Abstract
BACKGROUND AND PURPOSE: Patients with cancer of the oral cavity often present with advanced tumor stages, distant metastasis, or severe comorbidities, which render radical surgery infeasible. The purpose of this study was to investigate the response rate, technical feasibility, and safety of intra-arterial (IA) chemotherapy as palliative treatment in this situation.
METHODS: From November 1997 to December 2003, 64 patients with histologically proven oral squamous cell carcinoma, classified as inoperable, received IA high-dose chemotherapy with cisplatin as a palliative treatment at our institution. To minimize toxic side effects, sodium thiosulfat was given intravenously.
Twenty-eight percent of the patients were female; average age was 61.5 years. Clinical staging of primary tumors was TNM (tumor, nodules, metastases) stage IV in 89%, stage III in 6.3% and stage II in 4.7%. After local chemotherapy, additional radiation of the tumor area or radiochemotherapy was performed in 33 patients.
RESULTS: There were no major catheter-related complications or severe side effects of IA chemotherapy. After the first cycle, 10% percent of the patients had complete remission (CR), 35% had partial response (PR), and 43.3% presented with stable disease.
Mean follow-up interval was 11 ± 12.9 months. Forty-five patients died after a mean period of 7.6 ± 7.0 months (median, 5.1 months). The overall 1- and 2-year survival rates were 29.5% and 18%, respectively. There was a trend toward longer survival in patients who received subsequent radiation or radiochemotherapy after IA chemotherapy.
CONCLUSION: IA chemotherapy in patients with inoperable carcinoma of the oral cavity as palliative treatment was technically feasible and safe. The overall response rate after IA chemotherapy was 45% (CR 10%; PR 35%). Side effects could be minimized by neutralizing the cytotoxic agent by sodium thiosulfat.
Intra-arterial (IA) high-dose chemotherapy with cisplatin is an established therapeutic option in the multitechnique treatment of oral cancer (1–4). It affords the possibility to administer a much higher concentration of cisplatin at the tumor site compared with systemic administration while minimizing systemic side effects by neutralizing the cytostatic agent with intravenous infusion of sodium thiosulfat (5,6). The rationale for neoadjuvant intra-arterial chemotherapy is to inhibit local tumor growth, potentially reduce local relapse rate, and minimize tumor cell spread during subsequent operation (7). The first results of this neoadjuvant treatment have been published elsewhere (8,9).
Although modern treatment of squamous cell carcinoma of the oral and oropharyngeal cavity increasingly involves more nonsurgical modalities, radical tumor resection remains the most effective curative therapy (10). At the time of first diagnosis, however, many patients present with advanced tumor stages, with distant metastasis or with severe medical comorbidities, which render radical surgery infeasible. In most of these cases, it has to be stated that there is no chance even for nonsurgical effective but straining organ-preserving treatment regimens such as systemic chemotherapy by using TPF (taxan, platinum-based agent, fluorouracil) in three cycles. These incurable patients may benefit from IA chemotherapy as palliative treatment because the reduction of local tumor mass may result in less tumor bleeding and infection, improve oropharyngeal functions, and provide pain relief.
This report presents the short- and long-term results of 64 patients with inoperable cancer of the oral cavity. All patients received IA high-dose chemotherapy with cisplatin. Additional radiation-therapy or concomitant radiochemotherapy could be performed in approximately half of the patients.
Methods
From November 1997 to December 2003, a total of 289 patients with histologically proven, previously untreated oral or oropharyngeal squamous cell carcinoma underwent IA chemotherapy with high-dose cisplatin at our institution, followed by subsequent radical surgery in 225 cases.
Sixty-four patients with inoperable disease were recruited for this study. These patients were ineligible for surgery mainly because of advanced local tumor growth, distant metastasis, or severe medical comorbidities (Table 1). Twenty-eight percent were female, and the average age was 61.5 ± 11.3 years (range, 39–86 years; median, 67.5 years).
