied by intraabdominal free air: a rare complication of transcatheter arterial embolization of renal tumor: a case report [in Japanese]. Hinyokika Kiyo 1996;42:443–446 [PubMed] [Google Scholar]
- 10.Sandock DS, Seftel AD, Herbener TE, Goldstein I, Greenfield AJ. Perineal abscess after embolization for high-flow priapism. Urology 1996;48:308–311 [DOI] [PubMed] [Google Scholar]
- 11.Park S, Montoya A, Moreno N, Moran JF, Jacobs W, Pifarre R. Infective aortic endocarditis after percutanous balloon aortic valvuloplasty. Ann Thorac Surg 1993;56:1161–1162 [DOI] [PubMed] [Google Scholar]
- 12.Allison DJ, Jordan H, Hennessy O. Therapeutic embolistion of the hepatic artery: a review of 75 procedures. Lancet 1985;1:5–599 [DOI] [PubMed] [Google Scholar]
- 13.Stamm WE. Infections related to medical devices. Ann Intern Med 1978;89:764–769 [DOI] [PubMed] [Google Scholar]
- 14.Groff A. Experimental production of abscess of the brain in cats. Arch Neurol Neurosurg Psychiatry 1934;31:199–204 [Google Scholar]
- 15.Kurlan R, Griggs RC. Cyanotic congenital heart disease with suspected stroke: should all patients receive antibiotics? Arch Neurol 1983;40:209–212 [DOI] [PubMed] [Google Scholar]
- 16.Chen ST, Tang LM, Ro LS. Brain abscess as a complication of stroke. Stroke 1995;26:696–698 [DOI] [PubMed] [Google Scholar]