Abstract
BACKGROUND AND PURPOSE: Thiamine deficiency is extremely rare in infants in developed countries. To our knowledge, its MR findings in the brain have not been reported. The purpose of this study was to investigate the brain MR findings in infants with encephalopathy due to thiamine deficiency.
METHODS: The study group included six infants aged 2–10 months with encephalopathy who had been fed with solely soy-based formula devoid of thiamine from birth. All underwent MR evaluation at admission and follow-up (total of 14 examinations). In one patient, MR spectroscopy (MRS) was performed.
RESULTS: In five patients T2-weighted, fluid-attenuated inversion recovery, or proton-attenuated sequences showed bilateral and symmetric hyperintensity in the periaqueductal area, basal ganglia and thalami. Five had lesions in the mammillary bodies, and three, in the brain stem. In all six patients, the frontal region (cortex and white matter) was clearly involved. At presentation, MRS of the periaqueductal area showed a lactate doublet. On long-term follow-up, three of four patients had severe frontal damage; in two, this occurred as part of diffuse parenchymal loss, and in one, it was accompanied by atrophy of the basal ganglia and thalami.
CONCLUSION: Thiamine deficiency in infants is characterized by involvement of the frontal lobes and basal ganglia, in addition to the lesions in the periaqueductal region, thalami, and the mammillary bodies described in adults. MRS demonstrates a characteristic lactate peak.
Wernicke encephalopathy is caused by a deficiency of thiamine (vitamin B1). In adults, the disease is most commonly associated with alcoholism (1). Clinical manifestations include confusion, ataxia, and ocular motor disturbances. The typical MR findings are abnormal hyperintensity on T2-weighted images in the mammillary bodies, periaqueductal gray matter, periventricular thalamus, and hypothalamus (2). In children, thiamine deficiency is often associated with conditions involving prolonged malnutrition, such as cancer, chronic gastrointestinal diseases, and anorexia nervosa (3). Thiamine deficiency rarely occurs in infants in developed countries, and, to our knowledge, only one report describes the CT findings in children (4). The purpose of our study was to investigate the MR findings in six infants who developed encephalopathy due to thiamine deficiency after being fed soy formula devoid of thiamine.
Methods
Between September and November 2003, six infants aged 2–10 months were admitted to our center with encephalopathy of unknown cause. Extensive workup for a possible infectious disease yielded negative results. The patients’ weight and height were appropriate for their age. They had no history of metabolic disease. Lactate levels were elevated in CSF samples in all patients, and in blood samples in five. No electrolyte abnormalities were found. Only after the fifth child presented was a connection made between the similar neuroimaging findings and use of the same soy-based formula. The formula was analyzed and found to be devoid of vitamin B1. The diagnosis of Wernicke encephalopathy was established, and appropriate treatment with intramuscular thiamine injections was started.
All patients underwent MR imaging of the brain with a 1.5T unit (Intera; Philips, Eindhoven, the Netherlands). A total of 14 examinations were performed. The protocols varied at the discretion of the radiologists (L.K., M.S., O.K., G.H., C.H.), but all the examinations included standard axial T1- and T2-weighted imaging, as well as fluid-attenuated inversion-recovery (FLAIR) or proton-attenuation sequences. Intravenous contrast material (gadopentetate dimeglumine 0.2 mL/kg; Dotarem, Roissy, France) was used in all studies at presentation, and in 50% (7) of the follow-up examinations. In 12 examinations, axial diffusion-weighted images (DWIs) were obtained by using two b values: 0 and 1000 seconds/mm2, and apparent diffusion coefficient (ADC) maps were generated. In one patient, single-volume proton MR spectroscopy (MRS) with a TR/TE of 1500/144 was performed in the periaqueductal and frontal areas.
Results
Table 1 summarizes the patients’ clinical data, and Table 2 summarizes their imaging findings.
TABLE 1:
Clinical findings in six infants with thiamine deficiency
| Pt.No. | Age(months) | Gender | Lactate Levels |
Outcome | Length of Follow-up (months) | |
|---|---|---|---|---|---|---|
| Blood | CSF | |||||
| 1 | 8.5 | F | ↑ | ↑ | Dysphagia; severe DD | 4 |
| 2 | 4.5 | F | ↑ | ↑ | Dysphagia; mild DD | 5 |
| 3 | 10 | M | ↑ | ↑ | Dysphagia; moderate DD | 4 |
| 4 | 4.5 | F | N | ↑ | Mild DD | 5 |
| 5 | 2 | F | ↑ | ↑ | Complete recovery | 2 |
| 6 | 5 | F | ↑ | ↑ | Mild DD; ataxia | 4 |
Note.—↑ indicates elevated; N, normal; DD, developmental delay.