TABLE 1:
Distribution of tumor stages and comorbidities of 64 patients with inoperable oral cancer
| n (%) | |
|---|---|
| Characteristics | |
| Locally advanced tumor | |
| Stage T3 | 8 (12.5) |
| Stage T4 | 47 (73.4) |
| Crossed midline | 27 (42.2) |
| Advanced lymph node involvement | |
| Stage N2 | 32 (50) |
| Stage N3 | 3 (4.7) |
| Distant metastasis | 3 (4.7) |
| Comorbidities | |
| Synchronous malignoma | 9 (14) |
| Reduced general conditions | 24 (37.5) |
| Cardiovascular/stroke | 6 (9.4) |
| Pulmonary | 2 (3.1) |
| Severe cachexia | 3 (4.7) |
| Korsakow | 3 (4.7) |
| Age > 80 years | 6 (9.4) |
In all patients, routine staging was performed by using clinical assessment, sonography, CT, and/or MR imaging to define local tumor growth and regional lymph node involvement. Positron-emission tomography was used for the diagnosis of secondary tumors, lymph node involvement, or metastases.
Eighty-four percent of the tumors were located in the oral cavity (floor of the mouth,n = 27; oral tongue, n = 14; gingiva,n = 6; buccal mucosa, n = 5; hard palate;n = 2), and 16% were in the oropharynx (base of the tongue,n = 7; tonsils,n = 2; soft palate,n = 1). Clinical staging of primary tumors by using the TNM system was stage IV in 89%, stage III in 6.3%, and stage II in 4.7%. In 42% of cases, the tumor had crossed the midline. Three patients with stage II tumors were included in the study even though their tumors were formally respectable. Two 81-year-old patients were ineligible for surgery because of severe cardiac failure and reduced general conditions. A third patient, a 75-year-old man of Turkish origin, refused both surgery and further adjuvant treatment and received one cycle of IA chemotherapy as palliation.
All patients received IA chemotherapy as palliative treatment. Patients were treated with more than one cycle of local chemotherapy in case of partial response (grade II) and/or when vascular tumor supply involved more than one vessel. This was mainly the case in very large tumors and when cancer had already crossed the midline.
The primary goal of IA chemotherapy was a reduction in tumor size. Therefore, the microcatheter was advanced as far distally as possible into the tumor-feeding artery. If this was not possible, chemotherapy was administered from a more proximal position. In this situation, regional lymph nodes could be affected from chemotherapy. This “side effect,” however, was not specifically intended or evaluated in clinical follow-up.
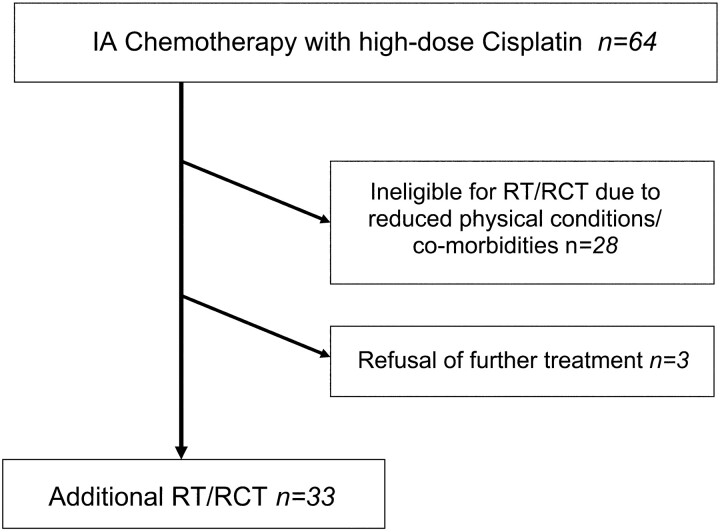
Additional radiation or radiochemotherapy of the tumor area and the regional lymph nodes over 5 weeks (51.3 Gy at 1.9 Gy per fraction) with systemic administration of docetaxel (25 mg/m2; Aventis Pharma, Antony Cedex, France) after intra-arterial chemotherapy was offered to patients when physical conditions were appropriate. This was the case in 33 patients. In 28 patients, supplementary treatment could not be performed because of severely reduced physical conditions or comorbidities. Three patients refused further therapy (Fig 1).
