Abstract
BACKGROUND AND PURPOSE: The long-term durability of Guglielmi detachable coil (GDC) embolization of cerebral aneurysms is still unknown. The purpose of this study was to evaluate the stability of anatomic occlusion of aneurysms treated with GDCs and assess the rate of recanalization and re-treatment.
METHODS: A multicenter study involving 650 patients with 705 ruptured aneurysms treated with GDCs between January 1998 to May 2003 was conducted. During this period, 63% of ruptured aneurysms were treated by the endovascular technique. The morbidity and mortality associated with this technique, procedural feasibility, acute angiographic occlusion results, and long-term angiographic follow-up were assessed.
RESULTS: Overall technical feasibility of GDC treatment was 96.9%. Upon admission, 25% of patients were Hunt and Hess grade IV or V. Acute angiographic results in 683 aneurysms demonstrated total occlusion in 496 cases (72.6%), subtotal occlusion in 171 cases (25.%), and incomplete occlusion in 16 cases (2.4%). All patients were controlled by angiography and MR imaging at 3 months, 1 year, and subsequent yearly examinations post-treatment. A second treatment was performed in 27 cases (recanalization, 4.7%). Long-term follow-up angiograms (mean, 36 months) were obtained in 571 aneurysms (95%). Of them, 422 aneurysms (73.9%) demonstrated complete occlusion, 148 aneurysms (25.9%) demonstrated subtotal occlusion, and only 1 aneurysm was incompletely occluded. Overall mortality was 11.4% for all patients, with procedural mortality evaluated at 1.4%. Overall morbidity was calculated at 8.6%. Only one rebleeding occurred in our study, with a second procedure performed without vital consequences for the patient.
CONCLUSION: Our multicenter study confirms the stability of aneurysm embolization with GDC, with only 4.7% of aneurysms requiring re-treatment.
Since 1991, endovascular treatment (EVT) of cerebral aneurysms has experienced a revolution, with the introduction of platinum coil technology (1–2). During the past 10 years, significant study of the feasibility of this technique has been performed, and clinical results of EVT have been published by endovascular specialists worldwide (2–8). Most recently, the results of the International Subarachnoid Hemorrhage Trial (ISAT) demonstrated the clinical superiority of endovascular treatment to standard surgical management of ruptured intracranial aneurysms (9–10). The main question of this technique is the stability of the occlusion with detachable coils and the efficacy in providing protection against growth or regrowth of the aneurysm and consequent bleeding, in ruptured aneurysms. The only remaining downside is the recanalization of aneurysms due to coil compaction, mostly because of a large neck or the size of the lesion. The aim of this study was to better assess the long-term durability of endovascular coiling of cerebral aneurysms with Guglielmi detachable coils (GDCs) and the real rate of second treatment for ruptured aneurysms alone.
Methods
A consecutive series was analyzed retrospectively: from January 1998 to May 2003, 705 intracranial ruptured aneurysms in 650 patients were considered for endovascular treatment at five neuroradiologic centers. During this same period, 37% of all ruptured aneurysms were surgically clipped (total aneurysms collected, 1,119). Endovascular treatment was performed by five physicians, each with a minimum of 5 years of experience performing EVT with GDC at beginning of study. Endovascular management for all cases treated as part of this study series were similar in technique approach and follow-up. Treatment protocol, device used, and follow-up were determined by consensus before the start of study. All aneurysms were embolized with GDC 10 or GDC 18 platinum coils (Boston Scientific/Neurovascular, Fremont, CA). For large aneurysms (>15 mm), GDC 18 coils were used as the first coils to obtain optimal packing. Angiographic data were collected prospectively in all cases by a specific physician independent of the five others practitioners, by using written reports of procedures. This physician only reported and collected data, without judgment or evaluation of occlusion. For follow-up, all written reports were sent to the collecting physician. Occlusions were evaluated by treating physicians by consensus, during repeated meetings.
Patients and Clinical Data.
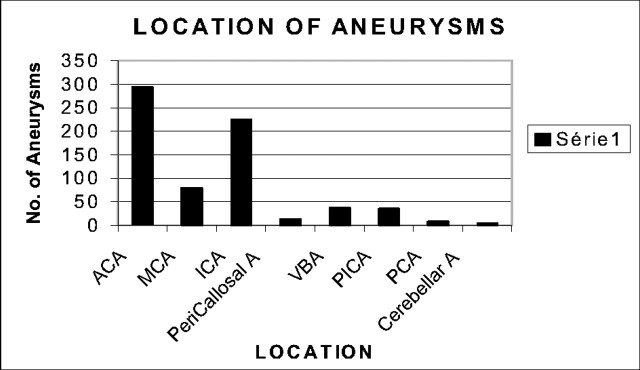
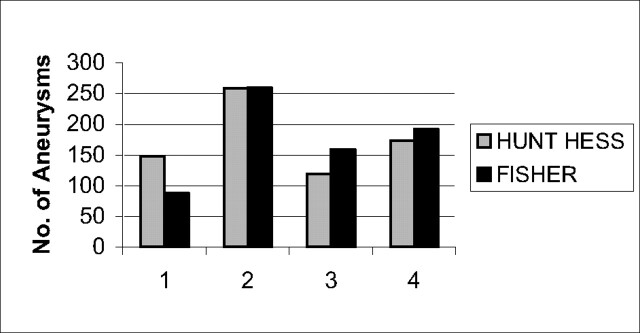
Patients ranged in age from 15 months to 84 years, with a mean age of 57 years. Women represented 57% of the study population. Upon admission, a cranial CT scan was performed on all patients, who were then examined by a neurosurgeon and neuroanesthetist. In cases presenting with hydrocephalus, external CSF drainage was initiated before endovascular treatment. A consensus between the referring neurosurgeon and the neurointerventionalist to treat the patient via an endovascular approach was reached in all cases. Clinical presentation, symptoms, aneurysm location, size and shape of the aneurysm sac, and patient age were considered. Locations of treated aneurysms are detailed in Fig 1. Aneurysms located in the anterior circulation represented 88% of all aneurysms treated. The severity of subarachnoid hemorrhage (SAH) was clinically assessed at the time of admission by using the Hunt and Hess grading scale (11). Upon admission, 21% of patients were admitted with clinical grade I. Patients presenting with Fisher grade III or IV represented 60% of our series population. Seventeen percent of patients presented with grade II, 35% with grade III, and 25% with grade IV. We made no distinction between grade IV and V patients at admission, because an apparent grade V could become a grade IV after hematoma evacuation or external CSF.