TABLE 2:
Imaging findings in six children with thiamine deficiency
| PatientNo. | Date ofMR | Periaqueductal | DW | Brain stem | DW | Tectum | MammillaryBodies | Thalami | DW | Caudate | DW | Putamen | DW | Frontal Cortexand WM | DW | VolumeLoss |
| 1 | 25.9.03 | − | N | − | N | + | + | − | N | − | N | − | N | − | N | − |
| 1.10.03 | + | C,V | + | N | + | + | + | C,V | + | C | + | C | + | C | − | |
| 7.11.03 | +Imp | N | − | N | Necrosis | Atrophy | +Necrosis | N | Hem Atrophy Necrosis | N | Hem Atrophy Necrosis | N | Laminar necrosis Leukomalacia | N | + | |
| 2 | 5.11.03 | + | N | + | C | − | + | + | C | − | C | − | N | + | C | − |
| B1→ | ||||||||||||||||
| 10.11.03 | − | NA | − | NA | − | + | +Imp | NA | − | NA | − | NA | Imp cortex | NA | − | |
| 26.11.03 | − | N | + | N | − | Atrophy | − | N | − | N | − | N | Laminar necrosis Leukomalacia | N | + | |
| 3 | 3.11.03 | + | NA | − | NA | − | − | + | NA | − | NA | + | NA | + | NA | + |
| B1→ | ||||||||||||||||
| 17.11.03 | +Imp | N | − | N | − | Atrophy | +Imp | N | +Worse | C | + | C | +Imp | N | Worse | |
| 4 | 25.9.03 | + | V | + | N | − | + | + | V | − | N | + | N | + | V | + |
| 8.11.03 | − | N | − | N | − | − | Imp | N | + | N | + | N | +Imp | N | Worse | |
| 5 | 6.11.03 | − | N | − | N | − | NA | − | N | − | N | − | N | − | C | − |
| 6 | 13.10.03 | + | N | − | N | − | + | + | N | − | N | + | N | + | N | − |
| B1→ | ||||||||||||||||
| 17.11.03 | − | N | − | N | − | − | − | N | − | N | Necrosis | N | + | N | − | |
| 9.2.04 | − | N | − | N | − | − | − | N | − | N | Atrophy | N | + | N | + |
Note.—+ indicates high signals on T2, FLAIR, or PD; −, normal signals; DW, diffusion-weighted images; C, cytotoxic edema; V, vasogenic edema; WM, white matter; NA, not available. N, normal or no signs of acute edema; Imp, improvement; Hem, hemorrhage; B1, start of treatment with B1.
Four patients presented with gastrointestinal symptoms (vomiting or diarrhea), accompanied by apathy, apnea, and seizures in two. The other two patients rapidly deteriorated to the same condition. In the remaining patients, apathy, apnea and/or seizures were the presenting symptoms. In two patients, neurologic examination revealed vertical nystagmus.
On MR examination at presentation, T2-weighted images showed hyperintensity of the mammillary bodies, periaqueductal gray matter, thalami, and basal ganglia in four patients, and images showed hyperintensity of the brainstem in three (Figs 1A and 2A). In one infant, involvement of the deep gray matter structures was visualized on follow-up study performed 5 days later because of clinical deterioration (Fig 1B–D). All patients had abnormal hyperintensity in the frontal regions. Four had diffuse involvement of both cortex and white matter up to the central gyrus (Figs 2B and 3). All lesions were bilateral and symmetric. In one infant, only the superior frontal region was involved, and in our youngest patient, only the precentral region (Fig 4A). Of note, optimal visualization of the mammillary bodies was not achieved in this patient.
