Abstract
BACKGROUND AND PURPOSE: Neonates with vein of Galen aneurysmal malformations (VGAMs) presenting with cardiac failure have high morbidity and mortality, and outcomes are significantly better in those presenting in later childhood. Neurologic outcomes in survivors are perceived to be uniformly poor, which may lead to the neonate being denied treatment. We assessed outcomes of modern neonatal intensive care and endovascular embolization in a consecutive series of such neonates presenting with cardiac failure.
METHODS: Between 1996 and 1998, five infants (three male, two female) were diagnosed with symptomatic VGAMs in the first week of life, four of whom had intractable, high-output cardiac failure and underwent initial endovascular treatment. There were 15 endovascular procedures and one neurosurgical clipping in these five patients. Transarterial and transvenous routes were required, using multiple embolic agents. We emphasized the use of sonographically guided, percutaneous transtorcular-venous–access, moveable-core guidewire as an embolic agent; routine MR imaging; and MR angiography.
RESULTS: Immediate outcomes included control of cardiac failure with normal neurologic function in four (80%) patients and one (20%) death from intractable cardiac failure. On follow-up examination, three (60%) infants showed no evidence of neurologic abnormality or cardiac failure; one (20%) infant showed moderate developmental delay. Two have had no further shunting on angiography, one has minimal flow, and one is awaiting follow-up imaging.
CONCLUSION: Endovascular therapy with modern neuroanesthetic and neurointensive care can provide good outcomes even in the highest-risk neonates with VGAMs and cardiac failure. If medical management of cardiac failure fails, and there is no evidence of gross cerebral parenchymal damage on imaging, urgent endovascular treatment is feasible and can reduce the almost-100% mortality otherwise expected, without invariably severe morbidity. Use of multiple embolization strategies in multiple stages usually is necessary in these patients, and novel approaches and embolic agents may be necessary.
Vein of Galen aneurysmal malformations (VGAMs) are rare congenital abnormalities that can cause severe morbidity and mortality, particularly in neonates but also in infants and older children (1−8). Its presentation in neonates usually includes high-output cardiac failure, most often fatal despite the best medical management (3, 4, 9). Surgery offers little improvement, with fatal outcomes in 80% to 100% of cases (3, 4, 10, 11). Consequently, there has been reluctance to treat these patients, because of perceived unacceptable morbidity likely to follow the rare successes in treating the cardiac failure. Results have improved markedly over recent years with endovascular management in infants and children, but mortality and morbidity remain high in the neonatal group, with mortality ranging from 23% to 75% and morbidity from 21% to 88% in larger series (7, 11−14). Morbidity can be reduced by selecting patients with no evidence of cerebral parenchymal damage or severe multisystem failure (6, 7, 11), although mortality can be increased in the neonatal period if no treatment is offered. In some patients with persistent cardiac failure, multisystem failure can be prevented by urgent endovascular treatment; these patients still may have an acceptable neurologic outcome. The purpose of our study is to present a consecutive series of neonates with VGAMs, of whom four of five required emergency endovascular embolization to control heart failure. Novel aspects include preoperative MR angiography and imaging, the use of guidewire as an embolic agent, and direct, sonographically guided percutaneous puncture of the dural venous sinuses.
Methods
The clinical details of the five consecutive cases, diagnosed between 1996 and 1998, are summarized in Table 1. All patients presented with cardiac failure. Seizures were present at diagnosis in one case, but none presented with intracranial hemorrhage or hydrocephalus. Associated anomalies included a sinus venosus defect detected by echocardiography. All patients were diagnosed with VGAMs by transcranial sonography; four patients also underwent MR imaging and angiography to detail the anatomy. One patient, diagnosed by antenatal sonography, developed pericardial effusion, cardiac failure, and a dilated right ventricle and atrium. All VGAMs were classified as choroidal (15) or type 3 (16), as would be expected in perinatal presentation with cardiac symptoms (Fig 1).
