Abstract
BACKGROUND AND PURPOSE: Benign tumors of the internal auditory canal (IAC) may leave the confines of the IAC fundus and extend into inner ear structures, forming a dumbbell-shaped lesion. It is important to differentiate dumbbell lesions, which include facial and vestibulocochlear schwannomas, from simple intracanalicular schwannomas, as surgical techniques and prognostic implications are affected. In this article, the imaging and clinical features of these dumbbell schwannomas are described.
METHODS: A dumbbell lesion of the IAC is defined as a mass with two bulbous segments, one in the IAC fundus and the other in the membranous labyrinth of the inner ear or the geniculate ganglion of the facial nerve canal, spanned by an isthmus. Twenty-four patients with dumbbell lesions of the IAC had their clinical and imaging data retrospectively reviewed. Images were evaluated for contour of the mass and extension into the membranous labyrinth or geniculate ganglion.
RESULTS: Ten of 24 lesions were facial nerve dumbbell lesions. Characteristic features included an enhancing “tail” along the labyrinthine segment of the facial nerve and enlargement of the facial nerve canal. Dumbbell schwannomas of the vestibulocochlear nerve (14/24) included transmodiolar (8/14), which extended into the cochlea, transmacular (2/14), which extended into the vestibule, and combined transmodiolar/transmacular (4/14) types.
CONCLUSION: Simple intracanalicular schwannomas can be differentiated from transmodiolar, transmacular, and facial nerve schwannomas with postcontrast and high-resolution fast spin-echo T2-weighted MR imaging. Temporal bone CT is reserved for presurgical planning in the dumbbell facial nerve schwannoma group.
Schwannoma is a benign neoplasm of the nerve sheath and is the most common neoplasm of the internal auditory canal (IAC) and cerebellopontine angle (1–6). “Acoustic” schwannomas most often arise from the vestibular division of the vestibulocochlear nerve. This tumor most commonly originates near the vestibular ganglion, at the junction of the central and peripheral myelin near the fundus of the IAC. Schwannomas may arise from any of the cranial nerves within the IAC, however, including the facial nerve (1, 5). Typical IAC schwannomas are intracanalicular without extension into the membranous labyrinth of the inner ear.
Treatment of patients with simple IAC schwannomas involves surgical resection with a goal of preserving hearing and facial nerve function (7–12). Classically, these schwannomas are treated surgically by using one of three approaches (translabyrinthine, middle fossa, or retrosigmoid/suboccipital), depending on the size and location of the mass, as well as level of hearing. When schwannomas leave the confines of the IAC and extend into the inner ear, surgical approaches and prognostic implications are affected.
A “dumbbell” lesion of the IAC is defined as a mass with two bulbous segments, one in the IAC fundus and the other in the membranous labyrinth of the inner ear or the geniculate ganglion of the facial nerve canal, spanned by an isthmus. Hearing preservation surgery is not an option when a lesion extends into the labyrinth, as removing a tumor from the labyrinth would be expected to result in profound sensorineural hearing loss (4). Should the dumbbell lesion extend to the geniculate ganglion, this would indicate a facial nerve neuroma and would influence the approach, as well as preparation, for facial nerve repair. Therefore, it is imperative to recognize when a lesion of the IAC is not a simple intracanalicular schwannoma.
Optimal therapeutic decisions for patients with IAC schwannomas require accurate imaging with precise lesion localization. MR imaging is the examination of choice for depicting of the IAC in patients with a suspected vestibulocochlear schwannoma (13–15). Both contrast-enhanced and high-resolution fast spin-echo (FSE) T2-weighted imaging are invaluable in the evaluation of IAC lesions. High-resolution FSE T2-weighted imaging provides excellent demonstration of the intracanalicular segments of the facial and vestibulocochlear nerves and is a useful adjunct to conventional contrast-enhanced studies of the IAC (16–18).
The objectives of our study were to define the radiologic features and determine the surgical and prognostic implications of IAC schwannomas that extend beyond the confines of the IAC and involve the facial or vestibulocochlear nerves.
