Abstract
BACKGROUND AND PURPOSE: Predominant cerebellar involvement has not been previously reported as a common neuroradiologic feature in pediatric mitochondrial cytopathies. Here we report the neuroradiologic findings of predominant cerebellar volume loss in children with various mitochondrial disorders.
METHODS: A retrospective analysis of the medical records of 400 consecutive patients referred for evaluation of mitochondrial encephalomyopathies was performed. In 113 cases, definite diagnosis of mitochondrial disease was based on the modified adult criteria that include clinical, histologic, biochemical, functional, molecular, and metabolic parameters.
RESULTS: Predominant cerebellar volume loss with progressive cerebellar atrophy and, less often, cerebellar hypoplasia were found in a heterogeneous group of patients with mitochondrial disease that consisted of four patients with complex I deficiency; four patients with multiple respiratory chain deficiencies; two patients with combined complex I + III and II + III deficiencies, including one patient with partial coenzyme Q10 deficiency; three patients with complex II deficiency; two patients with complex IV deficiency; one patient with mitochondrial neurogastrointestinal encephalomyopathy; and two patients with mitochondrial encephalomyopathy, lactic acidosis, and strokes.
CONCLUSION: Our retrospective study shows that isolated or predominant cerebellar involvement can be found in various respiratory chain defects or mitochondrial disorders expanding the classical neuroradiologic findings observed in mitochondrial encephalomyopathies. The diagnostic workup in patients with neuromuscular features whose brain MR imaging exhibits cerebellar volume loss should include the evaluation for mitochondrial encephalomyopathies.
Mitochondrial respiratory chain defects (RCDs) are a heterogeneous group of disorders of energy metabolism that can present at any age, with a wide and nonspecific range of clinical symptoms and with any mode of inheritance, originating from any organ or tissue, although tissues with high-energy requirements such as brain, skeletal muscle, and heart are particularly vulnerable (1, 2). The diagnosis of RCD can be particularly challenging to clinicians, especially when patients are children, in whom nonspecific neurologic symptoms are common; the neuroradiologic features may be atypical in the early stage, morphologic findings on skeletal muscle can be marginal, the interpretation of the analysis of mitochondrial respiratory chain (RC) enzymes is often difficult, and mitochondrial DNA (mtDNA) defects are often absent (3–5). Diagnostic criteria for pediatric RCD have been modified from an adult classification system—ie, the modified adult criteria (6). We previously studied the clinical course and outcome of 113 patients with mitochondrial disease diagnosed by using these modified criteria (7), representing one of the largest published series concerning the pediatric age group. Patients with mitochondrial disease often present a combination of abnormalities from different patterns on their MR imaging of the brain. Cerebral white matter abnormalities in combination with lesions in the basal ganglia and brain stem are relatively frequent (4, 8, 9). We identified these previously described MR imaging abnormalities in our cohort of patients (7). In addition, we have identified a substantial number of patients with definite mitochondrial disorders who exhibit cerebellar volume loss with either progressive cerebellar atrophy (in most patients) and cerebellar hypoplasia. The cases presented here definitely add RCD to the group of biochemically and/or molecularly defined disorders that either could cause progressive cerebellar atrophy or could disrupt early cerebellar development causing cerebellar hypoplasia, expanding the spectrum of neuroradiologic features in patients with RCD.