Fig 1.
Location of ruptured aneurysms. ACA: Anterior Communicant Artery; MCA: Middle Cerebral Artery; ICA: Internal Carotid Artery; Pericallosal A: Pericallosal Artery; VBA: Vertebro Basilar Artery; PICA: Posterior Inferior Cerebellar Artery; PCA: Posterior Communicating Artery; Cerebellar A: Cerebellar Artery.
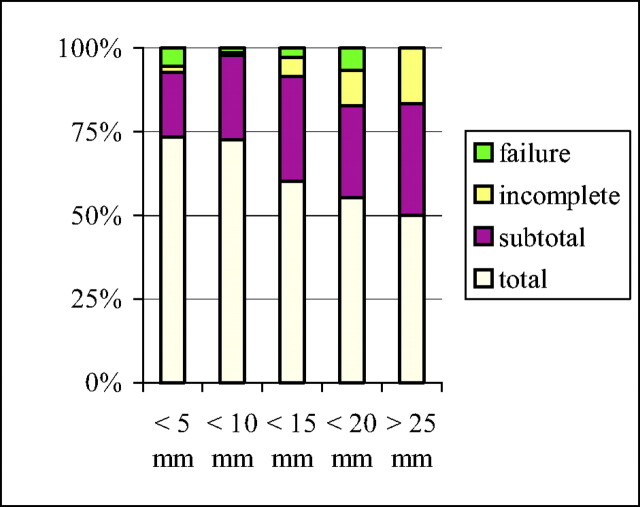
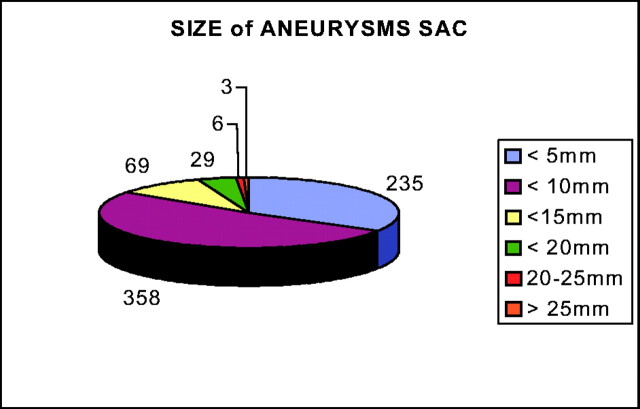
All aneurysm dimension measurements were made on diagnostic angiograms by using an internal reference by the treating physician. The known size of the internal carotid or basilar artery was compared with the largest size of the aneurysmal sac. Small aneurysms (measuring <5 mm) represented 33% of all cases. Aneurysms measuring 5–9 mm represented 52.3% of all cases, 9.4% of aneurysms measured 10–14 mm, and 4% of aneurysms measured 15–19 mm. Aneurysms <10 mm represented 85% of all aneurysms in this series. A total of nine aneurysms were defined as giant. Dome-to-neck ratios were not calculated for all aneurysms, but the balloon remodeling technique was used in the treatment of 43 aneurysms (6%).
Procedure.
Diagnostic angiograms were performed by using local anesthesia when clinical conditions were good (Hunt and Hess grades I and II). Once the best endovascular treatment recommendation was determined, patients received general anesthesia. A femoral puncture was used as first choice for all patients except two, in whom a direct carotid puncture was used because of vessel tortuosity. A 6F introducer was placed in the femoral artery with the Seldinger technique and bilaterally when the remodeling technique was used. Heparin was administered as an intravenous bolus to achieve an activated clotting time (ACT) approximately twice that of normal (perfusion of heparin 20 IU per kilogram of body weight/h). Prevention of proximal vasospasm was done by perfusion of papaverine during the procedure (through a guiding catheter into the carotid or vertebral artery). A 6F guiding catheter was selectively placed in the artery supplying the aneurysm. Once the optimal angiographic projection was defined, aneurysm catheterization was performed by advancing a microcatheter over a microguidewire. Coils were placed through the microcatheter into the aneurysm and detached under fluoroscopy. This process was repeated until the aneurysm had been embolized as well as possible. In cases in which the balloon remodeling technique (12–18) was used, a nondetachable balloon was placed at the aneurysm neck. In all cases, coil size, coil type, and total number of coils used were recorded. Procedural complications were also recorded. All patients were held postprocedurally in the neurosurgical intensive care unit for 3–6 days. Poor clinical grades were observed in the intensive care unit until stabilization of hemodynamic parameters. Anticoagulation was routinely continued for the first 48 hours postprocedure.
Follow-Up.
In consensus, treating physicians decided that each patient would be scheduled for follow-up digital subtraction arteriography (DSA) and MR imaging at 3 months, 12 months, and subsequent annual examinations. Multiple projections with selective injections served to define any residual lesions. At each center, the treating physician was responsible for this follow-up and review of each patient after DSA and MR imaging. When DSA and MR angiography (MRA) demonstrated concordance, patients received annual MRA control and DSA every 2 years posttreatment. In the case of discordance of results, or in patients with a potential risk of coil compaction, additional follow-up angiography was performed. Mean follow-up was 36 months (range, 6 months to 5 years). Angiograms were evaluated by the same physicians in consensus. In the event of modification of compaction, all control imaging was reviewed by all physicians in consensus. We decided to re-treat all aneurysms with increase in the size of remnant and opacification of neck.