TABLE 1:
Summary of clinical data for five neonates treated for VGAMs presenting with cardiac failure
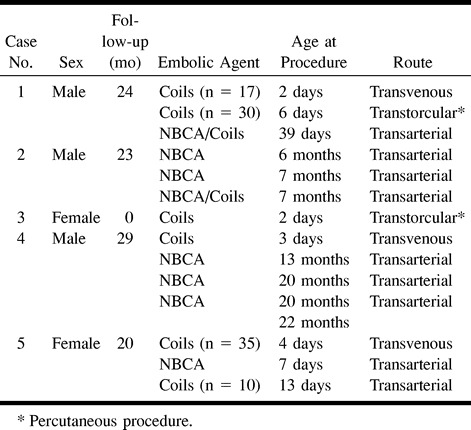
fig 1.
Representative case of VGAM in a neonate with cardiac symptoms.
A, Lateral MR angiography immediately after birth shows numerous dilated arteries and a large VGAM centrally, draining by high-positioned falcine sinus (*) into patent transverse sinuses. Note anterior cerebral artery contribution (arrow) via subfornical branch and markedly dilated posterior choroidal arteries (arrowhead).
B, Frontal projection of the MR angiography data gives more perspective on the contribution of deep perforating arteries.
C, Lateral projection shows transfemoral transvenous catheterization of the VGAM, with the catheter tip within the aneurysmal component. The injection follows partial occlusion from this transvenous approach, with coil mass seen anteriorly, some coils projecting posteriorly into the falcine sinus. Both transverse sinuses are patent.
D, Lateral projection after transarterial embolization, with glue casts seen in posterior choroidal branches and the cast extending into the malformation. Further transvenous approach is achieved with a larger (4F) catheter than is typically used, which enables more thrombogenic, larger coils to be used and coaxial embolization with platinum microcoils. Catheter tip (arrow) marks the posterior limit of the coil mass.
Detailed Case History Highlighting Specific Procedural Issues
A normal pregnancy was followed by normal vaginal delivery of a male infant (case 1). On day 2, cardiac failure developed with tachypnea, hypoxia, and cardiomegaly. Transcranial Doppler sonography showed a VGAM, 3 cm in diameter. MR imaging and angiography showed a large, midline varix with a diameter of 3 cm, an absent straight sinus, and an enlarged falcine sinus, which drained into bilaterally patent transverse sinuses and patent internal jugular veins. Bilateral supply from the posterior choroidal arteries (medial and lateral), anterior cerebral arteries (distal subfornical branch), anterior choroidal arteries, and transdiencephalic perforating arteries allowed classification as choroidal VGAM. Supply from the middle cerebral artery was impossible to prove on the MR angiography but subsequently was shown on digital subtraction angiography (DSA). There was no evidence of major cerebral parenchymal damage, hydrocephalus, or hemorrhage. Cardiac failure was treated with dopamine, furosemide, and enalapril but was not controlled. The multidisciplinary team agreed that an endovascular approach offered the best possible outcome. We fully discussed the procedure, alternatives, and risks with his parents, who provided informed consent.
The patient was transferred for angiography with femoral venous access in place, and because of the large number of arteries involved in this unstable neonate, transvenous embolization was planned as the first stage. A 4F catheter was navigated to the sigmoid sinus, but coaxial passage of a Tracker-18 failed until the larger catheter was repositioned at the torcular herophili. Embolization then was performed with a mixture of fibered and nonfibered coils: three 30 × 100-mm, eight 7 × 70-mm complex cloverleaf, five 6 × 60-mm complex spiral, and one 14 × 300-mm Guglielmi detachable coil (GDC)-18. The endpoint was a sustained increase in systemic blood pressure.
By day 4, the requirement for inotropic agents had decreased and urinary output was good. By day 5, however, cardiac failure had worsened markedly and the patient required emergency embolization. Neither arterial nor venous peripheral access could be obtained. Using a small sonography probe (ATL, 7.5-MHz probe) over the posterior fontanelle, a freehand puncture of the torcular was performed with an open 20-gauge needle, and a 4F catheter was placed in the falcine sinus using the Seldinger technique. The position was confirmed with contrast injection, showing a high flow rate and no venous occlusions. A Tracker-18 catheter was placed, and the remaining stock of microcoils that had been procured in the days after the initial treatment were used: five 5 × 300-mm, five 7 × 70-mm, three 3 × 30 mm, a Tornado 5/2 mm, and two 14 × 300-mm GDC coils. This was insufficient to correct the cardiac failure. Use of further GDCs was considered impractical, because the detachment time was excessive in the presence of Gianturco coils (>1 hour, requiring force to separate), and there was still very high flow through the falcine sinus. Through a guide catheter, we inserted a moveable core guidewire (Cook, Australia) soaked in thrombin (total length, 200 cm), because it was the only available material of sufficient volume and length to remain in the VGAM, soft enough to be unlikely to perforate the malformation, and removable if the coil mass inadvertently migrated distally. This was successful in slowing flow and increasing blood pressure, and the patient returned to intensive care. At 2 weeks of age, he was extubated on room air, feeding well, and gaining weight, with moderate ventriculomegaly but stable head circumference, requiring only diuretics.
