Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to assess supplementary motor area (SMA) activation during motor, sensory, word generation, listening comprehension, and working memory tasks by using functional MR imaging (fMRI). Human supplementary motor area (SMA) has been shown to play roles in motor control and other various functions such as sensory, speech expression, and memory. However, topographical localizations of these functions in the SMA remain unclear. The purpose of this study was to assess SMA activation during motor, sensory, word generation, listening comprehension, and working memory tasks by using functional MR imaging (fMRI).
METHODS: Sixteen healthy right-handed subjects (nine men and seven women) were imaged on a Siemens 1.5T system. Whole-brain functional maps were acquired by using blood oxygenation level–dependent echo-planar imaging sequences in the axial plane. Each paradigm consisted of five epochs of activation versus the control condition. The activation tasks consisted of left-finger complex movement, heat sensory stimulation of the left hand, word generation, listening comprehension, and working memory. The reference function was a boxcar waveform. Activation maps were thresholded at an uncorrected P = .0001. The thresholded activation maps were placed into MNI (Montreal Neurologic Institute) stereotactic coordinates, and the anatomic localization of activation within the SMA was compared across tasks.
RESULTS: SMA activation was observed in 16 volunteers for the motor task, 11 for the sensory task, 15 for the word generation task, five for the listening comprehension task, and 15 for the working memory task. Although not statistically significant, qualitative differences in the location of activation within the SMA were present by task. The rostral aspects of the SMA tended to activate during word generation and working memory tasks, and the caudal aspect of the SMA tended to activate during the motor and sensory tasks. Right (contralateral) SMA activation was observed during the motor and sensory tasks, and left SMA activation during the word generation and memory tasks.
CONCLUSION: Our results suggest that SMA is involved in a variety of functional tasks, including motor, sensory, word generation, and working memory. Some are tasks that are traditionally associated with this area (such as motor and sensory), and others are not (such as word generation and working memory). Qualitatively, the anterior and posterior portions of the SMA appeared to be engaged by different types of tasks.
The supplementary motor area (SMA) occupies the medial portion of Brodmann cortical area 6. As defined by electrical stimulation, this area is located anterior to the primary motor area of the foot and superior to the cingulate sulcus (1–5). Studies of the human SMA by using a variety of brain mapping methods, including functional MR imaging (fMRI) and positron-emission tomography (PET), have shown it to be involved in aspects of motor control, including task sequencing, intrinsic task complexity, and movement initiation (3–18). Responses elicited by electrical stimulation include bilateral movements of the extremities with assumption of characteristic postures, vocalization, sensory symptoms, aphasia, and autonomic changes (1–3). Evidence exists to indicate that the SMA is involved in various other functions, including sensory, listening comprehension, speech expression, and working memory (1–3, 18–31). The topographic relationships among areas activated by different functional tasks, however, are unclear. The purpose of this study was to assess SMA activation during motor, sensory, word generation, listening comprehension, and working memory tasks by using fMRI.
Methods
Sixteen healthy right-handed volunteers (nine men and seven women, 25–41 years old) were studied. All had English as a second language, no history of neurologic disorder, and at least a college-level education. Scanning was performed with a 1.5T whole-body MR imaging system (Vision; Siemens, Erlangen, Germany) by using a standard head coil. The participants were instructed to hold their heads still. Sponges and straps were used to stabilize the head.
Anatomic 2-mm-thick reference axial images were acquired with a magnetization-prepared rapid-acquisition gradient echo pulse sequence with parameters of 9.7 s/4 ms/1 (repetition time/echo time/excitations), a 240 × 240 field of view (FOV), and a 256 × 256 matrix. Functional MR imaging studies with 20 axial sections covering the whole brain were performed by using a multisection gradient recalled echo single shot echo-planar imaging pulse sequence. During the acquisition of the echo-planar images, five rest periods were alternated with five task periods. Each period was 21.9 seconds in duration. Functional MR images were acquired with parameters of 3599 ms/41 ms (repetition time/echo time), a 220 × 220 mm FOV, a 64 × 64 matrix, and a 6-mm section thickness. A total of 1200 images were acquired (60 images per section) during the 219 seconds required for each functional MR imaging run.