Methods
The studies were approved by our institutional review board. The diagnosis of mitochondrial disease was based on the use of an objective classification scheme based on assigning major or minor criteria for clinical, pathologic, enzymatic, molecular, functional, and metabolic parameters (6). Patients with two major or one major and two minor criteria were assigned with a definite diagnosis and were included in the study (7). Evidence of at least two relatively independent types of investigations (ie, clinical, histologic, biochemical, or molecular) was required to establish a definitive diagnosis. A muscle biopsy was performed as part of the diagnostic evaluation in most patients when a known pathogenic mtDNA mutation was not identified in blood. Mitochondrial RC assays were performed in 5% homogenates of frozen muscle specimens by using standard spectrophotometric assays (10–13). Coenzyme Q10 (CoQ10) was analyzed in skeletal muscle by using high-performance liquid chromatography with ultraviolet detection (275 nm) and by using coenzyme Q9 as an internal standard (14, 15). All patients were screened for mtDNA deletions/duplications and common point mutations (MELAS A3243G and T3271C, MERRF A8344G and T8356C, NARP T8993G and T8993C, cardiomyopathy G8363A, and LHON G11778A, G3460A, T14484C, and G14459A) (16, 17). Patients with a neurologic or neuromuscular phenotype were examined by using MR imaging. The MR studies were performed by using a 1.5T unit (Philips, Intera, Best, the Netherlands). T1-weighted images were obtained in sagittal plane, and T2-weighted and FLAIR-images were obtained in axial and coronal planes. We specifically looked for changes in the signal intensity and size of the basal ganglia and brain stem in T1- and T2-weighted images, cerebral and cerebellar volume loss, focal lesions, and the stage of myelination.
Results
Diagnosis of definite mitochondrial disease was made in 113 patients (66 boys and 47 girls), ranging in age from 2 weeks to 18 years of age. Sixty-eight patients with predominant neuromuscular features (70% of the cohort) who underwent brain imaging studies had abnormal brain MR imaging findings. After a careful retrospective review of neuroimaging data by an experienced neuroradiologist, the findings of this study in our group showed a wide spectrum of abnormalities that included cerebellar and cerebral atrophy, cerebellar hypoplasia, brain stem, and basal ganglia lesions, infarct-like bilateral parasagittal lesions, agenesis of the corpus callosum, and white matter abnormalities (7). Eighteen patients (26%) exhibited predominant cerebellar volume loss with the cerebrum less affected than the cerebellum. The observed cerebellar volume loss included cerebellar atrophy and cerebellar hypoplasia (Table 1). Two patients exhibited cerebellar hypoplasia (patients 5 and 7; Table 1), whereas the remainder exhibited progressive cerebellar atrophy. All of these patients had early neurologic findings with a mean age of symptom onset of 5 years. Cerebellar ataxia was the presenting feature in all but one patient (patient 6; Table 1). The average time from symptom onset to MR imaging was 3 years.
Clinical, biochemical, molecular, and neuroradiologic findings of the patients
| Patient/ Gender | Age at Diagnosis (months) | Presentation | RC/Molecular Defect | Neurological Symptoms | MR Findings |
|---|---|---|---|---|---|
| 1/F | 295 | NSME | CI–CIV | A, C, PR | Cerebellar atrophy |
| 2/F | 67 | NSME | CI | A, C, H, HL, PR, SZ | Cerebellar atrophy |
| 3/F | 85 | NSME | CI | A, C, H, SZ | Cerebellar atrophy |
| 4/F | 20 | NSME | CI–CIV | A, C, D, H, SZ | Cerebellar atrophy |
| 5/F | 7 | NSME | CI + III, II + III | A, C, H, HL, SZ | Pontocerebellar hypoplasia |
| 6/F | 85 | NSME | CI + III, II + III (CoQ10 deficiency) | C, H, PR, SZ | Mild cerebellar atrophy |
| 7/F | 13 | NSME | CI–CIV | A, C, H, HL | Cerebellar hypoplasia |
| 8/M | 48 | NSME | CI | A, C, H | Cerebellar atrophy |
| 9/M | 31 | NSME | CI | A, C, H, SZ | Cerebellar atrophy |
| 10/M | 45 | NSME | CI | A, C, H, HL, PR | Cerebellar atrophy |
| 11/F | 162 | NSME | CII | A, C, H | Cerebellar atrophy |
| 12/M | 35 | NSME | CIV | A, C, H, HL | Cerebellar atrophy |
| 13/M | 31 | NSME | CII | A, C, D, H, SZ | Cerebellar atrophy |
| 14/M | 43 | NSME | CII | A, C, H, HL, SZ | Cerebellar atrophy |
| 15/M | 16 | LS | CIV SURF1 (Q195X/A56G) | A, C, H | Cerebellar atrophy/ bilateral basal ganglia lesions |
| 16/M | 216 | MNGIE | TP (homozygous IVS1-1G→C) | A, H, O, P, PR | Cerebellar atrophy/ leukoencephalopathy |
| 17/F | 104 | MELAS | 3243A→G | A, C, H, HL, ST, SZ | Cerebellar atrophy/stroke like lesions |
| 18/F | 206 | MELAS | 3243A→G | A, C, H, HL, ST | Cerebellar atrophy/stroke-like lesions |
Note.—CI through CIV indicates defect of the RC enzyme complex I to IV; CI–CIV, combined RC enzyme defects; CoQ10, coenzyme Q10; NSME, nonspecific mitochondrial encephalomyopathy; LS, Leigh syndrome; MNGIE, mitochondrial neurogastrointestinal encephalomyopathy; MELAS, mitrochondrial encephalomyopathy with lactic acidosis and strokelike episodes; A, ataxia; C, cognitive impairment; D, dystonia; H, hypotonia; HL, hearing loss; O, external ophthalmoparesis; P, paresthesia; PR, pigmentary retinopathy; ST, strokelike episodes; SZ, seizures.