Methods
We retrospectively reviewed the clinical data and imaging characteristics of 24 patients with dumbbell lesions over a period of 10 years, from March 1989 through December 1999. Clinical data collected included hearing loss, tinnitus, dizziness, dysequilibrium, vertigo, facial twitching, and facial paralysis. Audiologic testing included pure tone audiogram, speech discrimination testing, and stapedial reflexes.
Patients were examined with a 1.5-T magnet. All patients were imaged with thin-section contrast-enhanced MR imaging through the IAC (800/27/2 [TR/TE/excitations], 3-mm slice thickness, 0-mm interslice gap, and fat saturation). High-resolution FSE T2-weighted imaging was performed using phased-array surface coils. FSE T2-weighted images were obtained using either 2D acquisition (4000/102/6, 2-mm slice thickness,−1-mm interslice gap, echo train length = 32) or 3D acquisition (4000/130/1, 0.8-mm slice thickness, 0-mm interslice gap, echo train length = 64). Patients who were managed conservatively were followed up with high-resolution FSET2-weighted imaging. Patients with suspected facial nerve lesions were also examined with thin-section temporal bone CT (1-mm axial/coronal). Images were evaluated for size and contour of masses and extension into the cochlea, vestibular structures, or geniculate ganglion. For those patients who underwent surgical resection, diagnosis was confirmed by histologic examination of the tumor. The diagnosis of the remaining patients was presumed schwannoma and made on the basis of clinical and imaging characteristics.
For the purposes of definition, we separated dumbbell lesions into two major groups: those involving the facial nerve (cranial nerve VII) and those involving the vestibulocochlear nerve (cranial nerve VIII). We defined a facial nerve dumbbell as an IAC mass that involved the labyrinthine segment of the facial nerve with extension to the geniculate ganglion (Fig 1). We defined a vestibulocochlear dumbbell as an IAC mass that involved the vestibulocochlear nerve with extension into the cochlea or vestibular structures or both. We identified three subgroups of the vestibulocochlear nerve schwannomas: transmodiolar, transmacular, and combined. Transmodiolar lesions extended from the IAC through the modiolus into the cochlea (Fig 2). Transmacular lesions extended from the IAC fundus through the macula cribrosa into the vestibule. Finally, a combined transmodiolar/transmacular lesion extended from the IAC fundus into both the cochlea and vestibule.
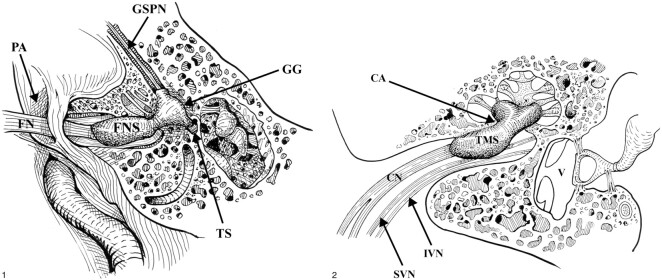
fig 1.
Dumbbell facial nerve schwannoma. The facial nerve schwannoma (FNS) dumbbells through an enlarged labyrinthine facial canal to involve the geniculate ganglion (GG). Minimal extension of the schwannoma may be present along the greater superficial petrosal nerve (GSPN). The tympanic segment of the facial nerve (TS) is usually not involved. The cisternal portion of the facial nerve (FN) is seen as it courses toward the IAC through the porus acusticus (PA), the entrance to the IAC. Used by permission (37).fig 2. Transmodiolar dumbbell schwannoma. The transmodiolar schwannoma (TMS) extends through the cochlear aperture (CA) and modiolus into the cochlea. The schwannoma involves the cochlear nerve (CN). The normal superior vestibular nerve (SVN) and inferior vestibular nerve (IVN) are present within the IAC. V: Vestibule. Used by permission (37)
Results
Twenty-four patients with the diagnosis of IAC dumbbell schwannoma were found. The patients ranged in age from 16 to 79 years (mean age,42 years); 13 were female and 11 were male patients. There were 10 facial nerve dumbbell lesions and 14 vestibulocochlear lesions.