The activation tasks consisted of left-finger complex movement, heat sensory stimulation of the left hand, word generation, listening comprehension, and working memory. For complex movement, the left thumb was apposed against each of the other fingers a different number of times, twice against the index finger, once against the middle finger, three times against the ring finger, and twice against the little finger, and then repeated in reversed order (7). For the heat sensory stimulation task the investigator alternately placed and removed a glove filled with water heated to 50°C on the left palm of each subject. For the word generation task, the subject silently generated as many words as possible beginning with a presented letter for each task period. For the listening comprehension task, materials were sampled from an English-language novel. The novel was divided into five blocks, and the five blocks had story continuity. Although all of the volunteers comprehended English, their native language was Korean. The same set of language material was used in all studies. The volunteers were instructed to pay attention to the story, to understand the contents of the hearing blocks, to relax, and to make an effort to not recall or think about the contents of the sentences that had been presented (23). The two-back memory task was used to activate working memory. The stimuli consisted of numbers shown as a random sequence and displayed at the center of a screen. N-back refers to how far back in the sequence of stimuli the subject had to recall. The two-back working memory task required subjects to continually update their mental set while responding to stimuli presented two sequences earlier. The remainder of the working memory paradigm was conducted with eyes open at rest (29–31).
The SMA was defined as the area in the medial portion of the superior frontal gyrus (Brodmann cortical area 6) in front of the primary motor cortex and superior to the cingulate sulcus. The midline defined its medial limit, and its anterior boundary was defined by a line passing perpendicularly through the rostrum of the corpus callosum (1–4). The SMA was divided into a rostral and caudal aspect by the V line. The V line is a vertical line traversing the posterior margin of the anterior commissure.
The time course of the signal intensity in each pixel over 219 seconds was plotted and compared with a reference function by cross-correlation analysis. Time-series images were motion-corrected by using a realignment program. Images were normalized to a standard space by using an eight-parameter linear transformation implemented in SPM 99 (Welcome Department of Cognitive Neurology, London, United Kingdom). Normalized images were smoothed by using an 8.0-mm Gaussian kernel in SPM. The fMRI SPM 99 statistics program was used to estimate the effects of conditions at each voxel according to the general linear model. The analysis was entered as an epoch design of the fixed response/boxcar form. Activation maps were thresholded at an uncorrected P = .0001. Activated pixels on functional images were overlaid on to corresponding anatomic reference images by using an image processing program. The resulting activation maps for each subject were then standardized into MNI (Montreal Neurologic Institute) stereotactic coordinates by using SPM 99 (32–34). Axial, sagittal, and volume-rendered activation maps were generated with the group analysis tools in SPM 99.
The number of volunteers demonstrating any activation of the SMA as defined was tabulated and compared across the various tasks. The Hotelling T2 test was used to test that the spatial location of the activation maximum within the SMA differed among tasks within each subject.
Results
SMA activation was observed in 16 volunteers for the motor task, 11 for the sensory task, 15 for the word generation task, five for the listening comprehension task, and 15 for the working memory task. The coordinates of these activated regions in MNI space were computed, and the local maxima of the activation clusters inside the SMA are listed in Table 1.