Fig 1.
Flowchart of the study design. Patients with inoperable cancer of the oral cavity received IA chemotherapy as palliative treatment. Additional regional radiotherapy (RT) or radiochemotherapy (RCT) was performed when physical conditions were appropriate.
The regional ethics committee approved the protocol. All patients signed informed consent before treatment.
IA catheterizations were performed via a transfemoral arterial approach by the Seldinger technique. A diagnostic catheter (4F or 5F) was introduced into the common carotid artery, and a diagnostic angiogram of the carotid bifurcation and the branches of the external carotid artery was performed by using a standard digital subtraction technique. The coaxial technique was used to place a 2.7F microcatheter (eg, Tracker-18, Target Therapeutics, San Jose, CA; Leger 18, Terumo, Leuwen, Belgium) into the appropriate tumor-feeding artery. The tip of the catheter was advanced distally as far as possible into the vessel to prevent unnecessary infusion into the mucosal or muscular branches (Fig 1). Before the start of IA chemotherapy, another test injection was performed to control proper positioning of the tip of the microcatheter and to ensure that the catheter was not occlusive.
High dose IA chemotherapy was administered with 150 mg/m2 cisplatin (Medac, Hamburg, Germany) dissolved in 500 mL saline solution 0.9% under controlled pressure over 5–15 minutes in combination with simultaneous intravenous sodium thiosulfat 9 g/m2 after a delay of 10 seconds for peripheral neutralization. For anesthesia, 0.1–0.3 mg of fentanyl were delivered intravenously or 5–15 mg mepivacain were injected intra-arterially into the perfused artery on demand.
Before IA chemotherapy, all patients were given 74 mg dolasetron intravenously and 75 mg prednisolone intravenously. Patients also received intravenous hydration pre- and post-IA chemotherapy with normal saline solution (0.9% NaCl with 20 mmol KCl). Thrombosis prophylaxis was achieved with subcutaneous heparin. Routine laboratory checks were made on alternate days. In-hospital stay was 4–6 days. Side effects of IA chemotherapy were noted.
Three weeks after the first cycle, treatment response was assessed in every patient clinically by inspection and palpation of the local tumor and by CT. The primary objective was the reduction of local tumor mass as classified according the World Health Organization (WHO) criteria:
(1) Complete remission—complete disappearance of the local tumor mass.
(2) Partial remission—>50% reduction of local tumor mass.
(3) Stable disease—<50% reduction of the local tumor mass.
(4) Progressive disease—continued growth of the tumor.
Overall response rate was calculated as the sum of the rates of complete and partial remissions. Stopping tumor growth (stable disease) was considered a positive treatment result in a palliative situation.
Clinical follow-up was performed each month in the first year and every second month in the following year(s). Survival rates were calculated by means of the Kaplan-Meier method and the χ2 test.
Results
A total of 117 IA catheterizations with local chemotherapy were performed on 64 patients. Thirty-five patients received more than one cycle of intra-arterial chemotherapy because of promising but incomplete local tumor response (grade II) or bilateral/multiple vessel blood supply of the tumor. Eighteen patients had two cycles, 16 patients had three cycles, and one patient had four cycles. The tumor-feeding artery was selectively catheterized in all cases. Two peri-interventional complications occurred: one arterial occlusion of the right leg (puncture side), which resolved completely after conservative treatment, and one transient ischemic attack. In one case, intra-arterial infusion had to be stopped prematurely because of acute alcohol toxic delirium.