On day 39, he returned with worsening cardiac failure. Through left femoral arterial access, a Fastracker-18 was placed without a guiding catheter into the left vertebral artery, left posterior choroidal branch. Placement of a 2 × 40-mm fibered platinum coil slowed flow through the large fistula, allowing embolization with n-butyl 2-cyanoacrylate (NBCA) diluted with iodized oil (lipiodol); supply from a posterior branch of the middle cerebral artery also was embolized with NBCA-lipiodol. Catheterization of the very tortuous anterior cerebral artery or contralateral posterior choroidal arteries was impossible within contrast limits, and terminal basilar perforators were too small for safe embolization. Cardiac failure was controlled, and the patient left the hospital shortly thereafter.
At 15 months, there was no evidence of cardiac failure, he did not require any cardiac medications, and he had normal head circumference and neurologic development. There was no imaging evidence of brain parenchymal damage. Follow-up DSA at 14 months showed no arteriovenous shunting, but prominent terminal basilar perforators still were present.
Results
There were 15 endovascular procedures and one surgical clipping in the five patients; all of the survivors had more than one procedure (median, three). The timing of the first procedure was determined by clinical state, and occurred between days 2 and 4 in the four cases of refractory cardiac failure (six procedures within first week of life; median age at first procedure, 3 days). All patients treated in the first week of life were treated because of cardiac failure that worsened despite maximal medical therapy. The only patient not treated when diagnosed with cardiac failure (features of cardiac failure noted by antenatal sonography) was managed medically until 6 months of age, when a series of three transarterial treatments was performed. This patient had severe stenosis of the main venous outflow through the falcine sinus from the median vein of the prosencephalon, which would have decreased the flow.
Four of five patients underwent staged embolization, the interval depending on the postembolization anatomic and clinical results. A single procedure was performed in the one patient who died in this series. Transarterial embolization was the preferred route of treatment, used alone or with other approaches in nine (60%) of 15 procedures; the preferred agent was NBCA, used alone (seven procedures) or with coils (two procedures [Table 2]). The embolization was performed through a 4F catheter alone (venous), a coaxial system with microcatheter (venous and arterial), or a microcatheter alone (arterial). Various embolic agents were used with the transvenous approach, including fibered and nonfibered platinum microcoils of varying diameter and configuration (from 30 × 100 mm to 3 × 300 mm), GDCs, Gianturco .035-in steel coils, moveable-core guidewire cut into lengths, and silk sutures.
TABLE 2:
Endovascular approach for embolization of vein of Galen malformations
Heart failure was well controlled in three of the four patients treated in the first week of life, although relapse required further staged embolization in two cases. The one infant who died had asystolic cardiac arrest during transtorcular venous embolization, in whom gross cardiac failure, associated with persistent pulmonary hypertension, never was adequately controlled.
Three patients now have no evidence of developmental delay or heart failure; one with sinus venosus defect awaits elective surgical correction. The other survivor (case 4) has moderate developmental delay at 15 months of age (Table 3). In this patient, initial transvenous embolization resulted in good control of cardiac failure, and the next embolization was to be performed at 6 months of age with regular clinical assessment until then. However, the next treatment occurred at month 13, with difficult vascular access limiting treatment to embolization of a single large pedicle. Over the next 1 to 2 months, it became apparent that milestones were not being reached; there were recurrent, severe seizures; and a shunt procedure was performed for persistent hydrocephalus. Imaging revealed significant enlargement of CSF spaces, some parenchymal calcification, and hypodensity consistent with venous hypertension and ischemia. After prolonged discussions with the family about the long-term prognosis, further embolization was undertaken in an attempt to control the seizures and prevent further deterioration. Clinicians noted improvement, which was considered marked by the family, and performed a fourth embolization of arterial feeders followed by surgical clipping of the remaining anterior cerebral artery supply.