Coordinates (x, y, z) represent the local activation maxima in the SMA by task for each subject in the coordinate system of the MNI atlas
| V | Mo |
Se |
Wo |
Me |
Li |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | |
| 1 | −6 | −8 | 56 | −8 | −4 | 60 | −8 | 4 | 58 | −5 | 0 | 57 | x | x | x |
| 2 | 8 | 5 | 56 | 11 | 3 | 52 | −7 | 13 | 60 | 4 | 9 | 49 | x | x | x |
| 3 | −3 | −9 | 60 | 1 | −2 | 50 | −7 | 4 | 48 | −6 | −2 | 61 | x | x | x |
| 4 | −4 | −7 | 45 | x | x | x | 1 | 20 | 46 | 3 | 11 | 54 | 5 | 8 | 54 |
| 5 | 8 | 6 | 59 | 8 | −1 | 49 | −4 | 12 | 47 | −8 | 14 | 46 | x | x | x |
| 6 | 5 | −9 | 52 | 4 | 2 | 46 | x | x | x | 2 | 18 | 47 | x | x | x |
| 7 | 6 | 0 | 44 | 6 | −7 | 47 | −5 | 0 | 44 | −4 | 0 | 52 | x | x | x |
| 8 | −4 | −4 | 61 | x | x | x | 0 | 18 | 46 | −4 | −2 | 60 | x | x | x |
| 9 | 7 | 3 | 52 | −6 | −9 | 58 | −6 | 8 | 54 | x | x | x | x | x | x |
| 10 | −2 | −4 | 47 | 7 | 4 | 48 | −2 | 6 | 57 | 6 | 2 | 46 | 8 | −7 | 50 |
| 11 | 2 | −6 | 46 | x | x | x | −7 | 1 | 68 | 7 | 4 | 60 | −4 | 0 | 60 |
| 12 | 1 | 2 | 61 | x | x | x | 8 | 16 | 54 | 8 | 8 | 47 | x | x | x |
| 13 | 5 | 5 | 62 | 6 | −5 | 52 | −4 | 23 | 50 | −3 | 8 | 55 | 2 | 6 | 57 |
| 14 | 3 | −2 | 46 | x | x | x | −1 | 3 | 52 | −6 | 4 | 54 | x | x | x |
| 15 | −6 | 1 | 47 | −5 | 4 | 55 | −8 | 6 | 46 | −8 | 13 | 44 | x | x | x |
| 16 | 6 | −4 | 48 | 1 | −12 | 60 | 4 | 7 | 47 | −7 | −3 | 51 | −6 | −8 | 58 |
| Average | 1.6 | −1.9 | 53 | 2.3 | −2.3 | 53 | −3.1 | 9.4 | 52 | −2.2 | 5.6 | 52 | |||
| Stdev | 5.1 | 5.2 | 6.6 | 6.3 | 5.5 | 5.1 | 4.7 | 7.2 | 6.7 | 5.5 | 6.6 | 5.7 | |||
Note.—V indicates volunteer; Mo, complex motor; Se, hot sensory; Wo, word generation; Me, working memory; Li, listening comprehension. The coordinates were obtained after transforming the brain images of individual studies onto the MNI atlas and are shown in millimeters. x indicates distance to right (+) or left (−) or mid sagittal line; y, distance anterior (+) or posterior (−) to vertical plane through anterior commissure; and z, distance above (+) or below (−) anterior commissure—posterior commissure line.
Motor, sensory, word generation, and working memory tasks produced SMA activation by group analysis, but the listening comprehension task did not (Fig 1). Differences in the location of activation maxima were noted qualitatively among the tasks administered, although these differences did not achieve statistical significance. The rostral aspect of the SMA tended to activate during the word generation and working memory tasks, and the caudal aspect of the SMA tended to activate during the motor and sensory tasks (Fig 2). As would be expected, predominantly right (contralateral) SMA activation was observed in the motor and sensory tasks. Predominantly left-side activation was noted for the word generation and memory tasks (P < .05; Fig 3).
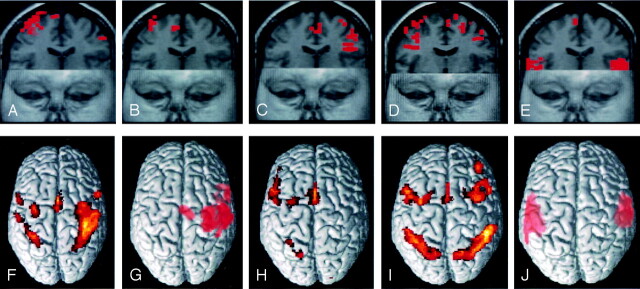
Fig 1.