Aneurysm occlusion was defined as follows in all cases: 100%, or complete, occlusion when the aneurysmal sac and neck were densely packed; 95%–99%, or subtotal, occlusion was defined as having a neck remnant; and incomplete occlusion was defined as having loose packing and partial opacification (5, 7, 8).
Statistical Analysis.
The qualitative and quantitative variables tested included patient baseline characteristics, aneurysm variables, and therapeutic and posttherapeutic factors. Patients were characterized by age, sex, initial clinical condition (Hunt and Hess scales and Fisher grades), aneurysm morphology (size and location), and number of aneurysms. Treatment feasibility was assessed and included coil characteristics such as total number of coils used, coil size, use of a nondetachable balloon, technical complications, and immediate posttreatment aneurysm occlusion. The statistical significance between occlusion and all parameters were analyzed by χ2 test, with a P value of 0.05 considered to indicate a statistically significant difference. When a test was calculated as statistically significant, we had calculated a coefficient “Phi” between 0 and 1, to determine the puissance from the association of two parameters. A high value indicates statistically significant relationship between the two parameters analyzed.
Results
Feasibility and Efficacy of Treatment
In 16 aneurysms, treatment failed because of tortuosity of cerebral vessels. No coils could be safely placed in any of these aneurysms. In six cases, the aneurysm was catheterized, but coil deployment failed because of coil instability inside the aneurysmal sac, which indicates a high risk of migration. These patients were subsequently referred for surgical clipping. Procedural feasibility of occlusion with GDC was 96.9% in our study (683 ruptured aneurysms were occluded by GDC).
Immediate Posttreatment Results
At the end of the initial procedure, occlusion was classified as complete, or total, occlusion in 496 cases (72.6%), subtotal in 171 cases (25%), and incomplete in 16 cases (2.4%). A second procedure was performed in 33 aneurysms because of an initial incomplete occlusion (n = 6) or secondary coil compaction (n = 27).
Evolution of 496 Aneurysms Showing Total Occlusion.
At the time this report was prepared, 359 completely occluded aneurysms remained stable, with no modification of the immediate posttreatment result at the final angiographic follow-up examination (Table 1). A small recurrence was observed in 52 cases of the follow-up time, between 3 months and 2 years after initial treatment. These patients were scheduled for follow-up by DSA and MRA. Coil compaction with remnant was observed in 14 cases. These patients also received re-treatment via endovascular treatment. Forty-seven patients died, and 24 patients were lost to follow-up.
TABLE 1:
Evolution of occlusion before and after second procedures
| Initial/Final Occlusion | Total | Subtotal | Incomplete | Lost to Follow-up | Dead |
|---|---|---|---|---|---|
| Total n = 496 | 359 | 52 | 14 | 24 | 47 |
| Subtotal n = 171 | 50 | 72 | 13 | 9 | 27 |
| Incomplete n = 16 | 5 | 6 | 1 | 4 | |
| Total n = 683 | 409 | 129 | 33 | 34 | 78 |
| After second treatment | 13 | 20 | 1 | ||
| Total n = 683 | 422 | 148 | 1 | 34 | 78 |
Note.—First column indicates initial occlusion; first line indicates evolution of occlusion.
Evolution of 171 Aneurysms with Subtotal Occlusion.
In 72 cases, no modification of occlusion from the initial treatment was noted. In 50 cases, there was a spontaneous thrombosis of the sac of the aneurysm resulting in a total occlusion. Nine patients were lost to follow-up, and 27 died. For 13 aneurysms with recanalization, a second procedure was successfully performed.
Evolution of 16 Patients with Incomplete Initial Occlusion.
In the 16 initial incomplete occlusions, 6 cases received a second procedure to ameliorate coil packing in the sac, 5 aneurysms had spontaneous amelioration of packing due to thrombosis, 4 patients died, and 1 patient was lost to follow-up. One patient experienced rebleeding 6 months after initial treatment, and a second treatment was successfully performed.
Recurrence with a Second Treatment.
We made a distinction between small recurrences (small changes in packing) with no blood flow into the aneurysmal sac and recanalization with flow into the aneurysm (Table 4). Recanalization may be a consequence of coil compaction, because of high arterial blood flow, aneurysm growth due to arterial disease, or coil migration in the thrombus surrounding the aneurysm. A simple change of packing does not automatically induce re-treatment. Despite an initial total occlusion, recanalizations inducing second procedures were observed in 14 aneurysms totally occluded and in 13 aneurysms with subtotal initial occlusion. In our series, a second treatment was performed in 33 (4.7%) cases (27 recanalizations and 6 initial incompletely occluded aneurysms) between 3 and 48 months after initial treatment (mean, 9.5 months). The overall rate of recanalization in this series was calculated at 4.7%. Of these, total occlusion was achieved in 13 cases, subtotal occlusion in 19 cases, and failure in 1 case.
Stability and Evolution of Occlusion
We obtained follow-up for 571 aneurysms. The evolution and stability of occlusion are presented in Table 4. Improvement at follow-up compared with immediate posttreatment angiographic results is defined as improvement of one outcome category. A worse angiographic follow-up result was defined as worsening of one category (from total to subtotal). Stability is defined as an unchanged occlusion at the longest follow-up obtained after initial treatment. Stability is evaluated from this series at 75.4%. Spontaneous amelioration was 9.6%, and the rate of recurrences (including small recurrences and recanalization) is 14.8%. Thirty-four patients (5% of cases) were lost to follow-up, and 11.4% of cases resulted in death (because of the severity of the initial hemorrhage).
For final outcomes, therefore, with the inclusion of the 33 cases requiring a second treatment, we obtained complete/total occlusion in 422 cases (74%), subtotal occlusion in 148 aneurysms (25%), and 1 incomplete occlusion.
Factors Influencing Initial Occlusion.
We correlated results of the initial occlusion which included patient age, Fisher scale, Hunt and Hess scale, and aneurysm location and size (Fig 4). The only parameter linked to occlusion was aneurysm size (χ2 test with P < .05). For berry aneurysms measuring <10 mm, a total or complete occlusion was obtained in 74% of aneurysms. For large aneurysms measuring >15 mm, complete occlusion was achieved in only 50% of those treated.
