Abstract
BACKGROUND AND PURPOSE: Our purpose was to specify the most severely affected brain structures in early treated phenylketonuria regarding volume loss and establish possible correlations between volume loss and plasma levels of phenylalanine (Phe).
METHODS: In 31 patients with early treated phenylketonuria and in 27 healthy volunteers, we acquired volumetric MR imaging data. Serum Phe concentrations at different times were measured as well. Semiautomatic volumetric postprocessing of the cerebellum, cerebrum (supratentorial brain tissue), hippocampus, intracranial volume, lateral ventricles, nucleus caudatus, nucleus lentiformis, pons, and thalamus, as well as the two-dimensional extension of the corpus callosum, was performed using the software BRAINS2. For each separate brain structure, the relative differences between the normal and the phenylketonuria group (Δrel) were calculated.
RESULTS: The cerebrum, corpus callosum, hippocampus, intracranial volume, and pons were significantly smaller in patients with phenylketonuria than in healthy patients. The volume of the lateral ventricles was significantly larger in patients with phenylketonuria than in healthy ones. The most severely affected structures were the pons (Δrel = 16%), hippocampus (Δrel = 14.5%), cerebrum (Δrel = 13%), and corpus callosum (Δrel = 10%). No significant differences were found for the basal ganglia, cerebellum, and thalamus. There were no significant correlations found between the volume of any of the different brain structures and the metabolic parameters.
CONCLUSION: The most severely affected brain structures in early-treated patients with phenylketonuria regarding volume loss are the cerebrum, corpus callosum, hippocampus, and pons.
Phenylketonuria is an inborn error of amino acid metabolism that results from mutations in the phenylalanine hydroxylase (EC 1.14.16.1) gene located on chromosome 12 at the locus 12q24.1 (1). The disease is transmitted as an autosomal recessive disorder with considerable variability in incidence among different ethnic populations. In the United States, the incidence is approximately 1 in 10,000 persons, ranging between 1/ 8000 persons among whites and 1/50,000 among African Americans (1). Phenylketonuria arises from a severe deficiency of phenylalanine hydroxylase that limits the hepatic hydroxylation of phenylalanine (Phe) to tyrosine (1). In affected patients, plasma and tissue levels of Phe and its related keto acids are elevated. Untreated patients usually exhibit epilepsy, microcephaly, severe mental retardation, and hyperactive behavior (2). Brain pathology of untreated patients is characterized by reduced brain weight and hypo- and demyelination of cerebral white matter (1). By the application of a Phe-restricted diet beginning in the first days of life, the phenotype of untreated phenylketonuria can be prevented. The aim of our MR imaging–based volumetric study was to determine which brain structures are affected in early-treated phenylketonuria regarding volume reduction. Furthermore, we searched for possible relationships between volume loss and plasma levels of Phe.
Methods
A total of 58 subjects were imaged, 31 adult patients with phenylketonuria (17 men, mean age, 30 years; 14 women, mean age 28 years) and 27 healthy volunteers (15 men, mean age, 30 years; 12 women, mean age, 27 years). The control group consisted of volunteers who were free of neurologic and other diseases involving the central nervous system such as metabolic disorders, traumatic brain injury, epilepsia, perinatal asphyxia, or drug abuse. The control group was recruited by asking friends and colleagues to acquire normal MR imaging data sets for this and other MR imaging–based studies. The data of patients and control subjects were accrued during the period from August 2001 to February 2003.
In all patients, the diagnosis of phenylketonuria was made within the first 5 days of life during routine neonatal screening for inborn errors of metabolism. Effective dietary treatment was started from day 4 to day 77 of life and was not stopped before 18 years of age. The onset of effective dietary treatment was defined as the day after birth when the serum Phe level was below 600 μmol/L for the first time. Patient treatment consisted of dietary restriction of the Phe supply to an amount that is absolutely necessary for synthesis of proteins and neurotransmitters by substitution of an amino acid composite free of Phe (Phe-free diet powder) and Phe-free food such as bread and pasta. Metabolic control of the patients complied with the recommendations of the guidelines of the German Working Group for Pediatric Metabolic Diseases (http://leitlinien.net). Accordingly, Phe levels should be in the range between 42 and 240 μmol/L until 10 years of age, between 42 and 900 μmol/L until 16 years of age, and below 1200 μmol/L in patients older than 16 years.