Functional MR images showing activation in the supplementary motor area during motor (A), sensory (B), word generation (C), working memory (D), and listening comprehension (E) tasks in volunteer 13. Volume-rendered functional MR images showing activation in the SMA for the entire group are illustrated for the motor (F), sensory (G), word generation (H), working memory (I), and listening comprehension (J) tasks.
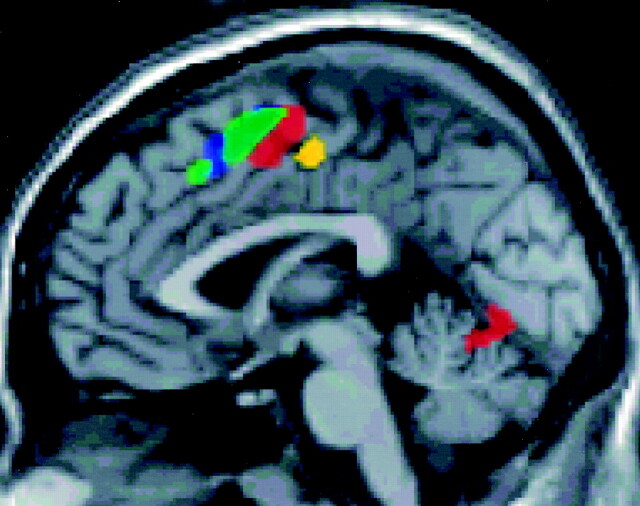
Fig 2.
Sagittal functional MR group maps of complex motor, hot sensory, listening comprehension, word generation, and working memory tasks. Functional MR images showing SMA activation during complex motor (red), heat sensory (yellow), word generation (green), and working memory (blue) tasks. The rostral aspect of the SMA tended to activate during word generation and working memory tasks, and the caudal aspect of the SMA during motor and sensory tasks. These differences, however, did not reach statistical significance.
Fig 3.
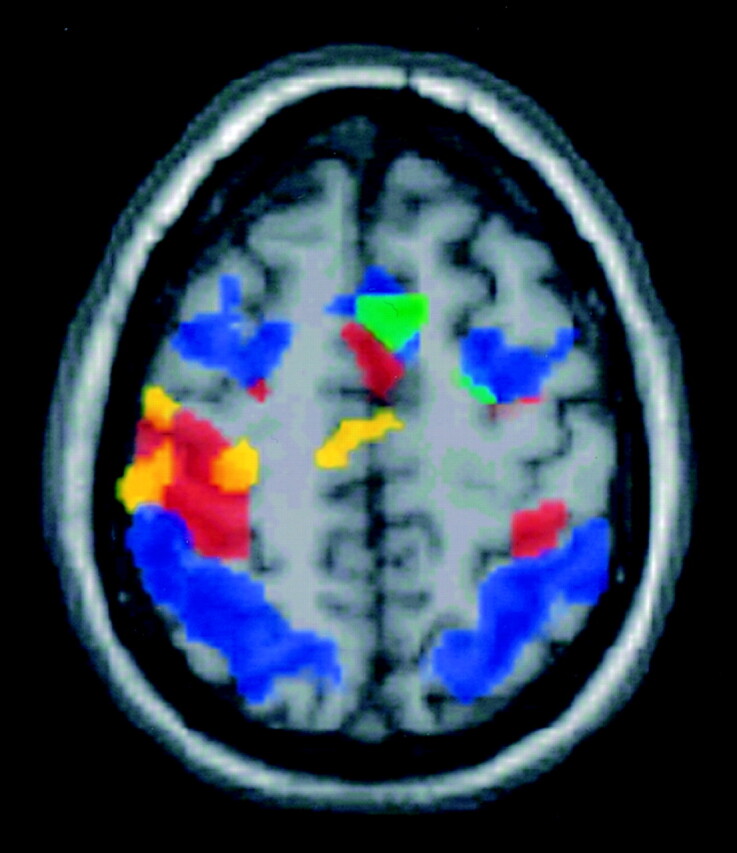
Axial functional MR group maps of complex motor (red), heat sensory (yellow), listening comprehension (magenta), word generation (green), and working memory (blue) tasks. Right (contralateral) SMA activation was observed during motor, and sensory tasks, and left SMA activation during word generation and memory tasks.