Fig 4.
Relation between size of aneurysms and % of initial occlusion.
Remodeling Technique
Wide-necked aneurysms with a maximal sac diameter–to–neck size ratio (SNR) of 1 are still difficult to embolize because of the risk of coil migration and/or coil protrusion into the parent artery. Such aneurysms also pose a risk for surgical clipping (13, 14). In 1994, Moret et al (12–15) described a new technique for treatment of these aneurysms. In our study, balloon remodeling was utilized in 43 aneurysms (6%). Thirty-three (76%) of 43 aneurysms measuring <10 mm and only 5.5% of small aneurysms were treated with nondetachable balloons. For medium, large, and giant aneurysms, this technique was used in 7.5%, 10%, and 22% of cases, respectively. The use of balloons did not result in significantly higher complication rate.
Complications Due to the Technique
The most frequent complications experienced in our study were thromboembolic events. Thirty-six cases (5.2%) experienced ischemic complications at the time of treatment with transient (n = 20) or permanent (n = 16) neurologic deficit. Six patients (0.8%) experienced ischemic cerebral lesions with evidence of clot with infarction resulting in death. When clots were visualized angiographically, mechanical recanalization was attempted with a microcatheter and microguidewire. Medical treatment of this complication consisted of arterial pressure elevation, intra-arterial vasodilatation, and fibrinolyse.
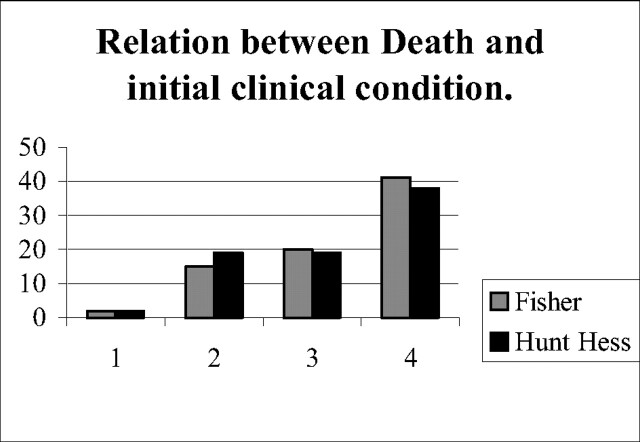
Aneurysm rupture during embolization occurred in 25 aneurysms (3.6%), which resulted in extravasation of contrast agent and clinical complications such as hematoma, elevation of blood pressure, and intracranial hypertension or hematoma that led to the deaths of four patients. Aneurysmal rupture resulted from insertion of the first coil in 22 cases (3.2%). Treatment of this complication was to continue further coil deposition into the aneurysmal sac. Total device-related events (1.6%) consisted of coil rupture for five cases, four cases of stretching of the coil, and two premature coil detachments. Procedural mortality was evaluated at 1.4%, and morbidity was evaluated at 8.9% for all procedures. Overall mortality was 11.4%, with vasospasm as the first etiology. Fig 5 indicates mortality of the initial pretreatment clinical grades.
Fig 5.
| Dead | Alive | % | |
|---|---|---|---|
| Fisher 1 | 2 | 89 | 2.4 |
| 2 | 15 | 263 | 5.7 |
| 3 | 20 | 157 | 12.7 |
| 4 | 41 | 196 | 20.9 |
| Hunt and Hess 1 | 2 | 149 | 1.3 |
| 2 | 19 | 261 | 7.3 |
| 3 | 19 | 119 | 16.0 |
| 4 | 38 | 176 | 21.6 |
Rebleeding.
Early rebleeding occurred in one case after embolization, without vital consequences for the patient. This patient had a symptomatic anterior communicating artery aneurysm with an initial subtotal occlusion. Six months after initial treatment, the patient presented with a SAH that necessitated a second treatment to achieve more complete coil packing. This patient had a good recovery.
Discussion
Since the ISAT preliminary report, which represents the first prospective, randomized, controlled trial, comparing the safety and efficacy of aneurysm clipping with coiling, it has been well demonstrated that the risk of death or dependence at 1 year post-treatment is significantly reduced with endovascular coil embolization compared neurosurgical aneurysm clipping (9, 10). Results of this trial demonstrated a 7.6% absolute risk reduction of death or dependency at 1 year after endovascular treatment compared with neurosurgical treatment (23.5% for coiling vs 31.1% for neurosurgery). Morbidity and mortality rates for endovascular coil embolization are less than for surgical morbidity and mortality rates (10).
Since 1997, endovascular coil treatment of ruptured aneurysms has been the first treatment choice at our respective centers for aneurysms from the anterior circulation or from basilar tip, even before the results of ISAT were published. The five endovascular practitioners have selected small aneurysms (85% <10 mm), 88% of which are from the anterior circulation for the EVT. These results are representative of a selected population of aneurysms. Some aneurysms were not accessible by EVT during this study and were clipped by neurosurgeons. In our center, 63% of ruptured aneurysms were embolized.
The evolution and long-term follow-up results of coiled aneurysms must be recognized now that feasibility of endovascular coiling has been established. Since 1991, published studies have focused on technical feasibility, angiographic results, and clinical outcomes from single-center experiences (3–8, 17–20). In most of these published reports, all aneurysms were included, (ie, ruptured and/or unruptured aneurysms). Treatment strategies for ruptured aneurysms are different from those for unruptured aneurysms. The technical procedure is made more difficult by existing factors such as vasospasm, hematoma, intracranial hypertension, and fragility of the aneurysm dome. We have prospectively collected all cases treated by EVT: one study involves the treatment of ruptured aneurysms and the other the treatment of unruptured aneurysms. We have made an autoevaluation of our results, like in other published series (3–8, 19, 20, 24, 25, 28–31, 42–45). Ideally, we should have an external observer to confirm our results, because interpretation of angiographic results involves subjective impressions.