TABLE 3:
Clinical outcomes after embolization
Final angiograms showed complete (two cases) or near-complete (two cases) angiographic obliteration in the four survivors. This closure rate is good, given the contribution of transdiencephalic feeders in these cases, but must await correlation with longer-term follow-up and clinical outcomes. The frequent combination of transarterial and transvenous embolization, and attempts to place NBCA from the arterial to intra-VGAM position, likely allowed closure even with significant contribution from perforators that were not embolized because of perceived high risk.
Discussion
Several proposed classification systems have been used to describe malformations of the vein of Galen (15–18). Of note, Lasjaunias and colleagues (15) have separated VGAMs from vein of Galen aneurysmal dilatations (VGADs)—the latter have a parenchymal arteriovenous malformation that drains through the vein of Galen, and patients with VGADs more often present with intracranial hemorrhage. Further separation of VGAMs into choroidal and mural types is based on the vasculature; choroidal VGAMs (the subject of this study) usually emerge in the neonatal period with cardiac failure; with abundant, usually bilateral blood supply from choroidal arteries and pericallosal arteries; and commonly with additional supply from transdiencephalic or transmesencephalic perforating vessels (usually thalamoperforating vessels) (15, 17). Middle cerebral artery supply, as seen in our case, is uncommon but more likely in the neonate than in older infants (17). The fistulas are extracerebral, in the velum interpositum.
In contrast, the blood supply for mural VGAMs comes from collicular and posterior choroidal arteries, with fistulas on the wall of the malformation draining into the median vein of the prosencephalon and then to the dural sinuses (15). Venous anomalies have been highlighted. The presence of a persistent falcine sinus and postulated development around the 10th intrauterine week indicate that the anomaly represents a persistent median prosencephalic vein of Markowski, with absent development of a normal vein of Galen (17, 19–22). Postulated causes of cerebral damage are reduced cerebral perfusion secondary to venous hypertension, abnormalities of CSF reabsorption leading to hydrocephalus and elevated intracranial pressure, and brain herniations (7, 10, 23) with the fetal venous anatomy contributing to the development of venous watershed ischemia (24, 25). Antenatal diagnosis has been reported, most commonly with transcranial sonography (26–28) but more recently with fetal MR imaging (26, 27). Antenatal diagnosis has been associated with improved outcomes in survivors (29).
In our series, we diagnosed aneurysm of the vein of Galen in all cases by transcranial sonography with color Doppler, which can quantify flow velocity, size the “aneurysm,” and provide a useful baseline for follow-up. We also performed MR imaging and MR angiography in four cases, which allowed classification as VGAM rather than VGAD and indicated the major vessels of supply (including perforators), tortuosity of arterial access, venous anatomy, and parenchymal/ventricular status (Fig 2). Performing MR imaging and MR angiography in the diagnostic phase aids in early endovascular intervention; the reduced need for diagnostic angiographic runs minimizes the duration of the procedure and amount of contrast material injected. Several reports describe the use of MR imaging and, more recently, MR angiography to monitor results of therapy (30–32). About 25 cases have reported MR imaging of neonates with VGAMs before intervention (19, 26, 27, 30, 31, 33–37) but only one case has reported preoperative MR angiography in such neonates (30).
fig 2.