Facial Nerve Schwannomas
Ten patients with facial nerve dumbbell schwannomas were reviewed. The patients ranged in age from 15 to 63 years (mean age, 43 years). There were six female and four male patients. Presenting clinical symptoms included hearing loss in seven patients and facial nerve paralysis in four patients. One patient also had tinnitus. All of the facial nerve dumbbell lesions extended from the IAC to the geniculate ganglion.
In all cases (10/10), MR imaging with contrast enhancement revealed a mass in the IAC fundus with an enhancing “tail” extending to the geniculate ganglion (Fig 3A). Three facial schwannomas also had extension anteromedially along the greater superficial petrosal nerve. High-resolution FSET2-weighted imaging (5/10) revealed a hypointense mass within the IAC along the facial nerve, extending to the geniculate ganglion (Fig 3B and C). CT (8/10) revealed a smoothly enlarged facial nerve canal involving the labyrinthine segment and geniculate ganglion region in all cases imaged (Fig 3D). Seven of the cases (7/10) were pathologically proven facial nerve schwannomas at surgery. The three remaining patients were managed conservatively and followed up radiologically.
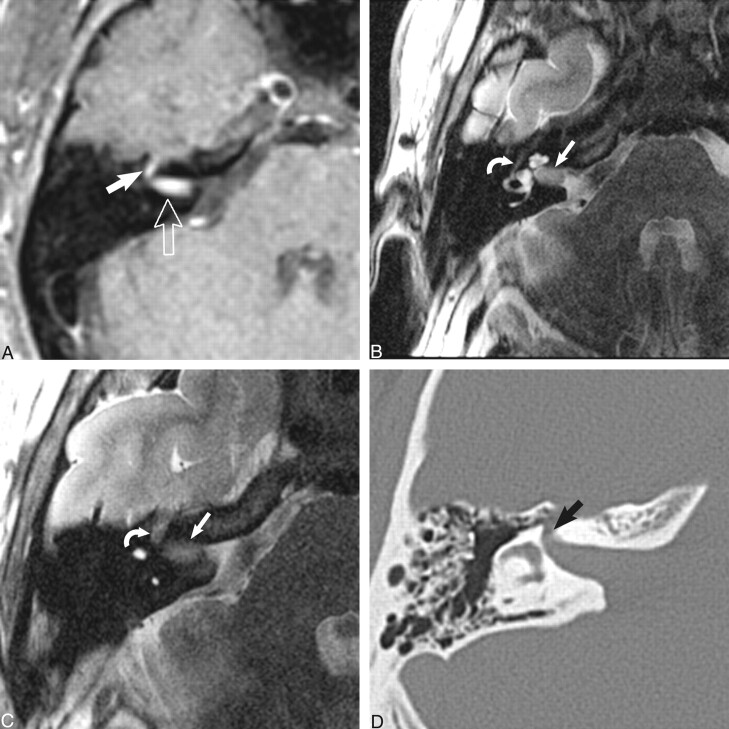
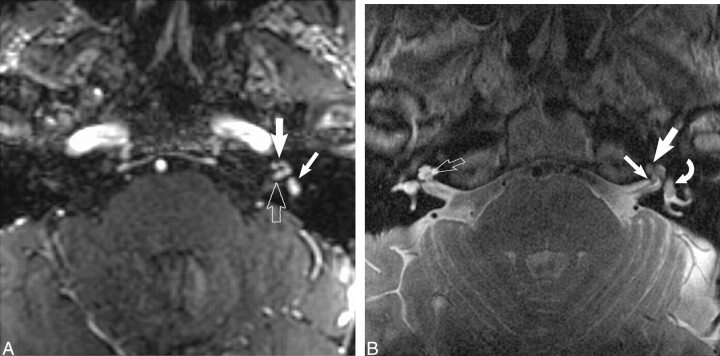
fig 3.