The aim of endovascular treatment is the prevention of rebleeding. Risk of rebleeding is significant 24 hours after the initial hemorrhage and at 7 days after initial hemorrhage, with a rebleeding mortality rate of 74% (21–23). The cumulative rate of rebleeding at 14 days is 27.7%, as reported by Juvela (22). In our institution, we typically treat an aneurysm within 24 hours to 4 days after the initial SAH, with a mean interval of 48 hours. Initial poor clinical conditions (Hunt and Hess grade IV or V) are not factored in terms of initiating treatment as soon as possible after the aneurysm’s initial rupture. In our study, feasibility of endovascular treatment is high, and we have experienced only 22 failures (3.1%). Some of these cases were subsequently treated surgically, and others experienced clinical conditions too poor to support brain surgery. This good rate of feasibility can be explained by the initial selection of aneurysms proposed to EVT (small aneurysms from the anterior circulation) and by the technical experience of all physicians: outcomes of endovascular coil embolization improve with the experience of its practitioners (24). In all of our participating centers, a neurosurgical consultation is provided in every case before EVT treatment is proposed to determine the best treatment option for every patient. Typically, basilar tip, vertebral artery, communicating artery, and internal carotid artery aneurysms are first considered for endovascular therapy. For middle cerebral artery, the technique used depends on the shape and morphology of the aneurysm: aneurysms with a large neck are more often referred for surgical treatment. Treatment of such indications, however, may improve with 3D angiography reconstruction and advances in endovascular technology, such as the introduction of coils with complex three-dimensional structures and liquid polymer techniques and the development of techniques using balloons and intravascular stents (8–10, 24–25).
The occlusion rate of aneurysms after endovascular treatment is very difficult to assess. This subjective evaluation includes the attenuation of coil packing and degree of possible neck remnant. Comparisons are difficult from one institution to another and from one published report to another, when definitions of complete occlusion and attenuated packing vary subjectively. Some studies indicate a percentage of occlusion, whereas other studies describe occlusion in terms of complete or total, subtotal, or incomplete. Complete or 100% occlusion indicates that there is no residual contrast material filling of the aneurysm. The difficulty lies in determining the difference between subtotal, >90%, or >95% occlusion. We decided to use the classification used by Cognard and colleagues (5, 6, 8) to determine rate of occlusion (100% for total; 95% for subtotal; and <95% for incomplete). In a recent publication, Raymond et al (25) have used another classification to define residual lesions (residual neck, residual aneurysm, minor, or major recurrence).
We used the maximum number of coils possible to treat each aneurysm, to prevent recanalization and rebleeding. Histologic studies have demonstrated that coil compaction is observed to be higher when the initial attenuation of the coil mass is <20%. Furthermore, an initial coil packing attenuation >20% protects the aneurysm from recanalization (26, 27). A study involving the evaluation of coil stability of aneurysms after GDC embolization has demonstrated that the embolized volume of unchanged aneurysms was 30.8% ± 10.2%, compared 19.9% ± 10.6% for recanalized aneurysms. There is a significant correlation between embolized volume and stability of embolized aneurysms (27). Attenuated coil packing, however, may also pose problems. We experienced some thromboembolic complications with occasional occlusion of the parent artery. We report 5.2% of thromboembolic complications (36 patients) resulting in a neurologic deficit for 16 patients and death in 6 patients. There were 25 aneurysmal ruptures with no clinical consequence for 21 patients, and 4 hematomas with intracranial hypertension. All complications are calculated at 10.5%. In other published reports (25–38), authors have demonstrated the same rate of global complications, about 10%; thromboembolic events are the most frequent (19–28). For Friedman et al (29), an increased risk of complications were seen with aneurysms originating from the posterior circulation. In our series, no relation between complications and location or size of aneurysms was significant. Furthermore, the remodeling technique does not increase the complication rate (17).
Mortality
Procedural mortality is reported at 1.4% in our series. Procedural mortality includes all complications related to EVT (ie, dome perforation, coil rupture, ischemic occlusion, and stretching coil). For ruptured aneurysms, it is important to distinguish between evolution of SAHs and device-related complications. In patients reporting with an initial poor clinical condition, death can occur due to vasospasm or initial hematoma. The real rates of morbidity and mortality of this technique will be known about results of unruptured aneurysms. Overall mortality was evaluated at 11.4%, because of poor initial clinical conditions, evidence of vasospasm, and complications due to EVT. More than half (52.5%) of patients who died were admitted with Fisher grade IV and 48.7% with Hunt and Hess grade IV. Like Bracard et al (31), who reported results involving severe hemorrhage, with a global mortality rate of 29%, mortality was correlated with the Hunt and Hess scale in our study: 20% of grade IV aneurysms have poor clinical outcome with death. Improvements in survival rates have been achieved, especially for patients with grade IV and V aneurysms. This improvement is less evident in the surgical series (32–33).
Comparison of Series
Numerous studies involving follow-up angiographic data have been published (Table 2). These studies have included a variety of aneurysm types, various locations, ruptured and/or unruptured aneurysms, and small or giant aneurysms. For comparison, we have summarized follow-up results of a large number of coiled aneurysms. In our series, initial results were complete or total in 72.6%, subtotal in 25%, and incomplete in 2.4% of cases. At final follow-up, 95% of all patients initially treated were reviewed. Seventy-four percent of patients demonstrated complete occlusion, 25% a subtotal, and 1% an incomplete occlusion. These results demonstrated a higher percentage of total occlusion at final follow-up due to the re-treatment of 27 (4.7%) aneurysms that were secondarily incomplete. The stability of occlusion is more important to consider than the rate of initial or final occlusion. We report 75.4% occlusion stability. Ninety-six percent of aneurysms observed to be completely occluded at 12 months remained stable at final follow-up (mean follow-up at 36 months, minimum, 3 months; maximum, 72 months). For Raymond et al (25), the detection of recurrences is correlated with the length of the angiographic follow-up period: 46.9% of all recurrences have been detected by 6 months, and 96.9% by 36 months. Because the long-term evolution of “coil therapy” has not been documented, we continue to keep on controlling all patients treated in the same manner as Raymond et al. To have good follow-up, we propose noninvasive imaging studies. In this study, no patient with normal control angiograms at 2 years has demonstrated recanalization at 3 years, although neck remnant growth was observed in 14.8% of aneurysms during the first year. On follow-up, improvement was observed in 9.6% of aneurysms. These results, as compared with others reported series (Table 7: Cognard [8], 14%; Gruber et al, 35%; Vanninen et al, 8%; Thornton, 18%; Raymond et al, 33.6% recurrences of treated aneurysms, with 20.7% major recurrences, and 0.8% of rebleeding; Murayama et al, 26.1%–17.2%) confirm the message that denser packing minimizes coil compaction and rebleeding.