For the estimation of patients’ compliance, at the date of the MR examination, we asked every patient (1) whether he or she was on the diet at present, (2) how long he or she had adhered to the diet in the past, (3) whether he or she was taking Phe-free diet powder, and (4) what composed his or her daily nutrition. According to this questionnaire, the quality of the diet of the patients was categorized as being on no diet, reduced diet, or strict diet. We acquired the following additional metabolic parameters: current serum Phe concentration (enzymatic assay, Quantase Phe assay, Porton, Cambridge, UK) as measured at the day of the scanning (PheCurr, μmol/L), mean serum Phe concentration until 5 years old (Phe5, μmol/L),2 mean serum Phe concentration until 12 years old (Phe12, μmol/L).2 In all patients serum Phe concentrations were measured in intervals of at most 14 days during the first 4 years of life and, after that, in intervals of at most 60 days. Neurologic testing was performed, and intelligence quotient (IQ) according to the Hamburg-Wechsler intelligence test for adults, including verbal and performance scale was measured, in all patients. All MR images were acquired by using a 1.5T unit (EDGE, Philips Medical Systems, Highland Heights, OH) with a standard head coil. The MR imaging protocol included a transverse dual spin-echo MR imaging sequence (TR/TE, 2248/20, 90; section thickness, 6 mm; flip angle, 90°; acquisition matrix, 256 × 256; field of view, 200 mm) and a 3D sagittal gradient-echo volumetric MR imaging sequence (TR/TE, 4.4/25; section thickness, 1.5 mm; flip angle, 20°; acquisition matrix, 256 × 256; field of view, 200 mm). The sagittal gradient-echo MR image was oriented parallel to the anterior commissure–posterior commissure line. The study protocol was approved by our institutional review board, and informed consent according to the declaration of Helsinki 2000 was obtained from each subject.
Volumetric measurements
Volumetric data of the patients and the normal group were anonymized before volumetric measurements. All measurements were performed by one person (N.P.). For volumetric measurements, we used the software BRAINS2 provided by the Image Processing Lab of the University of Iowa (http://www.psychiatry.uiowa.edu/ipl) (3). The volumetric MR imaging data were reoriented according to the BRAINS2 users’ guide to account for variability in rotation, head tilt, and roll. Before calculating volumes, we also picked Talairach parameters as described in the BRAINS2 standard work-up laboratory manual. Volumes were calculated on tissue-classified images (4). Measurements were performed by using the BRAINS2 masks, which are generated by a neural network and corrected by manual tracing. Masks are defined as objects that fill the volume of an image. When displayed, a mask obscures an area on all three imaging planes. The volumes of the hippocampus (hippo; 5), nucleus caudatus (caud; 6), thalamus (thal; 7), and nucleus lentiformis (lent; 6) consisting of putamen and globus pallidus were measured according to the manual tracing guidelines provided by BRAINS2. Furthermore, we defined additional measurement protocols as follows:
Brain (brain) was defined as intracranial volume including the whole supra- and infratentorial brain tissue superior to the foramen magnum and cerebrospinal fluid. Optic nerve, chiasma opticum, pituitary gland, and mamillary bodies were excluded. For that purpose, a BRAINS2 mask was generated and corrected by manual tracing.
Cerebellar volume (cbl) was defined as the infratentorial brain tissue volume excluding the pons. Those parts of the cerebellar peduncles that were located dorsally to the coronal section in which the vestibulocochlear nerve enters the brain stem at the pontomedullary sulcus were included in the cerebellar volume. A BRAINS2 mask was generated and corrected by means of manual tracing.
Cerebrum (cbr) was defined as the entire supratentorial brain tissue volume excluding subarachnoid spaces. The subarachnoid space was excluded by manual tracing based on the “brain” mask. The infratentorial structures were excluded manually as follows: The cerebellum was separated from the supratentorial structures by drawing a line along the tentorium on each coronal section. More ventrally, on each coronal section, a separating line was drawn from the floor of the third ventricle to the hippocampal sulcus perpendicularly to the pyramidal tract.