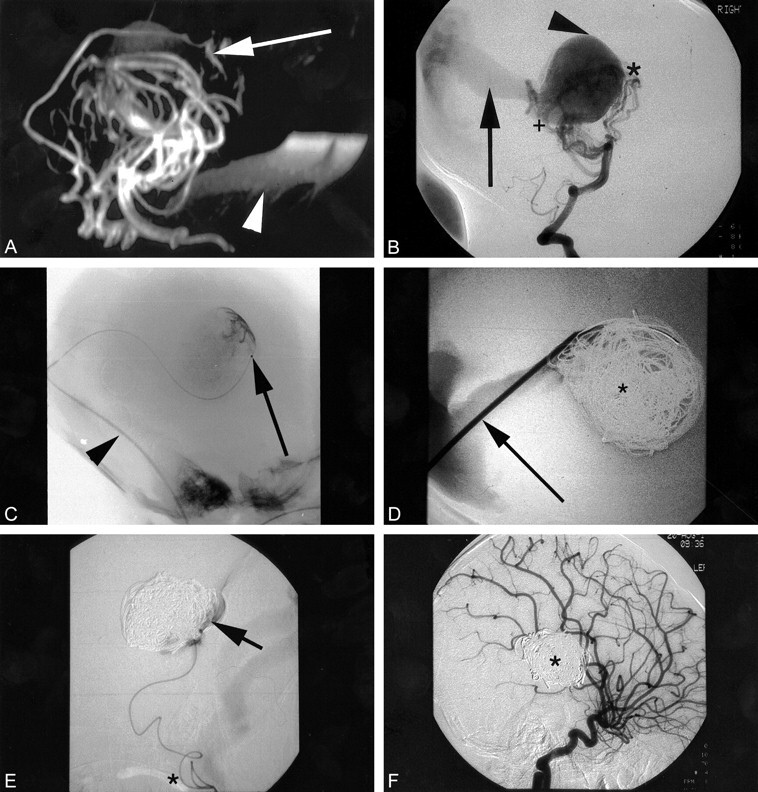
MR angiography in another neonate (case 1) with VGAM and cardiac symptoms.
A, Lateral MR angiography immediately after birth shows numerous dilated arteries and a large VGAM centrally (not seen well in this image, better shown on T2-weighted images). Note significant contribution (arrow) from subfornical branch, as in figure 1.
B, Lateral projection of a DSA run, injecting into the vertebral artery, shows good correlation with the MR angiography. Note the perforating vessels from the tip of the basilar artery and adjacent P1 segments of the posterior cerebral arteries, as well as the posterior choroidal supply.
C, Lateral projection shows transfemoral transvenous catheterization of the VGAM, guide catheter (arrowhead) passing to “torcular,” with microcatheter passed through this into the VGAM itself (arrow). Note the faint opacification of the VGAM.
D, Lateral projection shows direct percutaneous puncture of the torcular with the catheter (arrow) placed along falcine sinus into the central portion of the VGAM. The coil mass shown (*) comprises previous platinum coils from the transfemoral transvenous approach and the guidewire sections soaked in thrombin deployed as an embolic agent.
E, Lateral projection shows transarterial route through vertebral, basilar, posterior cerebral and posterior choroidal arteries. Note the extreme tortuosity of vessels (*) resulting from long-standing arteriovenous shunting and high flow. The tip of the catheter has been placed through the fistula into the malformation; contrast (arrow) confirms the position. NBCA can then be deployed, occluding the fistula and adjacent terminal portions of the artery and coil mass.
F, Delayed follow-up imaging, lateral projection, shows late arterial-phase carotid injection with no evidence of shunting. Coil mass shown subtracted (*).
Rodesch et al (29) reported on 16 newborns presenting with systemic cardiac manifestations as the first clinical symptoms of VGAM. Of these, 12 were managed effectively by digitalis diuretic treatment, embolized in infancy with 67% survival and normal neurologic development. The four infants not treated died shortly after birth from acute heart or multiorgan failure with extensive brain damage. The authors stressed the importance of transarterial embolization with NBCA and avoiding treatment in the neonatal period. Our results in neonates with VGAMs (four definite and one probable choroidal type) are comparable with those in recent series: 60% lacked neurologic impairment, 20% had moderate impairment, and one died. These results were obtained despite treatment of most cases (80%) within the first week of life and with combined transarterial and transvenous approaches. There is an excellent potential for normal neurologic development in these children presenting with high-output cardiac failure, achieved through the use of various imaging and endovascular techniques and specialized care by pediatric intensivists and anesthesiologists.
We agree that treatment in later infancy, by the transarterial approach, is ideal (6, 8) but have found the transvenous route useful in the first week of life, when there is an urgent indication for treatment of the neonate. Transfemoral and transtorcular embolizations of the vein of Galen (38, 39) have been described using various approaches, catheters, and embolic agents. In one of our patients, the transfemoral venous route was not possible. For this patient, we report the first successful songraphically guided percutaneous transtorcular embolization of a VGAM, and the use of sections of guidewire as an embolic agent.