Discussion
The SMA has traditionally been defined as an area in the frontal agranular cortex, corresponding to the medial part of Brodmann cortical area 6, which is involved with complex motor and motor planning tasks. The traditionally defined SMA has more recently been divided into rostral and caudal portions. The rostral SMA is particularly active during the learning of new sequential procedures. By contrast, the caudal SMA proper is active during the performance of sequential movements (2–5, 12–15). Recent studies, including the present one, show that the SMA should no longer be regarded as a functionally homogenous area, but rather as one composed of subregions with distinct functional roles (4, 11, 17–31). During complex motor and heat sensory tasks, activation tended to occur in the contralateral posterior portion of the SMA. By contrast, the word generation and working memory tasks tended to produce activation in the anterior portion SMA particularly on the left side. The listening comprehension task produced significant activation in only five of 16 volunteers.
PET studies on the lateralization of the SMA activation during motor tasks have typically shown bilateral activation that is predominantly contralateral to the moving hand (4, 7, 14, 15, 35). The results of the present study are in concordance; SMA was activated predominantly contralaterally in 10 subjects and ipsilaterally in six subjects during complex motor tasks (Table 1). Because we examined only left-hand complex motor tasks, it was not possible to determine whether the apparent lateralization of activity within the SMA was an effect of unilateral movement or of hemispheric dominance. To clarify this, it would be necessary to repeat this experiment using the right hand.
The SMA receives input not only from the motor and premotor cortices, but also from the sensory cortex (2, 36). PET studies have shown that painful heat sensory stimuli increase regional cerebral blood flow in the contralateral SMA (22). We observed activation in the contralateral SMA in eight subjects and in the ipsilateral SMA in three of 11 subjects during heat sensory stimulation task. Areas of sensory activation in the SMA tended to occur in the same location as motor responses (i.e., posterior and contralateral) (19). Of the five subjects who showed no SMA activation during the sensory task, all showed smaller-than-average volumes of activation during the motor task, which indicates that these subjects may be generically poor fMRI responders.
Speech disturbances are observed in patients with tumors involving the SMA and also in cases of anterior cerebral artery infarction (25, 26, 37–40). In functional activation comparisons of healthy control subjects and aphasic stroke patients, the most consistent compensatory activation found in patients was located in the SMA, more prominently on the left than on the right side. Significant activation of the left SMA in aphasic stroke patients, therefore, may indicate functional reorganization of speech to the SMA, which is still intact because of its location outside the territory of the left middle cerebral artery (41). The SMA is involved in the modulation and expression of speech, speech initiation, and the maintenance of speech fluency and volume. Speech disturbance is usually observed only after resection of the dominant SMA. The role of the nondominant SMA in speech production is controversial. For example, resection of the nondominant SMA may occasionally be associated with speech dysfunction (2, 25, 37, 40–43). In cases of unilateral SMA damage, the impairment of speech is often transient and the prognosis for recovery is usually good. This may result from bilateral participation of the SMA in the generation of speech (25). We observed SMA activation in the left side in 12 subjects and in the right side in three subjects during word generation tasks (Table 1).
Nakai et al (23) examined the relationship between the level of language comprehension and brain activation by using fMRI. SMA activation was observed in both English- and Japanese-language tasks for subjects who comprehended both languages. The SMA was not activated during language tasks that were not comprehended by the subject. Demand placed on syntactic or semantic processing during listening comprehension tasks may be greater for a non-native than a native speaker of the language in question. In our study, all subjects spoke English, but as a second language. During the listening comprehension task, we observed SMA activation in the right side in three and in the left side in two of the five subjects who did activate the SMA (Fig 1J).