Pons (pons) was defined as the infratentorial brain tissue volume superior to the foramen magnum excluding the cerebellar volume. The borderline to the cerebrum was drawn manually as described previously. Those parts of the cerebellar peduncles that were located rostrally to the coronal section in which the vestibulocochlear nerve enters the brain stem at the pontomedullary sulcus were included in the volume of pons.
The volume of the lateral ventricles (ventr) was completely traced manually according to UMC Utrecht 2001 guidelines (http://www.smri.nl/pdf/Brain_Morphology.pdf). Choroid plexus was excluded. In addition, we measured the two-dimensional extension of the corpus callosum in the midsagittal plane by manual tracing as described by Mitchell et al (8).
Reliability of the volumetric measurements
All measurements were performed by one person (N.P.) who had been intensively trained in cerebral volumetry before the study by S.H., a senior neuroradiologist with personal experience in more than 30,000 MR imaging studies of the brain. In addition, according to other groups (5, 6, 9–12) dealing with MR imaging–based volumetry, we calculated the intraclass correlation coefficients (SPSS reliability analysis, one-way random model) for inter- (N.P. vs S.H.) and intraobserver reliability of our measurements before, during, and after the study on 100 randomly chosen anatomic structures. Furthermore, the measurement prescriptions we used and all anatomic structures we have measured have been checked for eligibility and for visibility in previous studies (5, 6, 9).
Statistical analysis
All statistical tests, including calculation of mean, minimum, maximal values and SD for patients and controls, were performed with SPSS, version 11.0 (SPSS, Inc., Chicago, IL). The volumes of the different brain structures in the patient group were compared statistically with those of the normal group by using the Wilcoxon-Mann-Whitney U test.
For each separate brain structure with the exception of the corpus callosum, relative differences between the normal and the phenylketonuria group (Δrel) were calculated as follows: Δrel = (Volnormal –VolPKU) / Volnormal * 100%, where Volnormal is the mean volume of the brain structure of the normal group and VolPKU is the mean volume of the brain structure of the phenylketonuria group. Accordingly, the relative difference (Δrel) for the two-dimensionally measured extension of the corpus callosum was calculated. For those brain structures showing significant volume differences between the patient and the normal group, Spearman rank correlation tests were used to determine statistical correlations with metabolic parameters (PheCurr, Phe5, and Phe12) and IQ. The level of significance was set at P < .01 for all statistical tests.
Results
Clinical data
The neurologic status was normal in all patients except for subtle tremor, which was present in 13 patients. None of the patients had seizures or other complications in the history. Eleven (35.5%) of the 31 examined patients were classified as being on no diet, 10 patients (32.3%) were classified as being on reduced diet, and 10 patients (32.3%) were classified as being on strict diet. The other clinical and laboratory data are summarized in Table 1. PheCurr range was between 206 and 2603 μmol/L, which is typical for adult patients with phenylketonuria on and off diet (Table 1). The two indexes of dietary control Phe5 and Phe12 were closely correlated (r = .80, P < .0001). IQ was significantly correlated with the indexes of biochemical control Phe5 (r = −.519, P < .01) and Phe12 (r = −.463, P < .01).
TABLE 1:
Clinical and laboratory data of patients with PKU
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| IQ | 107.5 ± 18.7 | 64 | 148.0 |
| IQperf | 106.9 ± 17.7 | 76 | 149.0 |
| IQverb | 103.2 ± 16.1 | 56 | 134.0 |
| Phecurr | 1276.5 ± 490 | 205.7 | 2601.5 |
| Phe5 | 308.6 ± 102.2 | 181.5 | 570.5 |
| Phe12 | 399.3 ± 163.3 | 208.12 | 686.1 |
Note.—All volumes are given in mL. Phecurr, Phe5, and Phe12 are given in μmol/L. SD signifies standard deviation.