A summary of side effects of the chemotherapy is shown in Table 2. Mild and transient local toxicity was relatively rare. Vomiting was treated effectively with repeated doses of dolasetron. Hypokaliemia was treated with oral potassium. One patient developed transient renal failure (creatinine maximally 4 mg/dL), which resolved with conservative treatment after 10 days. There were no severe side effects (WHO grades III and IV) from chemotherapy, except one patient with grade III anemia who had to be treated with one blood transfusion.
TABLE 2:
Local and systemic side effects according the WHO classification after IA chemotherapy with cisplatin
| Acute side effects (WHO) | Percentage (%) |
|---|---|
| No measurable side effects | 6 |
| Local | |
| Swelling, stridor | 12 |
| Systemic | |
| Nausea grade I | 9 |
| grade II | 6 |
| Anemia grade I | 3 |
| grade III | 3 |
| Hypokalemia grade I | 24 |
| Serum ferrum decreased grade I | 18 |
| Serum creatinine elevated grade I | 18 |
| Transaminases elevated grade I | 9 |
| Hyperglycemia grade I | 3 |
| Hyperuricemia grade I | 3 |
| Hyperuremia grade I | 15 |
Sixty-three patients were available for clinical follow-up approximately 3 weeks after the first cycle of intra-arterial chemotherapy. Six patients (10%) had complete response, 22 (35%) had partial response, and 28 (44%) had stable disease. Seven patients (11%) showed tumor progression (Fig 2). Therefore, the positive overall response rate was 45%, and local tumor growth could be inhibited in 89% of patients.
Fig 2.
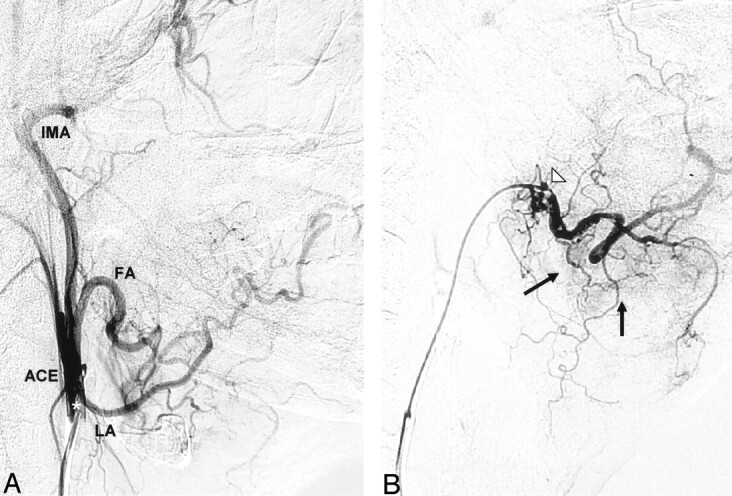
Superselective catheterization of a patient with advanced cancer of the gingiva of the lower mandible. A guiding catheter is introduced into the main stem of the external carotid artery (ACE) and a selective angiogram is performed (A). A coaxial microcatheter is then advanced into the facial artery (FA) via the guiding catheter. (B). Superselective angiography of the facial artery shows small tumor-feeding vessels originating from the main trunk and a submental branch of the FA (black arrows). LA indicates lingual artery; IMA, internal maxillary artery; white star, tip of the guiding catheter in the ACE stem; white triangle, tip of the microcatheter.
Thirty-three patients underwent radiation therapy. This decision nearly always depended on the fitness of the patients. Five patients had to truncate the radiation due to physical deterioration. Eleven patients were fit enough to start concomitant systemic chemotherapy. Three patients could receive all five planned cycles.
Follow-up data were available from 57 patients. Mean observation period was 11 ± 12.9 months (range, 0.5–54.2 months). Seven patients were lost to follow-up after a mean observation time of 5.7 ± 4.5 months.