Dumbbell facial nerve schwannoma.
A, Axial enhanced T1-weighted MR image (800/27/2) at the level of the IAC shows an avidly enhancing mass involving the IAC (open arrow) with an enhancing “tail” involving the labyrinthine segment of the facial nerve (white arrow) as it courses toward the geniculate ganglion.
B, Axial high-resolution FSE T2 MR image (4000/130/1) at the level of the IAC shows a hypointense mass filling the IAC (white arrow) displacing the normal cerebrospinal fluid. The tympanic segment of the facial nerve is barely visible in its bony canal (curved white arrow).
C, Axial high-resolution FSE T2-weighted MR image (4000/130/1) just cephalad to B shows the enlarged labyrinthine segment mass (curved white arrow) as it courses toward the geniculate ganglion. The hypointense intracanalicular portion is again seen (white arrow).
D, Axial CT image at the level of C confirms the enlarged labyrinthine portion of the facial nerve canal (black arrow). Used by permission (37).
Vestibulocochlear Schwannomas
Fourteen patients with vestibulocochlear schwannomas were reviewed. The patients ranged in age from 16 to 79 years (mean age, 54 years). There were seven male and seven female patients. All patients (14/14) presented with hearing loss and 13 of 14 presented with some combination of vestibular symptoms, including tinnitus, dizziness, dysequilibrium, and vertigo. Two patients also presented with facial nerve dysfunction (Table). Eight patients had transmodiolar dumbbell schwannomas, whereas only two patients had transmacular lesions. Four patients had combined lesions extending from the IAC into both the cochlea and vestibular structures. Five patients had pathologically proven schwannomas.
Presenting symptoms
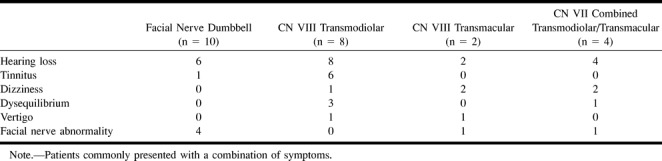
All patients were imaged with contrast-enhanced MR imaging, whereas the majority (11/14) also underwent high-resolution FSE T2-weighted imaging. Imaging of patients with transmodiolar lesions (n = 8) with contrast-enhanced T1-weighted MR imaging showed an enhancing mass in the IAC with extension into the cochlea. High-resolution FSE T2-weighted images showed a hypointense mass as a filling defect within the IAC extending to the cochlea (Fig 4). Imaging of patients with transmacular lesions (n = 2) with contrast-enhanced and high-resolution FSE T2-weighted MR imaging showed a mass extending from the IAC fundus to the vestibule (Fig 5). Finally, imaging of patients with combined transmodiolar/transmacular schwannomas (n = 4) showed a mass extending from the IAC to the cochlea and vestibule (Fig 6). One patient had a translabyrinthine mass extending from the IAC through the labyrinth into the middle ear (Fig 7).
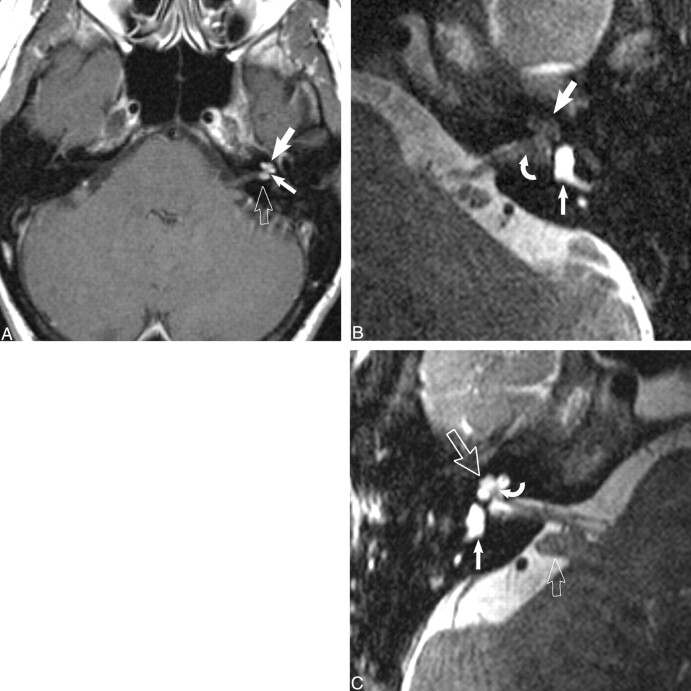
fig 4.