SMA activation was also observed in the working memory task. Prior N-back memory studies that specifically examined the relationship between working memory load and the SMA have demonstrated that increasing working memory load produces increasing left SMA activation (28–30). During the two-back working memory tasks, we observed the greatest activation in the left hemisphere, in the anterior portion of the SMA.
Conclusion
Our fMRI results suggest that significant functional heterogeneity exists within the SMA. The SMA participated in a variety of functional tasks, including motor, sensory, word generation, and working memory. Our results also support the notion that functionally specific subregions exist within the SMA.
Footnotes
Supported by Fund of Chonbuk National University Hospital Research Institute of Clinical Medicine.
References
- 1.Penfield W, Welch K. The supplementary motor area of the cerebral cortex: a clinical and experimental study. Arch Neurol Psychiatry 1951;66:289–317 [DOI] [PubMed] [Google Scholar]
- 2.Goldberg G. Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 1985;4:567–615 [Google Scholar]
- 3.Talairach J, Bancaud J. The supplementary motor area in man: anatomo-functional findings by stereo-electroencephalography in epilepsy. Int J Neurol 1966;5:330–347 [Google Scholar]
- 4.Tanji J. The supplementary motor area in cerebral cortex. Neurosci Res 1994;19:251–258 [DOI] [PubMed] [Google Scholar]
- 5.Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol 1996;6:782–787 [DOI] [PubMed] [Google Scholar]
- 6.Picard N, Strick PL. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb Cortex 2003;13:977–986 [DOI] [PubMed] [Google Scholar]
- 7.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 1980;43:118–136 [DOI] [PubMed] [Google Scholar]
- 8.Oliveri M, Babiloni C, Filippi MM, et al. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res 2003;149:214–221 [DOI] [PubMed] [Google Scholar]
- 9.Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature 1994;371:413–416 [DOI] [PubMed] [Google Scholar]
- 10.Righter W, Andersen PM, Georgopoulos AP, Kim SG. Sequential activity in human motor areas during a delayed cued finger movement task studied by time-resolved fMRI. Neuroreport 1997;8:1257–1261 [DOI] [PubMed] [Google Scholar]
- 11.Rao SM, Binder JR, Bandettini PA, et al. Functional magnetic resonance imaging of complex human movement. Neurology 1993;43:2311–2318 [DOI] [PubMed] [Google Scholar]
- 12.Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: an analysis using functional magnetic resonance imaging. J Neuroimaging 2002;12:172–178 [DOI] [PubMed] [Google Scholar]
- 13.Kansaku K, Kitazawa S, Kawano K. Sequential hemodynamic activation of motor areas and the draining veins during finger movements revealed by cross-correlation between signals from fMRI. Neuroreport 1998;9:1969–1974 [DOI] [PubMed] [Google Scholar]
- 14.Hikosaka O, Sakai K, Miyauchi S, et al. Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 1996;76:617–621 [DOI] [PubMed] [Google Scholar]
- 15.Humberstone M, Sawle GV, Clare S, et al. Functional magnetic resonance imaging of single motor events reveals human presupplementary motor area. Ann Neurol 1997;42:632–637 [DOI] [PubMed] [Google Scholar]
- 16.Weilke F, Spiegel S, Boecker H, et al. Time-resolved fMRI of activation patterns in M1 and SMA during complex voluntary movement. J Neurophysiol 2001;85:1858–1863 [DOI] [PubMed] [Google Scholar]
- 17.Cadoret G, Smith AM. Comparison of the neuronal activity in the SMA and the ventral cingulate cortex during prehension in the monkey. J Neurophysiol 1997;77:153–166 [DOI] [PubMed] [Google Scholar]
- 18.Chung GH, Han YM, Kim CS. Functional MRI of the supplementary motor area: comparison of motor and sensory tasks. J Comput Assist Tomogr 2000;24:521–525 [DOI] [PubMed] [Google Scholar]