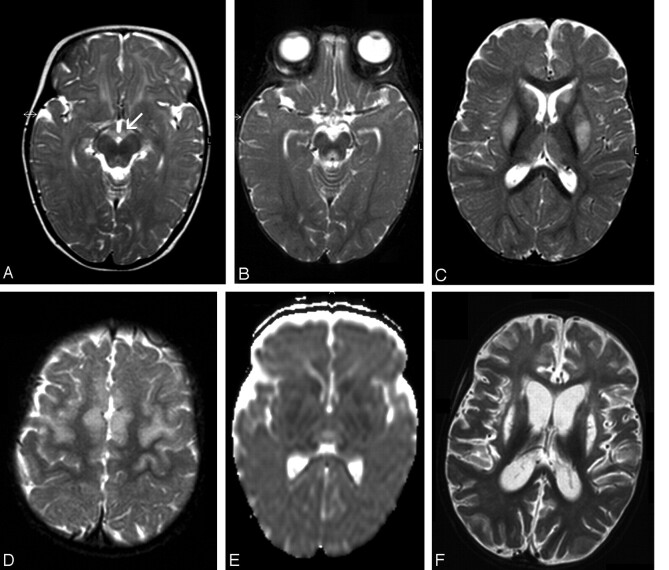
Fig 1.
Patient 1. Images at presentation (A) and follow-up 5 (B–E) and 45 (F) days later.
A, Axial T2-weighted image shows abnormal hyperintensity in the mammillary bodies (arrow) and tectum.
B–D, Axial T2-weighted images show abnormal hyperintensity in the periaqueductal region, thalami, basal ganglia, and frontal area. Lesions are bilateral and symmetric.
E, DWI (ADC) shows restricted diffusion in the basal ganglia. Thalami have hypointensity and hyperintensity, which are presumed to represent cytotoxic and vasogenic edema, respectively.
F, Axial T2-weighted image shows diffuse parenchymal loss, severe atrophy of the caudate nuclei, and necrosis of the putamina.
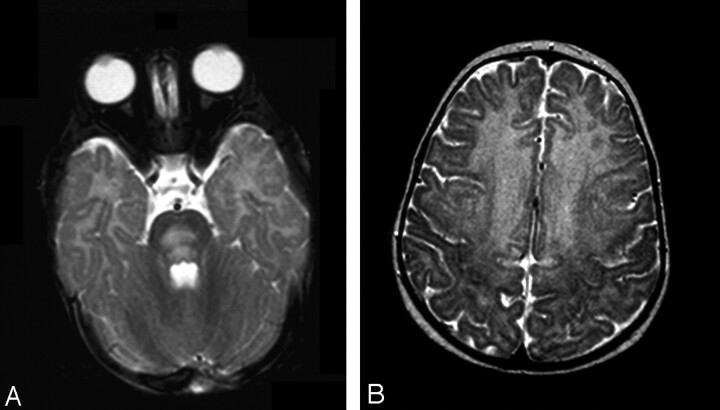
Fig 2.
Patient 2. Axial T2-weighted images.
A, At presentation, large area of hyperintensity is present in the pons.
B, Involvement of the frontal region, up to the motor cortex, is extensive.
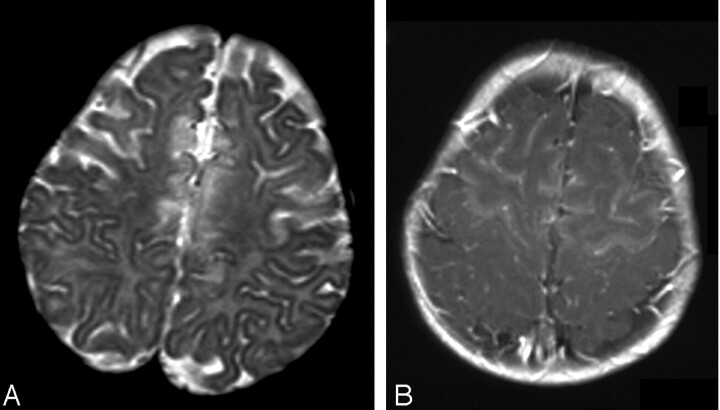
Fig 3.
Patient 4. Axial images at presentation.
A, T2-weighted image.
B, T1-weighted contrast-enhanced image shows extensive frontal injury. Note enhancement of both cortex and white matter.