Patients with a Nonspecific Mitochondrial Encephalomyopathy
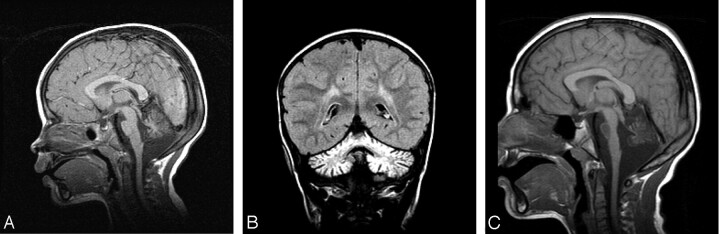
Fourteen patients with predominant cerebellar involvement and different RC defects of 30% or less of residual enzymatic activity on skeletal muscle, had a nonspecific mitochondrial encephalomyopathy (NSME) with neurologic symptoms ranging from psychomotor retardation, seizures, hypotonia, hypertonia, and sensorineural hearing loss, to ophthalmologic features such as pigmentary retinopathy, and external ophthalmoparesis. Thirteen patients among this group exhibited cerebellar ataxia and one of them, who had combined RCD, also exhibited marked dystonia (patient 4; Table 1). The one patient who did not present with cerebellar signs had combined complex I + III and II + III deficiencies, mild cerebellar atrophy, and a 50% reduction in the skeletal muscle CoQ10 content consistent with partial CoQ10 deficiency (patient 6; Table 1). One patient with isolated complex I + III and II + III deficiencies and predominant neurologic features including psychomotor retardation, ataxia, seizures, hypotonia, corneal opacity, and sensorineural hearing loss (patient 5; Table 1) exhibited pontocerebellar hypoplasia (Figs 1A and -B), and a second patient with combined RC defects and hypotonia, ataxia, developmental delay, sensorineural hearing loss, and pigmentary retinopathy (patient 7; Table 1) displayed isolated cerebellar hypoplasia and good preservation of the pons (Figs 2A and -B). The latter patient later developed a hypertrophic cardiomyopathy and renal tubular acidosis. The rest of the group exhibited progressive cerebellar atrophy (Figs 3A–C). Figure 3 exhibits an example of progressive cerebellar atrophy as mentioned in the text.
Fig 1.
Sagittal T1-weighted midline (A) and coronal (B) T1-weighted MR images through the posterior fossa show small cerebellar hemispheres and pontine hypoplasia with prominent cerebellar folia.
Fig 2.
A, Sagittal T1-weighted MR imaging demonstrates marked cerebellar volume loss with good preservation of the pons. B, Coronal T2-weighted sequence confirms cerebellar but not cerebral atrophy.
Fig 3.
Sagittal midline T1-weighted (A) and coronal fluid-attenuated (B) inversion recovery (C) images demonstrate evidence of progressive cerebellar atrophy when compared with interval sagittal T1-weighted midline image. Note evidence of pontine involvement with T1-weighted hypointensity returned from a pons diminished in size.
