Abstract
Summary: We report the CT findings in a patient with a lateral neck mass histologically shown to be a schwannoma but having certain radiographic features commonly considered pathognomonic for a type III second branchial cleft cyst. Our case, therefore, represents an exception to this long-established rule.
Branchial cleft cysts (BCCs) are both the most common cysts to arise in the neck and the most common congenital masses of the lateral neck (1–6). Other common benign cystic lateral neck masses that can mimic BCCs include thyroglossal duct cysts, ectopic thymic cysts, lymphangiomas, dermoid and epidermoid cysts, and cystic nerve sheath tumors (3, 4). Abscesses and necrotic adenopathy also can be difficult to distinguish from a BCC, particularly if it has previously been infected (3–5). Both BCCs and their benign cystic mimics are typically soft, slow growing, and painless, making clinical distinction difficult (3–6). Cross-sectional imaging has become the mainstay of diagnosis for these lesions (3, 4). This is a case report of a cystic schwannoma demonstrating the classic location and CT signs of a type III second BCC.
Case Report
Clinical History and Physical Examination Findings
A 44-year-old man presented to his family physician with a several-year history of a slowly enlarging, painless mass in the lateral right neck. The measured attenuation postcontrast was 35 HU. Evaluation by an otolaryngologist revealed a nontender, 3–4-cm, palpable, slightly mobile, level II–region mass. The remainder of the examination was unremarkable.
Radiographic Findings
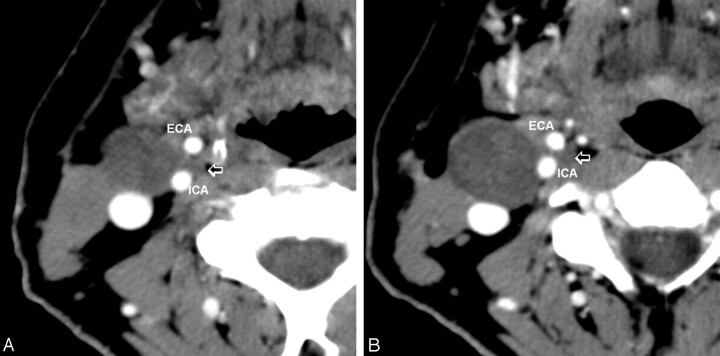
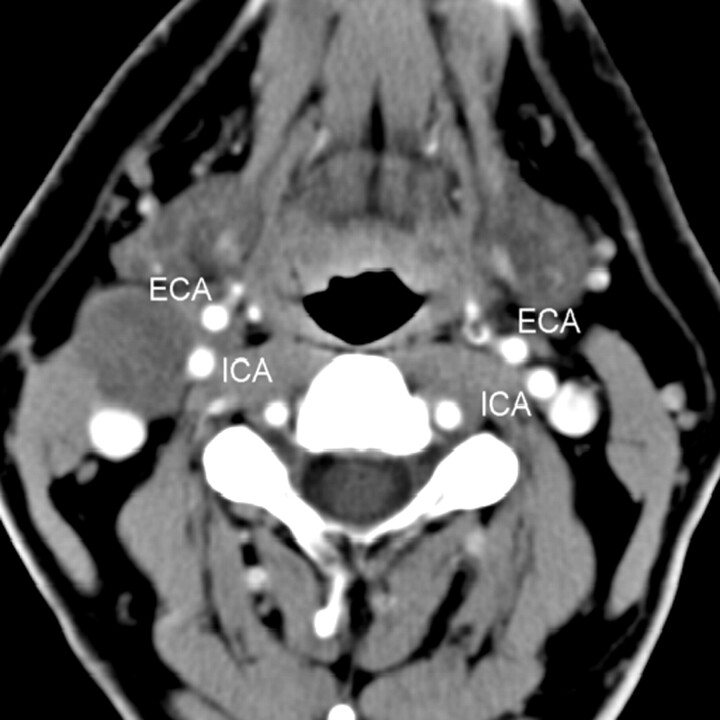
A contrast CT scan of the neck revealed a well-defined low-attenuation lesion anterior to the right sternocleidomastoid muscle without contrast enhancement. The lesion demonstrated a small “tail” extending between the internal carotid artery and the external carotid artery just above the carotid bifurcation (Figs 1 and 2). Mild posterior displacement of the internal jugular vein was also noted. A radiographic diagnosis of type III second BCC was made.
Fig 1.
Contiguous postcontrast CT images of the neck, showing the cystic tumor (small arrow) extending between the internal (ICA) and external (ECA) carotid arteries.
Fig 2.
Four types of second branchial cleft cyst. The cysts progressively move medially along a path between the anterior margin of the sternocleidomastoid muscle and the tonsillar fossa.
Histologic Findings
Histologic examination of the tumor was consistent with a cystic schwannoma. Antoni B areas were noted, which consisted of hypocellular tissue separated by edematous stroma. Verocay bodies and S-100 protein were present. No mitoses or areas of necrosis were identified. The cytology was bland.
Discussion
Second BCCs are by far the most common of the brachial cleft apparatus anomalies, representing about 95% of cases (1–4). Bailey and colleagues originally classified four types of BCC (3, 7). Type I is just deep to platysma muscle and the anterior lateral surface of sternocleidomastoid muscle. The type II BCC, more medial than type I, is seen along the anterior surface of the sternocleidomastoid muscle, just lateral to carotid space and posterior to the submandibular glands, arising on a line between the skin of the lateral neck and the ipsilateral faucial tonsil. Type III BCCs extend farther medially between the internal carotid artery and external carotid artery at the carotid bifurcation. Visualization of the cyst’s extension or “tail” between the internal carotid artery and external carotid artery has been considered pathognomonic for type III BCCs (2, 3, 8). Type IV is the least common and arises in the pharyngeal mucosal space just deep to the palatine tonsil, often extending upward from tonsil toward the skull base (8).