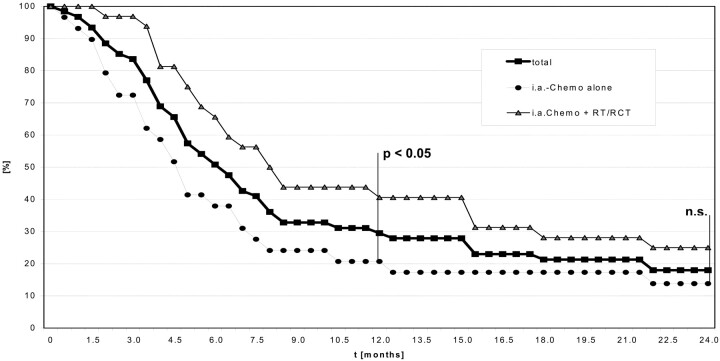
At the time of evaluation 12 patients (21%) were still alive. Mean survival in this subgroup was 25 ± 29.6 months (range, 1.9–54.2 months; median, 18.8 months). Forty-five patients (79%) died after a mean period of 7.6 ± 7.0 months (median, 5.1 months); death was directly tumor-related in 35 cases. The overall 1- and 2-year survival rates were 29.5% and 18%, respectively. There was a trend, however, toward longer survival in patients who received subsequent radiation or concomitant radiochemotherapy after IA chemotherapy than for patients with IA chemotherapy alone. Kaplan-Meier analysis revealed 1-year survival rates of 40.6% for patients with combined treatment and 20.7% after intra-arterial chemotherapy alone (P < .05) and 2-year survival of 25% versus 13.8%, respectively (n.s.,Fig 2).
Discussion
The central aims of palliative treatment for inoperable oral cancer are to improve local symptoms, achieve pain relief, and increase the duration of patient survival up to the point of not compromising quality of life.
Radiation therapy and systemic chemotherapy, alone or in combination, have been subject of several clinical trials concerning treatment of advanced head and neck cancer (12–15). Recently, Denis et al reported about improved overall 5-year-survival and locoregional control rates in stage III and IV oropharynx carcinoma after concomitant radiochemotherapy compared with radiation therapy alone (16). Both modalities, however, require sufficient patient cooperation because of repetitive treatment sessions and maintain severe toxicities (10,17). Therefore, radiation and systemic chemotherapy are not always feasible in patients with poor compliance or in extremely reduced physical conditions.
In contrast, local chemotherapy is capable of reducing and controlling tumor growth effectively whereas systemic side effects can be minimized by the neutralization of the cytotoxic agent. It is known that reduction in tumor volume—a primary effect of selective chemotherapy—has a major impact on functional improvement, pain relief, and mean survival (18,19). Therefore, local chemotherapy may be beneficial for this group of patients. Until now, there are only limited data concerning the feasibility and effectiveness of this technique in a true palliative situation.
Previous studies investigating IA chemotherapy in the treatment of advanced head and neck cancer reported overall response rates from 69%–95% and 5-year-survival rates as high as 39% (2,19–21).
Robbins et al reported about 213 patients with stage III and IV disease (20). Treatment included weekly IA infusion of cisplatin (150 mg/m2/week × 4) and conventional external-beam irradiation (180–200 cGy/fraction) to a total dose of 68–72 Gy before surgical treatment. Complete response at the tumor site was obtained in 80% of cases, and estimated 5-year survival was 38.8%.
Furutani et al retrospectively evaluated the results of a concurrent combination of selective IA (retrograde) chemotherapy and radiation therapy in 39 patients with locally advanced cancer of the tongue and tongue base (21). Thirty-eight percent and 12.8% of the patients were classified as having stage III and IV disease, respectively. In 37 of 39 patients in whom the combination therapy was completed, the response rate was 94.6%, and 3-year overall survival estimated by the Kaplan-Meier method was 58.9%.
Remission data of these studies was given after additional radiation. Therefore, the potential of IA chemotherapy itself could not be assessed precisely. Moreover, it must be stated that radiation doses of 60–70 Gy as administered in the studies discussed here cannot be considered as palliation but rather as attempts of curative treatment.