Leigh Syndrome and Cerebellar Volume Loss
The diagnosis of Leigh syndrome (LS) was made in one patient of our cohort (patient 15; Table 1) who exhibited cytochrome c oxidase deficiency in skeletal muscle and fibroblasts and was compound heterozygote for two mutations (Q195X/A56G) in the SURF-1 gene. Before the development of focal bilateral lesions in the basal ganglia, this patient was found to have predominant cerebellar atrophy on brain MR imaging and clinically, psychomotor retardation, hypotonia, and cerebellar ataxia.
Mitochondrial Neurogastrointestinal Encephalomyopathy
The patient reported with mitochondrial neurogastrointestinal encephalomyopathy ([MNGIE] patient 16; Table 1) had a classical clinical presentation, including gastrointestinal dysmotility, ptosis, and peripheral neuropathy, but without skeletal muscle involvement at morphologic, enzymatic, or mitochondrial DNA level, although gastrointestinal myopathy was present. The patient was homozygous for a novel thymidine phosphorylase gene (TP) splice acceptor–site mutation, IVS1–1G→C (18). MR imaging of the brain showed leukoencephalopathy, although the patient also exhibited predominant cerebellar volume loss with cerebellar and cerebral atrophy.
Mitochondrial Encephalomyopathy with Lactic Acidosis and Strokelike Episodes
The two patients with mitochondrial encephalomyopathy with lactic acidosis and strokelike episodes (MELAS) in our group (patients 17 and 18; Table 1) had the common 3243 A→G mutation in the mt tRNALeu gene with 30% and 43% heteroplasmy in peripheral blood, respectively. Both exhibited a typical clinical presentation with strokelike episodes preceded by seizures and accompanied by pancreatitis and diabetes mellitus in one of them (patient 18; Table 1). Extensive infarct-like lesions, not confined to vascular territories were present in these patients and cortical atrophy was observed. Both patients, however, also exhibited significant and marked progressive cerebellar atrophy.
Discussion
Different patterns of brain MR imaging abnormalities have been recognized and described in patients with mitochondrial disease (4, 8, 9). They comprise a pattern of energy failure, consisting of cortical pseudoinfarcts, basal ganglia lesions, and brain stem lesions in the acute phase as typically seen in MELAS, LS, and NARP; a second pattern of chronic neurodegeneration with global cerebral and/or cerebellar atrophy as observed in MERRF; a third pattern that consists of focal and diffuse white matter abnormalities, often with cystic degeneration and areas of contrast enhancement as observed in complex I, complex II, and complex IV deficiencies, and MNGIE; and, finally, a pattern of malformations that may be seen in severe variants of pyruvate dehydrogenase complex deficiency.
In our cohort, most of the patients with predominant cerebellar volume loss presented clinically with an unspecified encephalomyopathy with neurologic symptoms that ranged from developmental delay to seizures. Most of these patients had cerebellar ataxia, dysmetria, and dysdiadokokinesis, although one patient was not found to have cerebellar features. Classical mitochondrial syndromes in our pediatric group were uncommon as they occur more often in adults (19).
As previously mentioned, the patients with MELAS, LS and SURF1 deficiency, and MNGIE exhibited patterns in their brain imaging studies that have been previously described; however, they also displayed considerable cerebellar involvement, which was more predominant than cerebral atrophy and other features observed on their brain MR imaging studies.
MNGIE is defined as an autosomal recessive disorder caused by mutations in TP (20). Clinical diagnostic criteria have been established for MNGIE: severe gastrointestinal dysmotility, cachexia, ptosis, external ophthalmoparesis, peripheral neuropathy, and leukoencephalopathy (21). Leukoencephalopathy has always been described as the main neuroradiologic feature of patients with MNGIE (22); however, cerebellar atrophy has not been previously reported as a predominant neuroradiologic finding in addition to leukoencephalopathy, as observed in our case.
MELAS is characterized by strokelike episodes preceded by seizures. Short stature, diabetes mellitus, hearing loss, and slowly progressive mental impairment are common features (23). The most common mtDNA point mutation occurring in this syndrome is an A→G transition at the tRNALeu (UUR) 3243, which causes a defect in mitochondrial protein synthesis (24).