TABLE 2:
Follow-up results of a large number of published series
| Series | No. of A | Type of A | Initial Outcome (%) |
Progression (%) | Stable (%) | Remnant (%) | Recurrence (%) | Further tt gdc/S | Final Occlusion (%) |
Mean Follow-up | Rebleeding (%) |
Complications (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% | >95% | <95% | 100% | >95% | <95% | <6 Months | >6 Months | ||||||||||
| Casaco et al, 1993 | 71 | All | 83 | 17 | 73 | 27 | 5 | 3 | 83 | 12 | 5 | 13 | 2 | 0 | |||
| McDougall et al, 1996 | 33 | Basilar | 26.30 | 47.40 | 26.30 | 35.70 | 3 | 21 | 63 | 15 | 11.7 | 0 | 0 | ||||
| Raymond and Roy, 1997 | 70 | All | 43 | 40 | 17 | 12 | 8/3 | 46 | 42 | 12 | 31 | 5 | |||||
| Vinuela et al, 1997 | 403 | All | 70.8 | 21.4 | 4.2 | 33 | 9.18 | ||||||||||
| 31.2 | 41.6 | 10.4 | |||||||||||||||
| 35 | 57.1 | 5 | |||||||||||||||
| Eskridge and Song, 1998 | 150 | Basilar | 75 | 25 | 79 | 21 | 13.7 ruptured, 9.8 unruptured | 2 | 2 | ||||||||
| Kuether et al, 1998 | 77 | All | 41 | 50 | 9 | 26 | 67 | 7 | 37 | 14/1 | 44 | 46 | 13 | 17 | 2 | 0 | |
| Lefkowitz et al, 1999 | 23 | Wide-neck | 82 | 18 | 100 | 2/0 | 82 | 18 | 10 | 0 | 0 | ||||||
| Murayama et al, 1999 | 120 | 66 | 28 | 6 | 36 | 32 | 32 | 0 | 4/2 | 76 | 9 | 15 | 16.3 | 0 | 1 | ||
| Solander et al, 1999 | 79 | All | 68 | 32 | 40 | 45 | 15 | 2 | 3/2 | 79 | 21 | 10.7 | 0 | 0 | 13 | ||
| Bavinski et al, 1999 | 45 | Basilar | 40 | 43 | 17 | 44 | 56 | 17 | 8 | 33 | 50 | 17 | 6–72 | 1 | 0 | 4.40 | |
| Byrne et al, 1999 | 317 | Ruptured | 64 | 34 | 2 | 85.10 | 86 | 15 | 14.50 | 11/2 | 64 | 34 | 2 | 6 | 1 | 4 | |
| Cognard et al, 1999 | 203 | Small | 88 | 11 | 2 | 5 | 62 | 33 | 14 | 18 | 79 | 18 | 4 | 20.2 | 1 | 0 | 11 |
| Gruber et al, 1999 | 31 | Giant | 68 | 13 | 19 | 3 | 16 | 56 | 35 | 11/1 | 74 | 17 | 13 | 24.3 | 2 | 0 | 13.30 |
| Vanninen et al, 1999 | 52 | All | 55 | 38 | 6 | 10.20 | 8 | 3/8 | 67 | 28 | 5 | 3 | 1 | 0 | 2 | ||
| Thornton et al, 2002 | 196 | All | 39 | 46 | 15 | 46 | 26 | 28 | 18 | 3/10 | 61 | 22 | 17 | 16.6 | 1 | 0 | |
| Ng et al, 2002 | 160 | All | 46 | 16 | 38 | 44 | 28 | 9 | 12/4 | 6 months to 2 years | 0 | 2 | 6.9 | ||||
| Bracard et al, 2002 | 80 | Grade IV, V | 30 | 53.75 | 12.50 | 9 | 51 | 39 | 10 | 6 months | 1 | 0 | 6 | ||||
| Friedman et al, 2003 | 83 | Ruptured | 33 | 24 | 39 | 29 | 56 | 15 | 28/3 | 35 | 26 | 35 | 19.1 | 0 | 0 | 19 | |
| Vallée et al, 2003 | 55 | Basilar | 77 | 9 | 14 | 21 | 18 | 10 | 59 | 9 | 32 | 2 years | 0 | 0 | 11 | ||
| Murayama et al, 2003 | 916 | All | 55 | 35.4 | 3.5 | 46,4.8 | 20.9 | 3 months to 11 years | 0 | 12 | 8.4 | ||||||
| Henkes et al, 2004 | 1811 | All | 65.8 | 20.7 (90–99) | 5.5 (<90) | ||||||||||||
Note.—Authors and years of published series are reported in first column. A indicates aneurysms; t, treatment.
Appreciating recanalization is very subjective and depends on the physician’s experience. To develop more objective criteria to determine which patients needed second procedures, patients were reviewed collaboratively by physicians during repeated meetings. The most important criteria used were the change of packing and increase in the size of remnant. The rate of re-treatment was reported at 4.7% for all cases. Because of evidence of recanalization, 27 aneurysms were treated twice at a mean of 9.4 months. Fifty percent of recanalizations were re-treated at 6.5 months, and 73% of them were re-treated the 1st year. Factors supporting the low rate of recanalization include the technical expertise of the practitioners and the selection of aneurysms treated. Eighty-five percent of aneurysms treated were small aneurysms, which may be easier to pack than giant aneurysms. Our practice of attenuated packing has not resulted in higher complications than that reported in others studies.