In contrast to the cohort presented here, patients with distant metastasis, secondary malignancies, or severe concomitant diseases were for the most part excluded from the trials discussed here. Therefore, the aims of those studies differed significantly from our objectives.
With regard to the advanced local tumor stages and the medical comorbidity of the cohort of the present study, the overall response rate of 45% (10% complete and 35% partial response) was excellent. In an earlier discussion of 17 patients treated with IA chemotherapy alone, it could be demonstrated by comparison with a treatment-dependent prognosis index that this extremely well-tolerated technique had the same survival potential as compared with more straining palliative modalities such as radiation or tumor-reducing surgery (7). The remission rates reported here are in line with our more subjective findings, that approximately three-fourths of the study population reported substantial improvement from local symptoms after IA chemotherapy (eg, pain relief and/or improvement in swallowing and speech). Future (clinical) studies, however, should investigate the impact on quality of life more deeply.
Our data demonstrate that the procedure is safe and technically feasible in most patients. Because of the development of new catheter products, coaxial microcatheter technique, and continuous flushing of the catheter system, intervention-related complications have been reduced to a minimum. In our experience, the rate of severe complications in >360 intra-arterial chemotherapies did not exceed the 1% complication rate of routine diagnostic cerebral angiographies. The procedure is therefore much less risky than other neuroradiologic interventions.
A disadvantage of IA chemotherapy may be the lack of effect on regional lymph node and distant metastases. As a result, overall survival time may not be influenced satisfactorily in patients who are treated with IA cisplatin alone. Therefore, additional concomitant systemic chemotherapy and radiation may be useful to extend survival. In this study, the subgroup that underwent a combined therapeutic regimen had a statistical benefit in terms of mean period of survival and mean 1- and 2-year survival rates compared with patients with selective chemotherapy alone (P < .05). Following radiation and radiochemotherapy, four patients were still alive after a mean of 2 years of follow-up. Initially, all had clinically suspicious neck lymph nodes. These patients (4/64 patients) managed to change their status from palliation to curation. It should, therefore, always be attempted to add radiation to IA chemotherapy for palliation. Nevertheless, as has been discussed above, this is not possible in a large portion of patients who are recruited for palliative therapy.
Conclusion
Intra-arterial chemotherapy as palliative treatment in patients with inoperable carcinoma of the oral cavity is technically feasible and safe. The overall response rate was 45% (10% complete remission and 35% partial remission). Severe side effects could be minimized by the neutralization of the cytotoxic agent by sodium thiosulfat. Additional concomitant systemic chemotherapy and radiation may be useful to extend survival.
Fig 3.
Kaplan-Meier analysis of survival rates (in %) up to 24 months after intraarterial chemotherapy with high-dose cisplatin (total number of patients = 61, including lost follow-up); after selective chemotherapy alone (n = 29) and after IA chemotherapy followed by radiation (RT) or radiation combined with systemic chemotherapy (RCT) (n = 32).