Cerebellar atrophy in the context of MELAS has been described elsewhere (25). Enlargement of the 4th ventricle was the first sign of cerebellar atrophy, which was present only with severe disease, according to a previous publication (25). Although enlargement of the 4th ventricle was not detected in our patients with MELAS as a first sign of cerebellar atrophy, the demonstrated progressive cerebellar atrophy at a severe stage of the disease corroborates previous findings by Sue et al 1998 (25). These findings likely reflect a distinct disease process in MELAS, that of a slowly progressive degenerative process causing cerebellar as well as cerebral atrophy.
LS presents clinically as an early-onset progressive neurodegenerative disorder with a characteristic neuropathology consisting of focal, bilateral lesions in one or more areas of the central nervous system—including the brain stem, thalamus, basal ganglia, cerebellum, and spinal cord—accompanied by progressive impairment of cognitive and motor function, leading to loss of respiratory control and death in a few months or years (26). In recent years, deficiencies of pyruvate dehydrogenase complex, complexes I, II, and IV deficiencies, high levels of the T8993G mutation in the mtDNA ATPase 6 gene, and SURF1 mutations have been described as causes of LS (27). Brain MR imaging may demonstrate areas of altered signal intensity in the brain stem, cerebellum, and basal ganglia. Our patient with LS due to cytochrome c oxidase–associated SURF deficiency displayed progressive cerebellar atrophy and ataxia as presenting features. This presentation is quite uncommon and has been described elsewhere by Cacic et al (28), who reported a 2.5-year-old patient who presented with ataxic gait and tremor and developed an extrapyramidal syndrome with a head CT revealing marked cerebellar involvement. These cases demonstrate that marked cerebellar involvement can predate other neuroradiologic features of LS.
Although cerebellar involvement has been described as part of a wider array of central nervous system abnormalities in patients with mitochondrial disorders (4, 8, 9, 29), predominant cerebellar involvement (atrophy or hypoplasia) has not been commonly described as a significant neuroradiologic feature in children with mitochondrial RCD. One exception would be the cerebellar atrophy affecting both cerebellar hemispheres and vermis, observed in the ataxic syndrome caused by primary CoQ10 deficiency, a mitochondrial encephalomyopathy associated with deficiencies of quinone-dependent enzymes (complex I + III and II + III) and responsive to CoQ10 supplementation (30). One of the two patients with observed combined complex I + III and II + III deficiencies had mild cerebellar atrophy on MR imaging and manifested a partial deficiency of the quinone pool. The observed mild degree of atrophy could explain the absence of cerebellar symptoms in this patient. The mechanism responsible for the selective and progressive cerebellar atrophy in primary CoQ10 deficiency is not entirely known. In one study (31), the regional distribution of coenzyme Q10 in the brain of adult rats was investigated. The results showed that in rats the cerebellum contained the lowest level of CoQ10 of the seven brain regions that were investigated. Of the four regions of one human brain studied in the same paper, the cerebellum exhibited the lowest CoQ10 concentration. The investigators hypothesized that there could be a selective vulnerability of the cerebellum to a potential depletion of the quinone pool.
The neuroradiologic and biochemical findings in the patients in our series, however, demonstrated that predominant and progressive cerebellar atrophy could be associated mostly with other types of RCD or mitochondrial syndromes, expanding the spectrum of mitochondrial disorders associated with it. The etiology of this predominant cerebellar over cerebral involvement observed in such a heterogeneous group of patients remains unknown as of yet. We speculate that the specificity of pathogenic involvement of the cerebellum observed in this diverse group of mitochondrial disorders could be due to the result of the tissue-dependent expression of the underlying molecular and biochemical disorder. A population of neuronal mitochondria in the cerebellum could be more vulnerable to oxidative stress resulting from mitochondrial dysfunction in some patients with mitochondrial disorders because, as previously stated, the cerebellum exhibits the lowest concentration of CoQ10.