- 19.Lim SH, Dinner DS, Pillay PK, et al. Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol 1994;91:179–193 [DOI] [PubMed] [Google Scholar]
- 20.Korvenoja A, Huttunen J, Salli E, et al. Activation of multiple cortical areas in response to somatosensory stimulation: combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp 1999;8:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakigi R. Somatosensory evoked magnetic fields following median nerve stimulation. Neurosci Res 1994;20:165–174 [DOI] [PubMed] [Google Scholar]
- 22.Adler LJ, Gyulai FE, Diehl DJ, et al. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 1997;84:120–126 [DOI] [PubMed] [Google Scholar]
- 23.Nakai T, Matsuo K, Kato C, et al. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla: comprehension level and activation of the language areas. Neurosci Lett 1999;263:33–36 [DOI] [PubMed] [Google Scholar]
- 24.Chee MW, O’Craven KM, Bergida R, et al. Auditory and visual word processing studied with fMRI. Hum Brain Mapp 1999;7:15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zentner J, Hufnagel A, Pechstein U, et al. Functional results after resective procedures involving the supplementary motor area. J Neurosurg 1996;85:542–549 [DOI] [PubMed] [Google Scholar]
- 26.Pai MC. Supplementary motor area aphasia: a case report. Clin Neurol Neurosurg 1999;101:29–32 [DOI] [PubMed] [Google Scholar]
- 27.Casey BJ, Cohen JD, O’Craven K, et al. Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage 1998;8:249–261 [DOI] [PubMed] [Google Scholar]
- 28.Coull JT, Frith CD, Frackowiak RSJ, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 1996;34:1085–1095 [DOI] [PubMed] [Google Scholar]
- 29.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebl Cortex 1999;9:20–26 [DOI] [PubMed] [Google Scholar]
- 30.Jonides J, Schumacher EH, Smith EE, et al. The role of parietal cortex in verbal working memory. J Neurosci 1998;18:5026–5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas KM, King SW, Franzen PL, et al. A developmental functional MRI study of spatial working memory. Neuroimage 1999;10:327–338 [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ. Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab 1995;15:361–370 [DOI] [PubMed] [Google Scholar]
- 33.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 1993;30:161–173 [DOI] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human barin. 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme;1988;1–122
- 35.Kawashima R, Yamada K, Kinomura S, et al. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res 1993;623:33–40 [DOI] [PubMed] [Google Scholar]
- 36.Jurgens U. The efferent and afferent connections of the supplementary motor area. Brain Res 1984;63–81 [DOI] [PubMed]
- 37.Botez MI, Barbeau A. Role of subcortical structures, and particulary of the thalamus, in the mechanisms of speech and language. Int J Neurol 1971;8:300–320 [PubMed] [Google Scholar]
- 38.Arseni C, Botez MI. Speech disturbances caused by tumours of the supplementary motor area. Acta Psychiatr Neurol Scand 1961;36:279–299 [DOI] [PubMed] [Google Scholar]
- 39.Green JR, Angevine JB, White JC Jr, et al. Significance of the supplementary motor area in partial seizures and in cerebral localization. Neurosurgery 1980;6:66–75 [DOI] [PubMed] [Google Scholar]
- 40.Laplane D, Talairach J, Meininger V, et al. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci 1977;34:301–314 [DOI] [PubMed] [Google Scholar]
- 41.Karbe H, Thiel A, Weber-Luxenburger G, et al. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang 1998;64:215–230 [DOI] [PubMed] [Google Scholar]
- 42.Larsen B, Skinhoj E, Lassen NA. Variations in regional cortical blood flow in the right and left hemispheres during automatic speech. Brain 1978;101:193–209 [DOI] [PubMed] [Google Scholar]
- 43.Rostomily RC, Berger MS, Ojemann Ga, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg 1991;75:62–68 [DOI] [PubMed] [Google Scholar]