Transmodiolar dumbbell schwannoma.
A, Axial contrast-enhanced T1-weighted MR image (800/27/2) at the level of the IAC shows an enhancing mass in the fundus of the IAC (open arrow) with extension into the cochlea (large white arrow). The schwannoma extends through the cochlear aperture (small white arrow) and modiolus.
B, Axial high-resolution FSE T2-weighted MR image (4000/102/6) at the level of the IAC shows the transmodiolar schwannoma as a hypointense mass that has replaced the normal fluid of the cochlea (large white arrow). The intracanalicular portion of the mass displaced the normal fluid of the fundus of the IAC (curved white arrow). The vestibule is normal and fluid filled (small white arrow).
C, Axial high-resolution FSE T2-weighted MR image (4000/102/6) shows the normal right membranous labyrinthine structures. The normal cochlea (large open arrow) and vestibule (white arrow) are fluid-filled. The central bony modiolus (curved white arrow) through which the cochlear nerve fibers travel is well seen. The cerebellar flocculus (small open arrow) is a common cerebellopontine angle “pseudomass.” Used by permission (37).
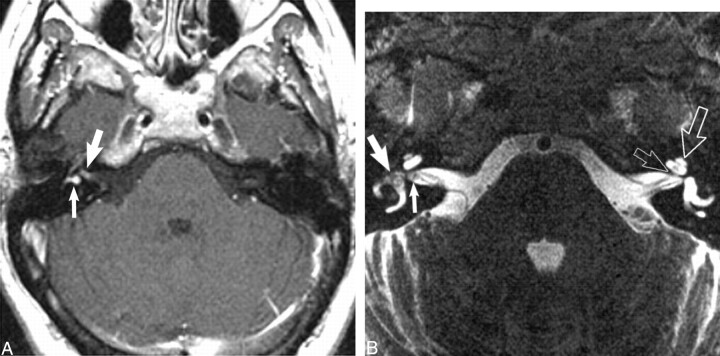
fig 5.
Transmacular dumbbell schwannoma.
A, Axial contrast-enhanced T1-weighted MR image (800/27/2) at the level of the IAC shows an enhancing mass in the fundus of the IAC (large white arrow) with extension into the vestibule (small white arrow).
B, Axial high-resolution FSE T2-weighted MR image (4000/102/6) at the level of the IAC shows a small nodular mass in the fundus of the IAC (small white arrow). The hypointense mass extends into the vestibule (large white arrow). In addition, the normal left fluid-filled cochlea (large open white arrow) and cochlear aperture (small open white arrow) are seen. Used by permission (37).
fig 6.
Combined transmodiolar/transmacular schwannoma.
A, Axial enhanced T1-weighted MR image (39/14/2) at the level of the IAC shows an enhancing mass in the fundus of the IAC (open arrow) with extension into the cochlea (large white arrow) and vestibule (small white arrow).
B, Axial high-resolution FSE T2-weighted MR image (4000/102/6) at the level of the IAC shows the hypointense mass in the cochlea (large white arrow) and vestibule (curved white arrow). A small nodule is also seen within the fundus of the IAC (small white arrow). The normal modiolus is well seen on the right (open right arrow). Used by permission (37).
fig 7.
Translabyrinthine schwannoma.