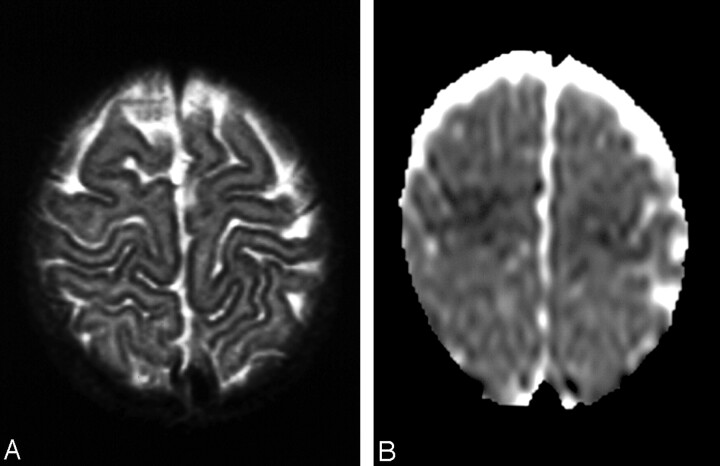
Fig 4.
Patient 5.
A, T2-weighted image shows subtle abnormal findings, slight hyperintensity of the anterior frontal region, and localized areas of blurring of the cortical stripe.
B, DWI (ADC) indicates restricted diffusion compatible with cytotoxic edema.
On admission, all patients but one (whose parents refused) were fed regular formula containing thiamine. On last follow-up, two patients had diffuse parenchymal loss, which was most severe in the frontal region, with leukomalacia (Fig 1F). T1 gyral hyperintensity compatible with laminar necrosis was also seen. In another patient, images showed shrinkage of the thalami and basal ganglia, with foci of necrosis and hemorrhage. In a third child, the lesions were less severe than in the others, with small areas of hyperintensity in the thalami, putamina, and parasagittal frontal cortex adjacent white matter. In a fourth child, only minimal frontal parenchymal loss and volume loss of the putamina were seen. Atrophy of the mammillary bodies was noted in three patients, and atrophy of the cerebellum and vermis was noted in one.
With therapy, improvement was observed in the brain stem in two patients, in the periaqueductal region in three patients, in the thalami in two, in the mammillary bodies in one, and in the frontal lobes in three. Enhancement after the injection of contrast agent was seen in the tectum in one patient and in the frontal cortex in two. DWIs showed cytotoxic edema in the brain stem in one patient, in the thalamus in two patients, in the basal ganglia in three, and in the frontal cortex in three (Figs 1E and 4B).
MRS of the periaqueductal lesion at presentation, performed in one patient, showed a lactate doublet with a slightly decreased ratio of n-acetylaspartate (NAA) to creatine (Cr) of 1.11 and slightly elevated ratio of choline (Cho) to Cr of 1.16. On the second examination 5 weeks later, with the normalization of T2 signal intensity, the lactate doublet disappeared. The NAA/Cr ratio improved to 1.51, and the Cho/Cr ratio increased to 1.39. Evaluation of an abnormal hyperintense area in the frontal white matter showed a metabolic pattern similar to that of the involved periaqueductal region (NAA/Cr ratio of 0.74 and Cho/Cr ratio of 1.46).
Clinical examination 2–5 months from the start of therapy revealed mild-to-severe developmental delay in five patients (Table 1).
Discussion
The clinical manifestations of thiamine deficiency are grouped under the name of beriberi, but they vary with the patient’s age and the organ system involved (5). In adults, Wernicke encephalopathy is characterized by ocular abnormalities, ataxia, and confusional state. In infants, thiamine deficiency may involve the cardiac or cerebral systems, or both. Patients with the cerebral form present with vomiting, nystagmus, and purposeless movements of the extremities and convulsions, all of which are nonspecific signs (5). Because the classical triad of Wernicke encephalopathy is not relevant in infants, imaging is crucial for proper diagnosis.
The literature contains only sporadic case reports of thiamine deficiency in infants. Most of the patients described were breastfed by mothers who were thiamine deficient themselves (5), or they were given nondairy formula low in thiamine (3, 4). To our knowledge, just one report discusses the imaging (CT) findings of thiamine deficiency in this age group. The sole abnormality was hypoattenuation of the putamina (4).