Schwannomas of the neck are, by contrast, uncommon tumors (9–11). They can arise from any peripheral, cranial, or autonomic nerve. As with BCCs, they commonly present clinically as insidious, slow-growing, painless masses, characteristics that often lead to an incorrect diagnosis (11). Most of these tumors are benign, but, if symptomatic, they may require surgical excision to preserve nerve function (10, 11). Diagnosis usually requires a combination of radiographic assessment and surgical pathologic correlation. The classic imaging appearance for a schwannoma of the neck is a large, sharply demarcated, fusiform enhancing mass in the region of the jugular foramen or parapharyngeal space (10, 11). In our review of the literature, this is the first reported case of a schwannoma extending between the internal and external carotid arteries mimicking a type III second BCC. Although rare, schwannoma should therefore be included in the differential diagnosis when a type III cyst is considered.
Several imaging techniques are available that can help differentiate between a schwannoma of the neck and a second BCC. These include MR spectroscopy (12–14) and MR diffusion-weighted imaging (15) or, alternatively, color Doppler sonography (16).
Cho et al evaluated the metabolic pattern of schwannomas and demonstrated (with MR spectroscopy) increased myoinositol (mI) in 11 cases of the 13 schwannomas they evaluated (12). This pattern alone appears to be very sensitive for the diagnosis of schwannoma. Cho et al observed elevated mI in almost every schwannoma they examined (12). In addition, Kinoshita and Yokota have noted enhanced mI from in vitro proton MR spectroscopy (13).
Wang et al were able to differentiate head and neck lesions with diffusion-weighted echo-planar MR imaging (15). They calculated the apparent diffusion coefficients from diffusion-weighted echo-planar MR images and noted that the mean of such coefficients of benign solid tumors was significantly smaller than for those of benign cystic lesions. This protocol could help differentiate a benign or malignant schwannoma from a BCC.
If further diagnostic accuracy is required, color Doppler sonography may be useful in distinguishing between a schwannoma and a BCC. Gritzmann et al reviewed the utility of sonography in evaluating soft-tissue masses of the neck. Their review concluded that BCCs have variable echogenicity, but on color Doppler imaging no vascularity is demonstrated inside the lesion (16). By contrast, schwannomas when evaluated with color Doppler imaging show internal vascularity that can easily be identified (16).
Conclusion
We present the case of a cystic schwannoma of the lateral neck, demonstrating what has been considered the classic CT findings of a type III second BCC. Consequently, although this “tail sign” is still strongly suggestive of a type III second BCC, it can no longer be considered pathognomonic for that disease entity.
References
- 1.Gold B. Second brachial cleft cyst and fistula. AJR Am J Roentgenol 1980;134:1067–1069 [DOI] [PubMed] [Google Scholar]
- 2.Lev S, Lev MH. Imaging of cystic lesions. Radiol Clin North Am 2000;38:1013–1027 [DOI] [PubMed] [Google Scholar]
- 3.Harnsberger H, Manusco A, Muraki A, et al. Branchial cleft anomalies and their mimics: computed tomographic evaluation. Radiology 1984;152:739–748 [DOI] [PubMed] [Google Scholar]
- 4.Coppens F, Peene P, Lemahieu SF. Diagnosis and differential diagnosis of branchial cleft cysts by CT scan. J Belge Radiol 1990;73:189–196 [PubMed] [Google Scholar]
- 5.Herman TE, McAlister WH, Siegel MJ. Branchial fistula: CT manifestations. Pediatr Radiol 1992;22:152–153 [DOI] [PubMed] [Google Scholar]
- 6.Albers GD. Brachial anomalies. JAMA 1963;183:399–409 [DOI] [PubMed] [Google Scholar]
- 7.Bailey H. Branchial cysts and other essays on surgical subjects in the facio-cervical region. London: HK Lewis;1929
- 8.Koeller KK, Alamo L, Adair CF, et al. Congential cystic masses of the neck: radiologic-pathologic correlation. Radiographics 1999;19:121. [DOI] [PubMed] [Google Scholar]
- 9.Weber AL, Montandon C, Robson CD. Neurogenic tumors of the neck. Radiol Clin North Am 2000;38:1077–1090 [DOI] [PubMed] [Google Scholar]
- 10.Colreavy MO, Lacy PD, Hughes J, et al. Head and neck schwannomas: a 10 years’ review. J Laryngol Otol 2000;114:119–124 [DOI] [PubMed] [Google Scholar]
- 11.Leu Y-S, Chang K-C. Extracranial head and neck schwannomas: a review of 8 years’ experience. Acta Otolaryngol 2002;122:435–437 [DOI] [PubMed] [Google Scholar]
- 12.Cho YD, Choi GH, Lee SP, et al. (1)H-MRS metabolic patterns for distinguishing between meningiomas and other brain tumors. Magn Reson Imaging 2003;21:663–672 [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Yokota A. Absolute concentrations of metabolites in the human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed 1997;10 ;2–12 [DOI] [PubMed] [Google Scholar]
- 14.Gill S, Thomas G, van Bruggen N, et al. Proton MR spectroscopy of intracranial tumors: in vivo and in vitro studies. J Comput Assist Tomogr 1990;14:497–504 [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology 2001;220:621–630 [DOI] [PubMed] [Google Scholar]
- 16.Gritzmann N, Hollerweger A, Macheiner P, et al. Sonography of soft tissue masses of the neck. Clin Ultrasound 2002;30:356–373 [DOI] [PubMed] [Google Scholar]