A, Axial enhanced T1-weighted MR image (800/27/2) at the level of the IAC shows a large enhancing mass within the cerebellopontine angle-IAC (open arrow) with extension into the cochlea (black arrow) and vestibule (white arrow).
B, Axial CT shows the soft-tissue mass extending into the middle ear (small black arrow) through an enlarged round window (large black arrow). Used by permission (37).
Discussion
The typical IAC schwannoma is a focal, lobulated, intracanalicular mass that is centered near the porus acusticus and extends toward the fundus of the IAC. Current treatment for these lesions usually involves surgical resection with the goal of preserving hearing and facial nerve function (7–12). In contrast, a dumbbell schwannoma extends beyond the confines of the IAC into the labyrinth, with involvement of the geniculate ganglion, cochlea, or vestibule. Intralabyrinthine schwannomas are rare but have been described (4, 19, 20). The extracanalicular portions of dumbbell schwannoma can be easily overlooked on routine imaging but are important to recognize because of their surgical and prognostic implications.
Imaging is critical for evaluation of dumbbell schwannomas. When contrast-enhanced MR imaging is completed, a bilobed enhancing mass within the IAC with extension to the geniculate ganglion, cochlea, or vestibule is identified. When the lesion is suspected to be a facial schwannoma, temporal bone CT is added to confirm the enlargement of the labyrinthine segment of the facial nerve canal and provide bony landmarks for the surgeon.
The use of high-resolution FSE T2-weighted imaging as a screening examination for IAC schwannoma requires attention to the less obvious finding of tumor extension beyond the IAC. Proper identification of these dumbbell schwannomas is critical; failure to identify the tumor leaving the IAC into either the inner ear membranous labyrinth or along the facial nerve to the geniculate fossa may lead to an incorrect diagnosis and inadequate surgery. When a mass is seen within the high signal fluid of the IAC, the radiologist must look for extension into the labyrinth. When the tumor extends into the high-signal fluid of the cochlea (transmodiolar schwannoma) or vestibule (transmacular schwannoma), it is readily visible as a filling defect. When the schwannoma extends along the labyrinthine segment of the facial nerve, however, it may be more difficult to see on FSE T2-weighted imaging, as it is surrounded by bone in this location. Temporal bone CT is used to confirm the enlargement of the bony canal in such cases.
Facial Nerve Schwannomas
Facial nerve schwannomas are rare lesions that must be recognized and differentiated from vestibulocochlear tumors. These patients usually present with facial nerve dysfunction or hearing loss, depending on tumor location and size (21–25). Facial nerve schwannomas presenting among patients with hearing loss can be confused clinically for a typical vestibulocochlear schwannoma (26, 27). Therefore, imaging becomes critical in the evaluation of these patients. The hearing loss may be either conductive or sensorineural, depending on the size and location of the tumor (21, 22). Conductive hearing loss is related to ossicular compression by involvement of the tympanic facial nerve segment. Sensorineural hearing loss results from compression of the cochlear nerve in the IAC or erosion into the membranous labyrinth (24, 25).
Facial nerve schwannomas usually involve the geniculate ganglion (21, 23, 28), but may involve any portion of the facial nerve (29). In our series, only dumbbell lesions from the IAC to the geniculate ganglion were included. These dumbbell facial nerve schwannomas can be confused clinically and radiologically with vestibulocochlear schwannoma. Therefore, facial nerve schwannomas can and should be differentiated by the imaging characteristics described herein. Characteristic MR imaging features include an enhancing “tail” along the labyrinthine segment of the facial nerve as it extends to the geniculate ganglion region or, on high-resolution FSE T2-weighted images, a hypointense mass involving the IAC facial nerve extending toward the geniculate ganglion. Temporal bone CT features include benign, sharply marginated remodeling with enlargement of the labyrinthine segment of the facial nerve canal, often with enlargement of the geniculate fossa (1, 25).