In adults, the pathognomonic imaging findings of Wernicke encephalopathy are hyperintensity on T2-weighted images in the mammillary bodies, periaqueductal gray matter, periventricular thalamus, and hypothalamus (2). Five of our patients also had involvement of these areas; in patient 5, the mammillary bodies were not optimally visualized. In addition, all six patients had involvement of the frontal lobes; in the youngest infant (patient 5), this was the only area affected. Frontal abnormalities are reported only rarely in both children (6) and adults (7–9) with thiamine deficiency. Of note, none of the three adults reported had alcoholism (7–9).
Involvement of the basal ganglia was noted in five patients in our series—a finding rarely reported in adults (9, 10). This finding seems to be more common in children (3, 4, 6, 11) with preferential involvement of the striatum and less involvement of the globus pallidus (10). In our group, the striatum was affected in three patients, and in one, additional lesions were found in the globi pallidi. In two patients, only the caudate nuclei or the putamina were abnormal. Three infants had lesions in the brain stem: two in the posterior pons and one in the tectum and central pons. Lesions in the tectum have been described in thiamine deficiency, but lesions in the posterior and central pons are unusual (12, 13). Other rarely reported sites of injury, such as the red nuclei and the dentate nucleus of the cerebellum (13), were not affected in our patients.
Tissues and cell types have a differential vulnerability to thiamine deficiency (14). The data derived from our small group indicated that the immaturity of the brain may play an important role in the susceptibility of specific brain areas. Our patients had preferential damage to the frontal lobes, followed by the basal ganglia.
All tissues require thiamine because it plays a role in several major metabolic processes: carbohydrate metabolism; biosynthesis of cell constituents, including neurotransmitters; production of reducing equivalents used in oxidant stress defenses; and synthesis of pentoses used as nucleic acid precursors (5, 14). Therefore, no single mechanism accounts for the neurologic damage thiamine deficiency induces. Increased lactic acid concentrations in the brain and associated acidosis, mitochondrial damage, alterations in levels of several neurotransmitters, induction of apoptosis, and neuronal cell death due to oxidative stress have all been implicated (5, 14).
The MRS spectrum reflected these metabolic changes. It showed a substantial lactate peak in injured areas in the diencephalon and the frontal white matter (due to the disturbances in carbohydrate metabolism), a decreased NAA/Cr ratio (indicative of neuronal damage), and a decreased Cho/Cr ratio (probably related to reduced incorporation of lipids into myelin). We know of no reports of the MRS findings in Wernicke encephalopathy in infants. The few case reports of thiamine deficiency in adults and rats describe similar findings (15).
Contrast enhancement is present in about 50% of reported cases, according to the review of Maschalchi et al (8) and to our investigation of more recent reports (6, 9, 12, 16–20). Enhancement depends on the stage and severity of disease because it is related to disruption of the blood-brain barrier, which may still be intact in the early acute stage (8). In our group, images in three patients demonstrated enhancement at diagnosis and in two on follow-up.
DWIs can depict edema in the earliest stages. Reports describe both vasogenic and cytotoxic edema in the thalami and periaqueductal region (9, 12, 18, 21–24). Follow-up after treatment showed complete resolution of the findings in some cases (18, 21, 24, 25), though the neurologic findings did not revert to normal. Our patients had mostly cytotoxic edema, in the basal ganglia in three patients, in the thalamus in two, and in the frontal region in three. Vasogenic edema in the frontal region was noted in an additional patient. The latter improved on MR studies obtained after her formula was changed. The cytotoxic edema in two patients (one treated and one untreated) was not reversible and progressed to atrophy and cortical necrosis. In the third patient (patient 5), no follow-up studies were available, but she is the only patient with complete neurologic recovery. Therefore, the presence of cytotoxic edema does not seem to imply irreversible damage.
We were able to observe the natural history of thiamine deficiency in patient 1, whose parents refused to change the formula (Fig 1). At presentation, physical examination revealed nystagmus, and abnormalities of the mammillary bodies and tectum were noted on MR imaging. Five days later, parallel to the clinical deterioration, repeated MR imaging showed involvement of the thalami, caudate, putamina, and frontal parenchyma, with signs of cytotoxic edema. Five weeks later, severe atrophy of all the involved areas was evident, with severe neurologic impairment; however, individual differences occur in the initial lesions and in the course of the disease. Several groups have documented interindividual variations in susceptibility to disorders related to thiamine deficiency (11, 14). Sensitive individuals may have a molecular defect in one of the enzymes that use thiamine as a cofactor (11, 14). Indeed, the deficient formula was given to 2%–6% of all infants in Israel, but only 19 developed clinical signs of thiamine deficiency (T. Schoenfeld, personal communication).