References
- 1.Kovács AF, Turowski B, Ghahremani MT, Loitz M. Intraarterial chemotherapy as neoadjuvant treatment of oral cancer. J Craniomaxillofac Surg 1999;27:302–307 [DOI] [PubMed] [Google Scholar]
- 2.Robbins KT.The evolving role of combined modality therapy in head and neck cancer. Arch Otolaryngol Head Neck Surg 2000;126:265–269 [DOI] [PubMed] [Google Scholar]
- 3.Hirai T, Korogi Y, Hamatake S, et al.Stages III and IV squamous cell carcinoma of the mouth: three-year experience with superselective intraarterial chemotherapy using cisplatin prior to definitive treatment. Cardiovasc Intervent Radiol 1999;22:201–205 [DOI] [PubMed] [Google Scholar]
- 4.Kovács AF, Schiemann M, Turowski B.Combined modality treatment of oral and oropharyngeal cancer including neoadjuvant intraarterial cisplatin and radical surgery followed by current radiation and chemotherapy with weekly docetaxel: three-year results of a pilot study. J Craniomaxillofac Surg 2002;30:112–120 [DOI] [PubMed] [Google Scholar]
- 5.Robbins KT, Storniolo AM, Kerber C, et al.Rapid superselective high-dose cisplatin infusion for advanced head and neck malignancies. Head Neck 1992;14:364–371 [DOI] [PubMed] [Google Scholar]
- 6.Robbins KT, Storniolo AM, Kerber C, et al.Phase I study of highly selective supradose cisplatin infusions for advances head and neck cancer. J Clin Oncol 1994;12:2113–2120 [DOI] [PubMed] [Google Scholar]
- 7.Kovács AF, Grüterich G, Wagner M.Long-term complete remission of oral cancer after anti-neoplastic chemotherapy as single treatment modality: role of local chemotherapy. J Chemotherapy 2002;14:95–101 [DOI] [PubMed] [Google Scholar]
- 8.Kovács AF.Intraarterial chemotherapy and chemoembolization in head and neck cancer: establishment as a neoadjuvant routine method. Cancer Ther 2003;1:1–9 [Google Scholar]
- 9.Kovács AF.Intraarterial induction high-dose chemotherapy with cisplatin for oral and oropharyngeal cancer: long-term results. Br J Cancer 2004;90:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulsen M, Aldren C, Tripcony L, Walker Q.Is surgery necessary in stage III and stage IV cancer of head and neck that responds to induction chemotherapy? Arch Otolaryngol Head Neck Surg 1996;122:467–471 [DOI] [PubMed] [Google Scholar]
- 11.Platz H, Fries R, Hudec M.Computer-aided individual prognoses of squamous cell carcinomas of the lips, oral cavity and oropharynx. Int J Oral Maxillofac Surg 1992;21:150–155 [DOI] [PubMed] [Google Scholar]
- 12.Al-Sarraf M, Pajak TF, Marcial VA, et al.Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck: an RTOG study. Cancer 1987;15:259.65. [DOI] [PubMed] [Google Scholar]
- 13.Merlano M, Corvo R, Margarino G, et al.Combined chemotherapy and radiation therapy in advanced inoperable squamous carcinoma of the head and neck: the final report of a randomized trial. Cancer 1991;67:915–921 [DOI] [PubMed] [Google Scholar]
- 14.Merlano M, Bessano M, Corvo R, et al.Five-year update of a randomized trial alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. J Natl Cancer Inst 1996;88:583–589 [DOI] [PubMed] [Google Scholar]
- 15.Munro AJ.An overview of randomized controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer 1995;71:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis F, Gauraud P, Bardet E, et al.Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radio-chemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004;22:69–76 [DOI] [PubMed] [Google Scholar]
- 17.Szepesi T, Stadler B, Hohenberg G, et al.Prognostic factors in the treatment of inoperable orofacial tumors with simultaneous radiotherapy and intra-arterial chemotherapy. Strahlentherapie 1985;161:299–307 [PubMed] [Google Scholar]
- 18.Pinto HA, Jacobs C.Chemotherapy for recurrent and metastatic head and neck cancer. Hematol Oncol Clin North Am 1991;5:667–686 [PubMed] [Google Scholar]
- 19.Imai S, Kajihara Y, Munemori O, et al.Superselective cisplatin (CDDP)-carboplatin (CBDCA) combined infusion for head and neck cancers. Eur J Radiol 1995;21:94–99 [DOI] [PubMed] [Google Scholar]
- 20.Robbins KT, Kumar P, Wong FS, et al.Targeted chemoradiation for advanced head and neck cancer: analysis of 213 patients. Head Neck 2000;22:687–693 [DOI] [PubMed] [Google Scholar]
- 21.Furutani K, Fuwa N, Kodaira T, et al.Continuous selective intraarterial chemotherapy in combination with irradiation for locally advanced cancer of the tongue and tongue base. Oral Oncol 2002;38:145–152 [DOI] [PubMed] [Google Scholar]