Knowing when to stop embolization can be difficult with transvenous embolization—stop too early, and recurrence is guaranteed; too late, and complete occlusion can paradoxically worsen heart failure because of acutely increased afterload. Transarterial embolization is our preferred route, and it is more effective in controlling heart failure when there is only one or a limited number of arterial pedicles. When there are numerous small arterial feeders, it often is impossible to achieve occlusion of more than one or two in the unstable neonate; in these cases, we believe transvenous embolization to offer a greater chance of temporary control of heart failure than transarterial embolization. Transvenous embolization then would be followed, at the second stage, by adjunctive transarterial embolization, which was the commonest procedure performed in our series.
If treatment is indicated in the newborn period, the timing of the first embolization procedure depends on the clinical presentation and response to medical management. Our case 2 was well controlled and had an excellent outcome when treated at 6 months with staged transarterial NBCA embolization. Our other cases could not be controlled, requiring treatment in the neonatal period. Of the four neonates treated in the first week, one died and three had a good outcome immediately afterward. One of the three survivors, however (case 4), showed delayed deterioration with eventual developmental delay and parenchymal damage. There was mild prominence of CSF spaces on the neonatal MR imaging but no evidence of significant delay during the first year of life. It seems unlikely that significant, irreversible parenchymal damage had occurred in utero that would have been a contraindication to treatment, although this cannot be proven. Damage occurring later, secondary to persistent or increased arteriovenous shunting or hydrocephalus, may have been avoided by earlier treatment, as had initially been planned at 6 months. In our opinion, treatment should be planned for 6 months of age even in the presence of apparently normal neurologic development, particularly if there is high flow suggested on imaging. If treatment is delayed beyond 6 months of age, we recommend frequent imaging of the brain, to supplement clinical assessments and detect any evidence of cerebral ischemia or hydrocephalus. Management would then include endovascular embolization as soon as practicable; hydrocephalus often responds to obliteration of the arteriovenous shunt. Shunt placement was avoided when possible in our patients but was performed for refractory hydrocephalus in this case. Reports have suggested worse outcome in patients with shunts (40), and hemodynamic theories have been proposed detailing worsened cerebral perfusion in the presence of a shunt and patent VGAM. After shunting, the epilepsy of the patient who had the single vessel clipped (the anterior cerebral artery) became worse and more difficult to control, probably due to worsened cerebral perfusion in this situation.
With the rise in skills of the interventional radiologist, improved embolization technologies and tools, and the noninvasive nature of the endovascular procedures, the role for surgery has diminished. Open surgery is technically very challenging in these young infants with engorged, hypervascular, fragile brains and patent vein of Galen malformations, and access to the feeding vessels is quite restricted. Nevertheless, if interventional techniques cannot reach or obliterate significant feeding vessels, operative clipping may be indicated. This is particularly applicable when the feeding vessels come from the anterior direction rather than deep to the malformation and when the conditions of the child and the brain are satisfactory. The management of neonates with vein of Galen malformations remains challenging and is best achieved by a multidisciplinary team that includes interventional radiologists, neurosurgeons, neonatologists, and anesthetists working in synergy.
Conclusion
Neonates with choroidal-type VGAMs and cardiac failure can be accurately and safely diagnosed and classified using sonography, MR imaging, and MR angiography. When cardiac failure cannot be controlled with medical therapy, various endovascular techniques allow safe, successful treatment of these infants. With appropriate selection of patients, good clinical outcomes can be achieved.
Footnotes
Address reprint requests to P.J. Mitchell, The University of Melbourne Department of Radiology, The Royal Melbourne Hospital, Melbourne, Australia 3050.