The principal differential diagnosis of thiamine deficiency is Leigh disease, which is caused by defects in different enzymes involved in energy metabolism (26), including thiamine dehydrophosphate (1). Leigh disease is characterized by a pattern of abnormalities similar to that of Wernicke encephalopathy, a common pathophysiology of mitochondrial damage, and laboratory findings of elevated lactate levels (26). Encephalitis, as well as acute disseminated encephalomyelitis, occasionally causes symmetric lesions; however, thiamine deficiency can be distinguished from these disorders by involvement of the mammillary bodies, a highly specific sign. Nevertheless, the diagnosis can be missed for technical reasons, as changes in signal intensity in the mammillary bodies are difficult to detect on axial T2 images because of partial-volume averaging with the suprasellar cistern. Therefore, findings on FLAIR or proton-attenuated sequences may be contributory. Contrast enhancement, if present, may also help. Furthermore, in patients in whom the diagnosis is delayed to the chronic phase, atrophy of the mammillary bodies may be noted (1). The diagnosis might also be missed because of the rarity of thiamine deficiency in infants, and routine evaluation of the brain in this age group may not always include scrutiny of the mammillary bodies.
Conclusion
Thiamine deficiency in infants causes abnormalities in the frontal lobes and basal ganglia, in addition to the lesions described in adults. MRS of the involved areas shows a lactate doublet owing to the disturbance in carbohydrate metabolism. Because the diagnosis relies heavily on neuroimaging, radiologists should be alerted to the different pattern of injury in this age group and to the possibility of thiamine deficiency, even in infants with no history of malnutrition.
Fig 5.
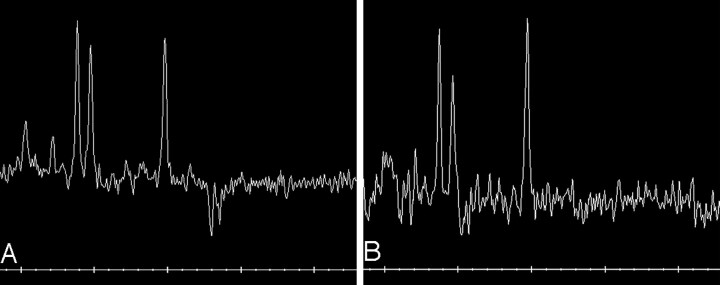
Patient 6. Proton MRS image from the periaqueductal region (TR/TE = 1500/144).
A, At presentation. Note the negative doublet of lactate. NAA/Cr ratio is reduced 1.11.
B, Five weeks later, the lactate doublet is no longer seen. NAA peak is higher than before.
References
- 1.Russell RM. Vitamin and trace mineral deficiency and excess. In: Harrison’s principles of internal medicine. 15th ed. New York: McGraw Hill;2001;461–464
- 2.Jack CR, Lexa FJ, Trojanowski JQ, et al. Normal aging, dementia and neurodegenerative disease. In: Atlas SW, ed. Magnetic resonance imaging of the brain and spine. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins;2002. :1177–1240
- 3.Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke’s encephalopathy. Pediatr Neurol 1999;20:289–294 [DOI] [PubMed] [Google Scholar]
- 4.Wyalt DT, Michael JN, Hillman RE. Infantile beriberi presenting as subacute necrotizing encephalomyelopathy. J Pediatr 1987;110:888–891 [DOI] [PubMed] [Google Scholar]
- 5.Tanphaichit V. Thiamin. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern nutrition in health and disease. 9th ed. Philadelphia: Lippincott, Williams & Wilkins;1999;381–389
- 6.D’Aprile P, Tarantino A, Sanatoro S, Carella A. Wernicke’s encephalopathy induced by total parenteral nutrition in patient with acute leukemia: unusual involvement of caudate nuclei and cerebral cortex on MR. Neuroradiology 2000;42:781–783 [DOI] [PubMed] [Google Scholar]
- 7.Yamashita M, Yamamoto T. Wernicke encephalopathy with symmetric pericentral involvement. MR findings. J Comput Assist Tomogr 1995;19:306–308 [DOI] [PubMed] [Google Scholar]