Although, for the most part, the patients in our cohort exhibited isolated cerebellar atrophy, two patients with RCD in our group exhibited cerebellar hypoplasia. Few cases of cerebellar hypoplasia and associated RCD have been previously described. A patient with a mitochondrial encephalomyopathy that presented with severe hypotonia, myoclonic seizures, and optic atrophy due to cytochrome c oxidase deficiency and hypoplasia of the cerebellum with rudimentary cerebellar hemispheres has been described elsewhere (32). Moreover, de Koning et al reported a case of lethal pontocerebellar hypoplasia associated with multiple RCD in skin fibroblasts (33). The cerebellum develops early in the first trimester of pregnancy, and anatomic differentiation of the major divisions of the cerebellum is completed within the first trimester (34). These previous reports in addition to our results suggest that ATP production and the biosynthetic pathways depending on a functional RC are crucial in a critical period of cerebellar differentiation.
In summary, the diagnostic work-up in patients with psychomotor retardation and neuromuscular features including early onset of cerebellar ataxia and predominant cerebellar involvement on brain MR imaging should include the search for mitochondrial disease. Even in disorders such as LS, caused by SURF1 mutations and associated with cytochrome c oxidase deficiency, progressive cerebellar volume loss may be the first neuroradiologic feature observed. In retrospect, because some of the patients of our cohort were ascertained in childhood rather than in early infancy, it is not possible to completely determine whether the cerebellar involvement is due to impaired differentiation leading to cerebellar hypoplasia, or merely progressive atrophy. Thus, a prospective volumetric study in our group will help us to better understand the different types and prevalence of cerebellar volume loss in disorders of energy metabolism. MR spectroscopy might also assist in demonstrating different patterns of metabolic change reflecting different substrates of neuronal mitochondrial activity leading to cerebellar volume loss.
Acknowledgments
We thank the Baylor College of Medicine Mental Retardation Research Center for its support.
References
- 1.Munnich A, Rustin P, Rötig A, et al. Clinical aspects of mitochondrial disorders. J Inherit Metab Dis 1992;15:448–455 [DOI] [PubMed] [Google Scholar]
- 2.Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2001;2:342–352 [DOI] [PubMed] [Google Scholar]
- 3.De Vivo DC. The expanding clinical spectrum of mitochondrial diseases. Brain Dev 1993;15:1–22 [DOI] [PubMed] [Google Scholar]
- 4.Valanne L, Ketonen L, Majander A, et al. Neuroradiologic findings in children with mitochondrial disorders. AJNR Am J Neuroradiol 1998;19:369–377 [PMC free article] [PubMed] [Google Scholar]
- 5.DiMauro S, Bonilla E, De Vivo DC. Does the patient have a mitochondrial encephalomyopathy? J Child Neurol 1999;14:S23–S35 [DOI] [PubMed] [Google Scholar]
- 6.Bernier FP, Boneh A, Dennett X, et al. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 2002;59:1406–1411 [DOI] [PubMed] [Google Scholar]
- 7.Scaglia F, Towbin JA, Craigen WJ, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics 2004;114:925–931 [DOI] [PubMed] [Google Scholar]
- 8.Lindner A, Hofmann E, Naumann M, et al. Clinical, morphological, biochemical, and neuroadiological features of mitochondrial encephalomyopathies: presentation of 19 patients. Mol Cell Biochem 1997;174:297–303 [PubMed] [Google Scholar]
- 9.Van der Knapp M, Valk J. Magnetic resonance in mitochondrial disorders. Euromit 6; Nijmegen, the Netherlands, July2004
- 10.King TE, Howard RL. Preparation and properties of NADH dehydrogenase from cardiac muscle. In: Estabrook R, Pullman M, eds. Methods in enzymology: oxidation and phosphorylation. New York: Academic Press;1967. :275–294