Volumetric measurements
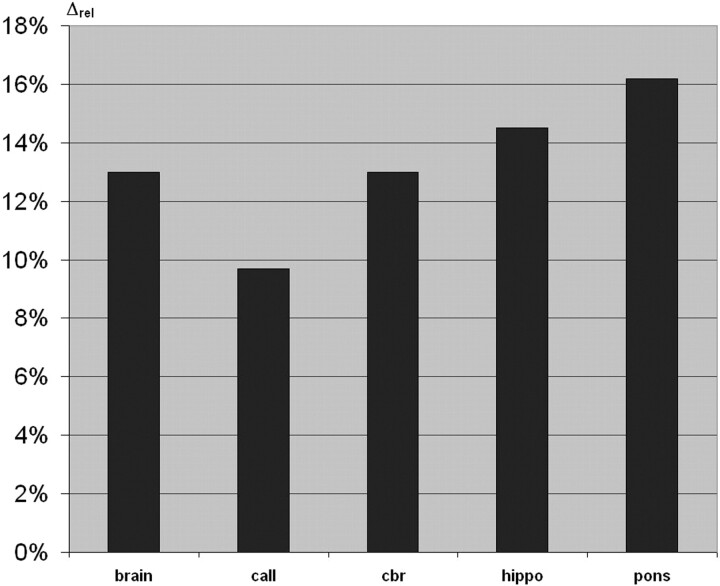
None of the MR images of the healthy volunteers showed abnormalities on visual inspection by two senior neuroradiologists (S.H., B.K.). The results of the volumetric measurements are summarized in Table 2. The strongest relative volume reduction in the phenylketonuria group with respect to the normal group was found for the pons (Δrel = 16%), hippocampus (Δrel = 14.5%), and cerebrum (Δrel = 13%) (Fig 1). In addition, the two-dimensional extension of the corpus callosum was lower by 10% in phenylketonuria with respect to normal controls (Fig 1). No significant differences were found for the nucleus caudatus, nucleus lentiformis, and thalamus. For the lateral ventricles, the relative difference between the phenylketonuria and the normal group was −8.8%. A significant correlation was found only between IQverb and the hippocampus (r = 0.471, P < .01) but not between the volume of any of the different brain structures and the metabolic parameters.
TABLE 2:
Volumetric data of the analyzed brain structures and two-dimensional extension of the corpus callosum, PKU vs control group
| Normal |
PKU |
P Value | |
|---|---|---|---|
| Mean value ± SD | Mean value ± SD | ||
| brain | 1674.2 ± 99.7 | 1460.32 ± 179.72 | <.001 |
| cbr | 1101.1 ± 95.5 | 960.64 ± 117.45 | <.001 |
| cbl | 140.3 ± 11.3 | 132.30 ± 9.35 | ns |
| pons | 34.9 ± 6.9 | 29.27 ± 6.38 | <.005 |
| call | 6.31 ± 0.74 | 5.70 ± 0.71 | <.005 |
| hippo | 6.13 ± 1.43 | 5.24 ± 0.80 | <.001 |
| caud | 7.42 ± 0.85 | 7.32 ± 1.01 | ns |
| lent | 9.96 ± 0.79 | 9.90 ± 0.70 | ns |
| thal | 11.75 ± 0.67 | 12.10 ± 0.88 | ns |
| ventr | 12.50 ± 2.15 | 13.71 ± 1.75 | <.005 |
Note.—All volumes are given in mL; call is given in cm2; ns, no significance. Abbreviations identified in “Volumetric measurements” section.
Fig 1.
Bar graph shows relative volume differences of several brain structures and relative difference of two-dimensional extension of the corpus callosum (call) in phenylketonuria versus the normal group. Pons and hippocampus (hippo) are the most severely affected structures. cbr = cerebrum.
Reliability of the volumetric measurements
Intraclass correlation coefficients for inter- and intraobserver reliability of our measurements were 0.97 for all structures, which indicate an excellent intra- and interobserver agreement.