- 8.Maschalchi M, Simonelli P, Tessa C, et al. Do acute lesions of Wernicke’s encephalopathy show contrast enhancement? Report of three cases and review of the literature. Neuroradiology 1999;41:249–254 [DOI] [PubMed] [Google Scholar]
- 9.Doss A, Mahad D, Romanovski C. Wernicke encephalopathy: unusual findings in nonalcoholic patients. J Comput Assist Tomogr 2003;27:235–240 [DOI] [PubMed] [Google Scholar]
- 10.Opdenakker G, Gelin G, De Surgeloose D, Palmers Y. Wernicke encephalopathy: MR findings in two patients. Eur Radiol 1999;9:1620–1624 [DOI] [PubMed] [Google Scholar]
- 11.Coe M, Carfagnini F, Tani G, Ambrosetto P. Wernicke’s encephalopathy in a child: case report and MR findings. Pediatr Radiol 2001;31:167–68 [DOI] [PubMed] [Google Scholar]
- 12.Halavaara J, Brander A, Lyytinen J, Setala K., Kallala M. Wernicke’s encephalopathy: is diffusion weighted MRI useful? Neuroradiology 2003;45:519–23 [DOI] [PubMed] [Google Scholar]
- 13.Bae SJ, Lee HK, Lee JH, et al. Wernicke’s encephalopathy: Atypical manifestation at MR imaging. Am J Neuroradiol 2001;22:1480–482 [PMC free article] [PubMed] [Google Scholar]
- 14.Singleton CK, Martin PR. Molecular mechanism of thiamine utilization. Curr Molec Med 2001;1:197–07 [DOI] [PubMed] [Google Scholar]
- 15.Rugilo CA, Uribe Roca MC, Zurru MC, et al. Proton MR spectroscopy in Wernicke encephalopathy. Am J Neuroradiol 2003;24:952–55 [PMC free article] [PubMed] [Google Scholar]
- 16.Ming X, Wang MM, Zee D, et al. Wernicke’s encephalopathy in a child with prolonged vomiting. J Child Neurol 1998;13:187–89 [DOI] [PubMed] [Google Scholar]
- 17.Bergui M, Bradac GB, Zhong JJ, et al. Diffusion weighted MR in reversible Wernicke encephalopathy. Neuroradiology 2001;43:969–72 [DOI] [PubMed] [Google Scholar]
- 18.Chu K, Kang DW, Kim HJ, et al. Diffusion weighted imaging abnormalities in Wernicke encephalopathy. Arch Neurol 2002;59:123–27 [DOI] [PubMed] [Google Scholar]
- 19.Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Wernicke encephalopathy: MR findings and clinical presentation. Eur Radiol 2003;13:1001–009 [DOI] [PubMed] [Google Scholar]
- 20.Sparacia G, Banco A, Lagalla R. Reversible MR abnormalities in an unusual paediatric presentation of Wernicke’s encephalopathy. Pediatr Radiol 1999;29:581–84 [DOI] [PubMed] [Google Scholar]
- 21.Chung TI, Kin JS, Park SK, et al. Diffusion weighted MR imaging of acute Wernicke’s encephalopathy. Eur J Radiol 2003;45:256–58 [DOI] [PubMed] [Google Scholar]
- 22.Kashihara K, Irisawa M. Diffusion-weighted magnetic resonance imaging in a case of acute Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry 2002;73:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doherty MJ, Watson NF, Uchino K, et al. Diffusion abnormalities in patients with Wernicke encephalopathy. Neurology 2002;58:656–657 [DOI] [PubMed] [Google Scholar]
- 24.Hong KS, Kang DW, Cho YJ, et al. Diffusion weighted magnetic resonance imaging in Wernicke’s encephalopathy. Acta Neurol Scand 2002;105:132–134 [DOI] [PubMed] [Google Scholar]
- 25.Niclot P, Guichard JP, Djomby R, et al. Transient decrease in water diffusion in Wernicke’s encephalopathy. Neuroradiology 2000;44:305–307 [DOI] [PubMed] [Google Scholar]
- 26.Barkovich AJ. Toxic and metabolic brain disorders. In: Pediatric neuroimaging. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2000;71–156