References
- 1.Friedman DM, Madrid M, Berenstein A, Choi IS, Wisoff JH. Neonatal vein of Galen malformations: experience in developing a multidisciplinary approach using an embolization treatment protocol. Clin Pediatr (Phila) 1991;30:621-629 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Monaco R, De Victor D, Mann C, Hannedouche A, Terbrugge K, Lasjaunias P. Congestive cardiac manifestations from cerebrocranial arteriovenous shunts. Endovascular management in 30 children. Childs Nerv Syst 1991;7:48-52 [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HJ, Chuang S, Hendrick EB, Humphreys RP. Aneurysms of the vein of Galen. Experience at The Hospital for Sick Children, Toronto. J Neurosurg 1982;57:316-322 [DOI] [PubMed] [Google Scholar]
- 4.Johnston IH, Whittle IR, Besser M, Morgan MK. Vein of Galen malformation: diagnosis and management. Neurosurgery 1987;20:747-758 [DOI] [PubMed] [Google Scholar]
- 5.Lasjaunias P, Rodesch G, Pruvost P, Laroche FG, Landrieu P. Treatment of vein of Galen aneurysmal malformation. J Neurosurg 1989;70:746-750 [DOI] [PubMed] [Google Scholar]
- 6.Lasjaunias P, Garcia-Monaco R, Rodesch G, et al. Vein of Galen malformation. Endovascular management of 43 cases. Childs Nerv Syst 1991;7:360-367 [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias P, Hui F, Zerah M, et al. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Childs Nerv Syst 1995;11:66-79 [DOI] [PubMed] [Google Scholar]
- 8.Halbach VV, Dowd CF, Higashida RT, Balousek PA, Ciricillo SF, Edwards MS. Endovascular treatment of mural-type vein of Galen malformations. J Neurosurg 1998;89:74-80 [DOI] [PubMed] [Google Scholar]
- 9.Amacher AL, Shillito J Jr. The syndromes and surgical treatment of aneurysms of the great vein of Galen. J Neurosurg 1973;39:89-98 [DOI] [PubMed] [Google Scholar]
- 10.Ciricillo SF, Edwards MS, Schmidt KG, et al. Interventional neuroradiological management of vein of Galen malformations in the neonate. Neurosurgery 1990;27:22-27 [DOI] [PubMed] [Google Scholar]
- 11.Lasjaunias P, Rodesch G, Terbrugge K, et al. Vein of Galen aneurysmal malformations. Report of 36 cases managed between 1982 and 1988. Acta Neurochir 1989;99:26-37 [DOI] [PubMed] [Google Scholar]
- 12.Casasco A, Lylyk P, Hodes JE, Kohan G, Aymard A, Merland JJ. Percutaneous transvenous catheterization and embolization of vein of Galen aneurysms. Neurosurgery 1991;28:260-266 [DOI] [PubMed] [Google Scholar]
- 13.Lylyk P, Vinuela F, Dion JE, et al. Therapeutic alternatives for vein of Galen vascular malformations. J Neurosurg 1993;78:438-445 [DOI] [PubMed] [Google Scholar]
- 14.Brunelle F. Arteriovenous malformation of the vein of Galen in children. Pediatr Radiol 1997;27:501-513 [DOI] [PubMed] [Google Scholar]
- 15.Berenstein A, Lasjaunias P. Surgical Neuroangiography. NewYork: Springer; 1993
- 16.Yasargil MG. Microneurosurgery. New York: Thieme; 1988
- 17.Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology 1989;31:109-128 [DOI] [PubMed] [Google Scholar]
- 18.Houdart E, Gobin YP, Casasco A, Aymard A, Herbreteau D, Merland JJ. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology 1993;35:381-385 [DOI] [PubMed] [Google Scholar]
- 19.Lasjaunias P, Garcia-Monaco R, Rodesch G, Terbrugge K. Deep venous drainage in great cerebral vein (vein of Galen) absence and malformations. Neuroradiology 1991;33:234-238 [DOI] [PubMed] [Google Scholar]
- 20.Truwit CL. Embryology of the cerebral vasculature. Neuroimaging Clin N Am 1994;4:663-689 [PubMed] [Google Scholar]
- 21.Raybaud CA, Strother CM. Persisting abnormal embryonic vessels in intracranial arteriovenous malformations. Acta Radiol Suppl 1986;369:136-138 [PubMed] [Google Scholar]