- 11.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. In: Estabrook R, Pullman M, eds. Methods in enzymology: oxidation and phosphorylation. New York: Academic Press;1967. :322–331
- 12.Yonetan T. Cytochrome oxidase: beef heart. In Estabrook R, Pullman M, eds. Methods in enzymology: oxidation and phosphorylation. New York: Academic Press;1967. :332–335
- 13.Srere PA. Citrate synthase. In Lowenstein J, ed. Methods in enzymology: oxidation and phosphorylation. New York: Academic Press;1969. :3–11
- 14.Laaksonen R, Riihimaki A, Laitila J, et al. Serum and muscle tissue ubiquinone levels in healthy subjects. J Lab Clin Med 1995;125:517–521 [PubMed] [Google Scholar]
- 15.Rousseau G, Varin F. Determination of ubiquinone-9 and 10 levels in rat tissues and blood by high-performance liquid chromatography with ultraviolet detection. J Chromatogr Sci 1998;36:247–252 [DOI] [PubMed] [Google Scholar]
- 16.Liang MH, Wong L-JC. Yield of mtDNA mutation analysis in 2000 patients. Am J Med Genet 1998;77:385–400 [PubMed] [Google Scholar]
- 17.Wong L-JC, Senadheera D. Direct detection of multiple point mutations in mitochondrial DNA. Clin Chem 1997;43:1857–1861 [PubMed] [Google Scholar]
- 18.Szigeti K, Wong L-JC, Perng C-L, et al. MNGIE with lack of skeletal muscle involvement and a novel TP splice site mutation. J Med Genet 2004;41:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoffner JM. Maternal inheritance and the evaluation of oxidative phosphorylation diseases. Lancet 1996;348:1283–1288 [DOI] [PubMed] [Google Scholar]
- 20.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999;283:689–692 [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Nishigaki Y, Marti R. MNGIE: a disease of two genomes. Neurologist 2004;10:8–17 [DOI] [PubMed] [Google Scholar]
- 22.Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology 1994;44:721–727 [DOI] [PubMed] [Google Scholar]
- 23.Pavlakis S, Phillips P, DiMauro S, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes: a distinctive clinical syndrome. Ann Neurol 1984;16:481–488 [DOI] [PubMed] [Google Scholar]
- 24.Goto Y, Nonaka I, Horai S. A mutation in the tRNA Leu (UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 1990;348:651–653 [DOI] [PubMed] [Google Scholar]
- 25.Sue CM, Crimmins DS, Soo YS, et al. Neuroradiological features of six kindreds with MELAS tRNALeu A3243G point mutation: implications for pathogenesis. J Neurol Neurosurg Psychiatry 1998;65:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahl HH. Getting to the nucleus of mitochondrial disorders: identification of respiratory chain-enzyme genes causing Leigh syndrome. Am J Hum Genet 1998;63:1594–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeviani M, Carelli V. Mitochondrial disorders. Curr Opin Neurol 2003;16:585–594 [DOI] [PubMed] [Google Scholar]
- 28.Cacic M, Willichowski E, Mejaski-Bosnjak V, et al. Cytochrome c oxidase partial deficiency-associated Leigh disease presenting as an extrapyramidal syndrome. J Child Neurol 2001;16:616–619 [DOI] [PubMed] [Google Scholar]
- 29.Bakker HD, van den Bogert C, Drewes JG, et al. Progressive generalized brain atrophy and infantile spasms associated with cytochrome c oxidase deficiency. J Inher Metab Dis 1996;19:153–156 [DOI] [PubMed] [Google Scholar]
- 30.Lamperti C, Naini A, Hirano M, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology 2003;60:1206–1208 [DOI] [PubMed] [Google Scholar]
- 31.Naini A, Lewis V, Hirano M, DiMauro S. Primary coenzyme Q10 deficiency and the brain. Biofactors 2003;18:145–152 [DOI] [PubMed] [Google Scholar]
- 32.Lincke CR, van den Bogert C, Nitjmans LGJ, et al. Cerebellar hypoplasia in respiratory chain dysfunction. Neuropediatrics 1996;27:216–218 [DOI] [PubMed] [Google Scholar]
- 33.De Koning TJ, de Vries LS, Groenendaal F, et al. Pontocerebellar hypoplasia associated with respiratory-chain defects. Neuropediatrics 1998;30:93–95 [DOI] [PubMed] [Google Scholar]
- 34.Larsell O, Jansen J. The comparative anatomy and histology of the cerebellum. Minneapolis: Lund Press;1972