Discussion
The most constant pathologic change in untreated phenylketonuria is a reduction in the weight of the brain down to 80%–90% of the average for age (13). It is still not known whether this reduced brain size mainly reflects a deficiency in neuronal cell bodies, dendrites, axons, or glial cells (2, 14). The major histopathologic changes in untreated phenylketonuria are (1) hypomyelination and gliosis of the white matter systems that myelinate late, (2) infrequent occurrence of progressive white matter destruction, and (3) developmental delay or arrest in cerebral cortex (14). Changes in cortical layering, tissue mass atrophy, and reduced dendritic arborization have been reported (13, 15). The number and spread of dendritic basilar processes of large pyramidal cells are reduced in hyperphenylalaninemical rat pups (14).
Typical neurologic and neuropsychological manifestations even in patients who received dietary treatment early in life include subtle deficits in cognition, problem solving, and motor coordination. The only other frequently observed neurologic finding is subtle tremor (1, 16). Epilepsy, spastic paraparesis, and dementia have been observed in sporadic cases (16). Despite the fact that primarily cerebral white matter is involved in phenylketonuria, many of the clinical manifestations of phenylketonuria are more typically those of cerebral gray matter than of white matter diseases. Especially the presence of cognitive deficits and epilepsy suggests dysfunction of the late-maturing regions of cerebral neocortex (13).
The typical MR imaging features of early treated phenylketonuria are hyperintensities on T2-weighted images that are located mainly in the occipital-parietal regions; in more severe cases, they may extend into the frontal and parietal lobes, including the arcuate fibers (17). An abnormal MR signal intensity is most markedly found in the Phe-sensitive oligodendrocytes, which are located in brain areas that normally myelinate postpartum (eg, in the subcortical and periventricular white matter, in the corpus callosum, in the optic tract, and in the forebrain). Rarely, the basal ganglia, brain stem, and cerebellum are affected (18–20). In contradiction to other groups (18, 21), in the study of Pietz et al (22), the extent of these hyperintensities has been shown to be correlated with metabolic control established by the plasma Phe values and with neuropsychological testing but not with IQ or visually evoked potentials.
There are reports about improvement of the MR imaging, changes over weeks after the reintroduction of dietary treatment, and the lowering of plasma Phe concentrations (23). In a study of early treated patients with phenylketonuria who were systematically evaluated by using MR imaging, Pietz et al (22) reported marked diffuse cortical atrophy in 3 (5.9%) of 51 patients. Except for increased Phe levels, however, MR spectroscopy revealed normal metabolic levels both in cerebral gray and white matter in that study (22). The link between pathology, MR imaging, and MR spectroscopy findings in phenylketonuria and clinical features is not clearly understood yet. This might be one reason for the fact that treatment policies for phenylketonuria are quite different among European countries, ranging from the French practice to discontinue diet at 5 years of age to the British recommendation for a lifelong diet (16). Treatment policies in the United States and Canada reveal similar inconsistencies (24). According to the German guidelines, our patient group underwent dietary treatment until the age of 18 years.
On the basis of other, nonquantitative imaging studies (14, 25–27), we found that the volume of the cerebrum (supratentorial brain tissue) and the intracranial volume are significantly reduced in early treated patients with phenylketonuria compared with healthy controls. Furthermore, our study shows that the volume of predominantly white matter structures (ie, the pons but also of the hippocampus, a predominantly gray matter structure) is reduced in early treated patients with phenylketonuria. In addition, the extension of the corpus callosum as measured two-dimensionally was smaller in patients with phenylketonuria than in healthy controls. To the best of our knowledge, however, it has not been previously reported that the size of the hippocampus is reduced in phenylketonuria. It could be speculated that this finding might be the morphologic correlate for impaired memory function in these patients. For other mainly gray matter structures such as the nucleus caudatus, nucleus lentiformis, and thalamus, we did not find a statistically significant difference. Our measurements revealed that not only supratentorial structures, but also the pons, were affected.