- 22.Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev 1986;9:177-216 [DOI] [PubMed] [Google Scholar]
- 23.Girard N, Lasjaunias P, Taylor W. Reversible tonsillar prolapse in vein of Galen aneurysmal malformations: report of eight cases and pathophysiological hypothesis. Childs Nerv Syst 1994;10:141-147 [DOI] [PubMed] [Google Scholar]
- 24.Andeweg J. The anatomy of collateral venous flow from the brain and its value in etiological interpretation of intracranial pathology. Neuroradiology 1996;38:621-628 [DOI] [PubMed] [Google Scholar]
- 25.Andeweg J. Consequences of the anatomy of deep venous outflow from the brain. Neuroradiology 1999;41:233-241 [DOI] [PubMed] [Google Scholar]
- 26.Yamashita Y, Abe T, Ohara N, et al. Successful treatment of neonatal aneurysmal dilatation of the vein of Galen: the role of prenatal diagnosis and trans-arterial embolization. Neuroradiology 1992;34:457-459 [DOI] [PubMed] [Google Scholar]
- 27.Campi A, Scotti G, Filippi M, Gerevini S, Strigimi F, Lasjaunias P. Antenatal diagnosis of vein of Galen aneurysmal malformation: MR study of fetal brain and postnatal follow-up. Neuroradiology 1996;38:87-90 [DOI] [PubMed] [Google Scholar]
- 28.Goelz R, Mielke G, Gonser M, et al. Vein of Galen malformation: prenatal diagnosis and noninvasive procedure. Z Geburtshilfe Neonatol 1996;200:72-75 [PubMed] [Google Scholar]
- 29.Rodesch G, Hui F, Alvarez H, Tanaka A, Lasjaunias P. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst 1994;10:79-83 [DOI] [PubMed] [Google Scholar]
- 30.Campi A, Rodesch G, Scotti G, Lasjaunias P. Aneurysmal malformation of the vein of Galen in three patients: clinical and radiological follow-up. Neuroradiology 1998;40:816-821 [DOI] [PubMed] [Google Scholar]
- 31.Leff SL, Kronfeld G, Leonidas JC. Aneurysm of the vein of Galen. Ultrasound, MRI and angiographic correlations. Pediatr Radiol 1989;20:98-100 [DOI] [PubMed] [Google Scholar]
- 32.Roosen N, Schirmer M, Lins E, Bock WJ, Stork W, Gahlen D. MRI of an aneurysm of the vein of Galen. AJNR Am J Neuroradiol 1986;7:733-735 [PMC free article] [PubMed] [Google Scholar]
- 33.Borthne A, Carteret M, Baraton J, Courtel J, Brunelle F. Vein of Galen vascular malformations in infants: clinical, radiological and therapeutic aspect. Eur Radiol 1997;7:1252-1258 [DOI] [PubMed] [Google Scholar]
- 34.Seidenwurm D, Berenstein A, Hyman A, Kowalski H. Vein of Galen malformation: correlation of clinical presentation, arteriography, and MR imaging. AJNR Am J Neuroradiol 1991;12:347-354 [PMC free article] [PubMed] [Google Scholar]
- 35.Desprechins B, Debaere C, Machiels F, Bougatef A, Osteaux M. A vein of Galen aneurysm with an abnormal drain system: MRI findings. Pediatr Radiol 1995;25:442-443 [DOI] [PubMed] [Google Scholar]
- 36.Baenziger O, Martin E, Willi U, Fanconi S, Real F, Boltshauser E. Prenatal brain atrophy due to a giant vein of Galen malformation. Neuroradiology 1993;35:105-106 [DOI] [PubMed] [Google Scholar]
- 37.Hurst RW, Kagetsu NJ, Berenstein A. Angiographic findings in two cases of aneurysmal malformation of vein of Galen prior to spontaneous thrombosis: therapeutic implications. AJNR Am J Neuroradiol 1992;13:1446-1450 [PMC free article] [PubMed] [Google Scholar]
- 38.Mickle JP, Quisling RG. The transtorcular embolization of vein of Galen aneurysms. J Neurosurg 1986;64:731-735 [DOI] [PubMed] [Google Scholar]
- 39.Dowd CF, Halbach VV, Barnwell SL, Higashida RT, Edwards MS, Hieshima GB. Transfemoral venous embolization of vein of Galen malformations. AJNR Am J Neuroradiol 1990;11:643-648 [PMC free article] [PubMed] [Google Scholar]
- 40.Zerah M, Garcia-Monaco R, Rodesch G, et al. Hydrodynamics in vein of Galen malformations. Childs Nerv Syst 1992;8:111-117 [DOI] [PubMed] [Google Scholar]