We experienced only one case of rebleeding (0.2%) and 27 neck remnants. Rebleeding was observed to be the result of incomplete or loose initial packing. This patient suffered a SAH but was rapidly re-treated without clinical consequence. In terms of rebleeding or coil compaction, incomplete occlusion is the result of inadequate embolization of the neck or the base of the aneurysm, but exclusion of the aneurysm dome seems to be the key in preventing short-term rebleeding (16, 29, 45).
For follow-up of these aneurysms, some authors have compared MR angiography with DSA (25, 46–50). Typically, follow-up examinations include both modalities, and MRA may replace DSA in the long-term follow-up of coiled cerebral aneurysms, when these examinations are concordant. We propose MRA with gadolinium and DSA examination between 3 and 6 months after treatment and again 1 year after. When occlusion is considered complete and remains stable, we propose follow-up by MRA each year and by DSA every 2 years. We perform angiography at different times in cases with residual filling of the neck of the aneurysm or when there is a mismatch between MRA and DSA. Mean follow-up of our series is 36 months, and we have controlled some patients for 5 years after the initial SAH. We have lost few patients to follow-up, because all of them live in nearby towns and rarely change addresses. Each treating physician was responsible for follow-up and performance of MRA and DSA. In each case, the physicians insisted that patients be evaluated clinically at 6–12 months and yearly. In this series, we have achieved good midterm follow-up for 571 aneurysms and have lost only 34 patients (5%) to follow-up. Elsewhere, Murayama et al (45) reported 11 years of experience with GDC, with an overall recanalization rate of 20.9%, but they have obtained follow-up in only 53.4% of patients. Raymond et al obtained a mean follow-up at 31.3 months for a total of 76.5% patients (25).
Factors Influencing Recanalization
We have evaluated our results with statistical study to determine the relationship between size, location, age, clinical, and radiologic status with occlusion. The only parameter influencing the initial rate of occlusion was the size of the aneurysmal sac. No parameters were linked with this rate of recanalization. For small aneurysms, total or complete occlusion was obtained in 75% of cases, for large aneurysms this rate of occlusion was obtained in only 50% of cases. For Murayama et al (45), overall recanalization was linked to size of the aneurysm: 5.1% for small aneurysms, 20% for small aneurysms with a wide neck, and 35.3% for large aneurysms. In their experience, most recanalization occurred within 3 months of treatment.
Conclusion
Endovascular treatment of ruptured cerebral aneurysms is associated with low morbidity and seems to facilitate good outcomes in patients with an aneurysmal SAH. The feasibility of this technique is good and should improve with new technology and greater experience of endovascular practitioners. With good selection of aneurysms to be treated, total initial packing is a condition for the long-term stability of occlusion. Intensive follow-up has permitted early re-treatment for 27 aneurysms at a mean of 9.4 months. In our population, 85.2% of aneurysms were stable or demonstrated progressive occlusion with time. Only 14.8% of aneurysms demonstrated a worsening of the initial occlusion, of which 4.7% had a second procedure. Follow-up is essential for all aneurysms, and MR imaging and DSA are proposed as the control technique for each year after treatment. The real rates of morbidity and mortality will be known from unruptured series.
Fig 2.
Fisher and Hunt and Hess grades at admission.
Fig 3.
Sizes of aneurysms’ sac are indicated in millimeters. Numbers of aneurysms are reported.
Acknowledgments
Particular thanks are expressed to Signe Haughton, for her editorial assistance, and Jean Michel Simon, who contributed to the data base and statistical analysis. We wish to thank the members of Groupes des Neuroradiologues Interventionnels du Centre Ouest, for the editorial collaboration and manuscript review.
References
- 1.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. II. Preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;77:515–524 [DOI] [PubMed] [Google Scholar]
- 3.Zubillaga A, Guglielmi G, Vinuela F, Duckwiler G. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatments results. AJNR Am J Neuroradiol 1994;15:815–820 [PMC free article] [PubMed] [Google Scholar]
- 4.Massoud T, Guglielmi G, Vinuela F, Duckwiler G. Endovascular treatment of multiple aneurysms involving the posterior intracranial circulation. AJNR Am J Neuroradiol 1996;17:549–554 [PMC free article] [PubMed] [Google Scholar]
- 5.Cognard C, Pierot L, Boulin A, et al. Intracranial aneurysms: endovascular treatment with mechanical detachable spirals in 60 aneurysms. Radiology 1997;202:783–792 [DOI] [PubMed] [Google Scholar]
- 6.Pierot L, Boulin A, Castaing L, Rey A, Moret J. Selective occlusion of the basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery 1996;38:948–954 [DOI] [PubMed] [Google Scholar]
- 7.Cognard C, Weill A, Castaings L, et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510 [DOI] [PubMed] [Google Scholar]
- 8.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–56 [DOI] [PubMed] [Google Scholar]
- 9.International Subarachnoid Aneurysm Trial. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 10.Sellar Robin. International Subarachnoid Aneurysm Trial (ISAT): preliminary report. Neurointerventionist 2002;3:82 [Google Scholar]
- 11.Hunt WE, Hess RMC, Schramm J. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 12.Moret J, Pierot L, Boulin A, Castaings L. “Remodeling” of the arterial wall of the parent vessel in the endovascular treatment of intracranial aneurysms (presented at the 20th Congress of the European Society of Neuroradiology). Neuroradiology 1994;36:83 [Google Scholar]
- 13.Moret J, Cognard C, Weill A, et al. The remodeling technique in the treatment of wide neck intracranial aneurysms. Interv Neuroradiol 1997;3:21–35 [DOI] [PubMed] [Google Scholar]