According to the results of Rupp et al (28) and Pietz et al (29), who found a strong linear correlation between blood Phe values and brain Phe values, a correlation between blood Phe values and brain volume could be expected, too; however, we found no such correlation between blood Phe values and brain volume. This lack of correlation in our study could be explained by the fact that the impact of Phe on brain development is most marked in the early years of life and blood-brain transport of Phe could be different in different patients. Our findings are supported by Koch et al (30) and Weglage et al (31), who found no correlation between blood and brain Phe levels and therefore favor an individual blood-brain barrier Phe transport system. Our finding that mean serum Phe concentration until 5 years of age and mean serum Phe concentration until 12 years of age correlated well with IQ is a well-known phenomenon: According to the data of Smith et al (32), each rise of blood Phe of 300 μmol/L between 5–8 years of age results in a fall in IQ of 4–6 points. The quite normal IQ values in the phenylketonuria group must be attributed partly to repeated testing effects: 8 years ago, the same 31 patients had been tested in another study (16), and a mean IQ of 97.8 was observed. In addition, brain volume reduction in phenylketonuria might be the result of a general malnutrition of proteins, minerals, and vitamins due to the dietary treatment (33, 34).
Further study is needed to determine the relationship between volumetric MR data, MR imaging, and clinical parameters in patients with phenylketonuria. Brain tissue volumetry performed before and after the cessation of the dietary treatment could clarify whether volume loss occurs in phenylketonuria after diet cessation.
Conclusion
The most severely affected structures with respect to volume loss in patients with phenylketonuria treated early in life are the cerebrum, corpus callosum, hippocampus, and pons. These volumetric findings may help to determine whether patients with phenylketonuria should be recommended to receive lifelong dietary treatment.
References
- 1.Scriver CR, Kaufman S, Eisensmith RC, Woo SLC. The hyperphenylaninemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. Metabolic basis of inherited disease. Vol 1. 17th ed. New York, NY: McGraw-Hill;1995:1015–1075 [Google Scholar]
- 2.Brenton DP, Pietz J. Adult care in phenylketonuria and hyperphenylalaninaemia: the relevance of neurological abnormalities. Eur J Pediatr 2000;159 (suppl 2):S114–S120 [DOI] [PubMed] [Google Scholar]
- 3.Magnotta VA, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph 2002;26:251–264 [DOI] [PubMed] [Google Scholar]
- 4.Harris G, Andreasen NC, Cizadlo T, et al. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999;23:144–154 [DOI] [PubMed] [Google Scholar]
- 5.Pantel J, O’Leary DS, Cretsinger K, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus 2000;10:752–758 [DOI] [PubMed] [Google Scholar]
- 6.Magnotta VA, Heckel D, Andreasen NC, et al. Measurement of brain structures with artificial neural networks: two- and three-dimensional applications. Radiology 1999;211:781–790 [DOI] [PubMed] [Google Scholar]
- 7.Spinks R, Magnotta VA, Andreasen NC, et al. Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging. Neuroimage 2002;17:631–642 [PubMed] [Google Scholar]
- 8.Mitchell TN, Free SL, Merschhemke M, et al. Reliable callosal measurement: population normative data confirm sex-related differences. AJNR Am J Neuroradiol 2003;24:410–418 [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr A, Knauth M, Weisbrod M, et al. [Similarity of the brains of twins]. Rofo 2001;173:515–521 [DOI] [PubMed] [Google Scholar]
- 10.Okugawa G, Takase K, Nobuhara K, et al. Inter- and intraoperator reliability of brain tissue measures using magnetic resonance imaging. Eur Arch Psychiatry Clin Neurosci 2003;253:301–306 [DOI] [PubMed] [Google Scholar]