- 14.McDougall CG, Halbach VV, Dowd CF, et al. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393–399 [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon assisted Guglielmi detachable coiling of wide-necked aneurysms. Part II. Clinical results. Neurosurgery 1999;45:531–538 [DOI] [PubMed] [Google Scholar]
- 16.Mericle RA, Wakhloo AK, Rodriguez R. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms: technical note. Neurosurgery 1997;41:975–978 [DOI] [PubMed] [Google Scholar]
- 17.Cottier JP, Pasco A, Gallas S, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol 2001;22:345–351 [PMC free article] [PubMed] [Google Scholar]
- 18.Mangiafico S, Cellerini M, 00000 0, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of cerebral aneurysms: a single center retrospective study. Intervent Neuroradiol 2002;8:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber A, Killer M, Bavinski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 20.Hallacq P, Piotin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of P2 segment aneurysms: retrospective review of a 10-year series. AJNR Am J Neuroradiol 2002. :1128–1136 [PMC free article] [PubMed]
- 21.Jane JA, Winn HR, Richarrdson AE. The natural history of intracranial aneurysms: rebleeding rates during the acute and long-term period and implications for surgical management. Clin Neurosurg 1977;24:176–184 [DOI] [PubMed] [Google Scholar]
- 22.Juvela S. Rebleeding from ruptured intracranial aneurysms. Surg Neurol 1989;32:323–326 [DOI] [PubMed] [Google Scholar]
- 23.Jomin M, Lesoin F, Lozes G. Prognosis with 500 ruptured and operated intracranial aneurysms. Surg Neurol 1984;l21:13–18 [DOI] [PubMed] [Google Scholar]
- 24.Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176–183 [DOI] [PubMed] [Google Scholar]
- 25.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–1403 [DOI] [PubMed] [Google Scholar]
- 26.Kawanabe Y, Sadato A, Taki W, Hashimoto N. Endovascular occlusion of intracranial aneurysms with GDC: correlation between coil packing density and coil compaction. Acta Neurochir 2001;143:451–455 (abstract). [DOI] [PubMed] [Google Scholar]
- 27.Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol 2002;23:762–767 [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton J, Debrun G, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002;50:239–250 [DOI] [PubMed] [Google Scholar]
- 29.Friedman JA, Douglas AN, Meyer FB. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol 2003;24:526–533 [PMC free article] [PubMed] [Google Scholar]
- 30.Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke 2002;33:210–217 [DOI] [PubMed] [Google Scholar]
- 31.Bracard S, Lebedinsky A, Anxionnat R, et al. Endovascular treatment of Hunt and Hess grade IV and V aneurysms. AJNR Am J Neuroradiol 2002;23:953–957 [PMC free article] [PubMed] [Google Scholar]
- 32.Duke BJ, Kindt GW, Breeze RE. Outcome after urgent surgery for grade IV subarachnoid hemorrhage. Surg Neurol 1981;54:146–150 [DOI] [PubMed] [Google Scholar]
- 33.Gumprecht H, Winkler R, Gerstner W, Lumenta CB. Therapeutic management of grade IV aneurysm patients. Surg Neurol 1997;47:54–59 [DOI] [PubMed] [Google Scholar]
- 34.Casasco AE, Aymard A, Gobin YP, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg 1993;79:3–10 [DOI] [PubMed] [Google Scholar]
- 35.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997;41:1235–1246 [DOI] [PubMed] [Google Scholar]
- 36.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 37.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the food and drug administration multicenter clinical trial. J Neurosurg 1998;89:81–86 [DOI] [PubMed] [Google Scholar]
- 38.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with GDC: a single center experience. Neurosurgery 1998;43:1016–1025 [DOI] [PubMed] [Google Scholar]
- 39.Murayama Y, Vinuela F, Duckwiler GR, et al. Embolization of incidental cerebral aneurysms by using the GDC system. J Neurosurg 1999;90:207–214 [DOI] [PubMed] [Google Scholar]
- 40.Solander S, Ulhoa A, Vinuela F, et al. Endovascular treatment of multiple intracranial aneurysms by using GDC. AJNR Am J Neuroradiol 1999;90:857–864 [DOI] [PubMed] [Google Scholar]
- 41.Bavinski G, Talazoglu V, Killer M. Gross and microscopic histopathological findings in aneurysms of the human brain treated with GDC. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 42.Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–663 [DOI] [PubMed] [Google Scholar]
- 43.Vanninen R, Koivisto T, Saari T, et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils: a prospective randomized study. Radiology 1999;211:325–336 [DOI] [PubMed] [Google Scholar]
- 44.Vallée JN, Aymard A, Vicaut E, et al. Endovascular treatment of basilar tip aneurysms with GDC: predictors of immediate and long-term results with multivariate analysis: 6-year experience. Radiology 2003;226:867–879 [DOI] [PubMed] [Google Scholar]
- 45.Murayama Y, Nien LY, Duckwiller G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years experience. J Neurosurg 2003;98:959–966 [DOI] [PubMed] [Google Scholar]
- 46.Mericle RA, Wakhloo AK, Rodriguez R. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms: technical note. Neurosurgery 1997;41:975–978 [DOI] [PubMed] [Google Scholar]
- 47.Nome T, Bakke SJ, Nakstad PH. MR angiography in the follow-up of coiled cerebral aneurysms after treatment with GDC. Acta Radiol 2002;43:10–14 (abstract) [DOI] [PubMed] [Google Scholar]
- 48.Boulin A, Pierot L. Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital substraction angiography. Radiology 2001;219:108–113 [DOI] [PubMed] [Google Scholar]
- 49.Brunereau L, Cottier JP, Sonier CB, et al. Prospective evaluation of time-of-flight MR angiography in the follow-up of intracranial saccular aneurysms treated with Guglielmi detachable coils. J Comput Assist Tomogr 1999;23:216–223 [DOI] [PubMed] [Google Scholar]
- 50.Cottier JP, Bleuzen-Couthon A, Gallas S, et al. Follow-up of intracranial aneurysms treated with detachable coils: comparison of plain radiographs, 3D time-of-flight MRA and digital subtraction angiography. Neuroradiology 2003;45:818–824 [DOI] [PubMed] [Google Scholar]
- 51.Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 2004;54:268–80 [DOI] [PubMed] [Google Scholar]