- 11.Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging 2002;16:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnack HG, van Haren NE, Hulshoff Pol HE, et al. Reliability of brain volumes from multicenter MRI acquisition: a calibration study. Hum Brain Mapp 2004;22:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer CA. Comments on the neuropathology of phenylketonuria. Eur J Pediatr 2000;159(suppl 2):S107–S108 [DOI] [PubMed] [Google Scholar]
- 14.Huttenlocher PR. The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 2000;159 (suppl 2):S102–S106 [DOI] [PubMed] [Google Scholar]
- 15.Dyer CA, Kendler A, Philibotte T, et al. Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol 1996;55:795–814 [DOI] [PubMed] [Google Scholar]
- 16.Pietz J. Neurological aspects of adult phenylketonuria. Curr Opin Neurol 1998;11:679–688 [DOI] [PubMed] [Google Scholar]
- 17.Ullrich K, Moller H, Weglage J, et al. White matter abnormalities in phenylketonuria: results of magnetic resonance measurements. Acta Paediatr Suppl 1994;407:78–82 [DOI] [PubMed] [Google Scholar]
- 18.Thompson AJ, Tillotson S, Smith I, et al. Brain MRI changes in phenylketonuria: associations with dietary status. Brain 1993;116:811–821 [DOI] [PubMed] [Google Scholar]
- 19.Cleary MA, Walter JH, Wraith JE, et al. Magnetic resonance imaging of the brain in phenylketonuria. Lancet 1994;344:87–90 [DOI] [PubMed] [Google Scholar]
- 20.Pearsen KD, Gean-Marton AD, Levy HL, Davis KR. Phenylketonuria: MR imaging of the brain with clinical correlation. Radiology 1990;177:437–440 [DOI] [PubMed] [Google Scholar]
- 21.Desmond DW. Cognition and white matter lesions. Cerebrovasc Dis 2002;13 Suppl 2:53–57 [DOI] [PubMed] [Google Scholar]
- 22.Pietz J, Kreis R, Schmidt H, et al. Phenylketonuria: findings at MR imaging and localized in vivo H-1 MR spectroscopy of the brain in patients with early treatment. Radiology 1996;201:413–420 [DOI] [PubMed] [Google Scholar]
- 23.Cleary MA, Walter JH, Wraith JE, et al. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J Pediatr 1995;127:251–255 [DOI] [PubMed] [Google Scholar]
- 24.Brenton DP, Tarn AC, Cabrera-Abreu JC, Lilburn M. Phenylketonuria: treatment in adolescence and adult life. Eur J Pediatr 1996;155 (suppl 1):S93–S96 [DOI] [PubMed] [Google Scholar]
- 25.Hanley WB, Feigenbaum AS, Clarke JT, et al. Vitamin B12 deficiency in adolescents and young adults with phenylketonuria. Eur J Pediatr 1996;155 (suppl 1):S145–S147 [DOI] [PubMed] [Google Scholar]
- 26.Villasana D, Butler IJ, Williams JC, Roongta SM. Neurological deterioration in adult phenylketonuria. J Inherit Metab Dis 1989;12:451–457 [DOI] [PubMed] [Google Scholar]
- 27.Leuzzi V, Trasimeni G, Gualdi GF, Antonozzi I. Biochemical, clinical and neuroradiological (MRI) correlations in late-detected PKU patients. J Inherit Metab Dis 1995;18:624–634 [DOI] [PubMed] [Google Scholar]
- 28.Rupp A, Kreis R, Zschocke J, et al. Variability of blood-brain ratios of phenylalanine in typical patients with phenylketonuria. J Cereb Blood Flow Metab 2001;21:276–284 [DOI] [PubMed] [Google Scholar]
- 29.Pietz J, Rupp A, Burgard P, et al. No evidence for individual blood-brain barrier phenylalanine transport to influence clinical outcome in typical phenylketonuria patients. Ann Neurol 2002;52:382–383; reply 383–384 [DOI] [PubMed] [Google Scholar]
- 30.Koch R, Moats R, Guttler F, et al. Blood-brain phenylalanine relationships in persons with phenylketonuria. Pediatrics 2000;106:1093–1096 [DOI] [PubMed] [Google Scholar]
- 31.Weglage J, Wiedermann D, Denecke J, et al. Individual blood-brain barrier phenylalanine transport determines clinical outcome in phenylketonuria. Ann Neurol 2001;50:463–467 [DOI] [PubMed] [Google Scholar]
- 32.Smith I, Beasley MG, Ades AE. Effect on intelligence of relaxing the low phenylalanine diet in phenylketonuria. Arch Dis Child 1991;66:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swayze VW 2nd, Andersen AE, Andreasen NC, et al. Brain tissue volume segmentation in patients with anorexia nervosa before and after weight normalization. Int J Eat Disord 2003;33:33–44 [DOI] [PubMed] [Google Scholar]
- 34.Arnold GL, Vladutiu CJ, Kirby RS, et al. Protein insufficiency and linear growth restriction in phenylketonuria. J Pediatr 2002;141:243–246 [DOI] [PubMed] [Google Scholar]