When the diagnosis of dumbbell facial nerve schwannoma has been made on the basis of imaging studies, a series of important treatment decisions must be initiated. Proper patient counseling prior to therapy is vital, given the likelihood of facial nerve dysfunction with treatment. If the patient does not have facial nerve dysfunction at the time of first diagnosis, s/he should be followed up with electrical testing and MR imaging until some facial nerve dysfunction surfaces. Because earlier treatment is often correlated with a better return of facial function (29, 30), surgery is offered at this time.
The surgical approach for dumbbell facial nerve schwannoma is selected on the basis of tumor size and the degree of residual hearing (29). The translabyrinthine approach is used in cases of large tumor or no serviceable hearing or both. This approach allows safe access to the IAC and labyrinthine, tympanic, and mastoid segments of the facial nerve. If the patient has residual hearing, the middle fossa approach is employed to access the IAC, labyrinthine segment, geniculate ganglion, and anterior tympanic portions of the facial nerve without injuring the cochlea. When the facial nerve schwannoma involves multiple, contiguous segments of the facial nerve, nerve grafting becomes necessary. Rarely, the tumor is small (less than 1 cm in length), and a primary anastomosis can be used. Facial nerve function is diminished by surgical intervention whether the facial nerve schwannoma is primarily removed or grafted (31).
Vestibulocochlear Schwannomas
Patients with dumbbell vestibulocochlear schwannomas usually present with hearing loss similar to patients with simple intracanalicular schwannomas confined to the IAC. Patients with these tumors often have vestibular symptoms. Rarely, they present with facial nerve symptoms related to compression from the tumor. The importance of recognizing extension of the tumor beyond the IAC preoperatively is obvious, as incomplete removal of tumor, early recurrence of tumor, and an increased rate of hearing loss as a complication of surgery all can result from overlooking this radiologic finding (4). In fact, the observation of vestibulocochlear schwannoma extending to the cochlear base, transmodiolar, or transmacular makes the possibility of hearing preservation at the time of surgery remote (32).
Vestibulocochlear dumbbell schwannomas can be differentiated from simple intracanalicular schwannomas by extension of the tumor into the cochlea or vestibule or both revealed by either high-resolution FSE T2-weighted or contrast-enhanced MR imaging. When screening FSE T2-weighted MR imaging reveals extension into the membranous labyrinth of the inner ear, a contrast-enhanced T1-weighted MR study is done for confirmation. Because complete hearing loss results from a surgical cure in this patient group, many are managed conservatively and followed up clinically and with high-resolution FSE T2-weighted imaging.
The majority of patients in our series had the transmodiolar type of dumbbell schwannoma, which is somewhat surprising, because most typical IAC schwannomas arise from the vestibular division of the vestibulocochlear nerve (5). We suspect that dumbbell schwannomas represent a different type of lesion. The fact that there is a predominance of transmodiolar lesions suggests these lesions may arise along the distal cochlear division of the vestibulocochlear nerve in the vicinity of the modiolus. In addition, there were several combined transmodiolar/transmacular schwannomas that had involvement of the cochlea, as seen in Figure 6. In these cases, the schwannoma fills the cochlea with direct contiguous intralabyrinthine spread into the vestibule.
Patients with schwannomas confined to the IAC usually undergo surgical resection using a translabyrinthine, retrosigmoid, or suboccipital approach, depending on the tumor size and location and the degree of hearing loss present. Because surgical treatment of dumbbell vestibulocochlear schwannoma guarantees complete hearing loss in the affected ear, these patients are initially managed conservatively. When disabling vertigo is part of the clinical picture, or if follow-up imaging reveals tumor growth down the IAC into the cerebellopontine angle, surgical resection using a translabyrinthine approach is completed. No growth or slow growth of vestibulocochlear schwannoma is common, however, and many of these tumors never require surgery (33–36).
Conclusion
Dumbbell schwannomas may extend from the confines of the IAC into the various segments of the labyrinth or facial nerve canal. It is critical to recognize extension of IAC schwannomas into the modiolus (transmodiolar), the macula (transmacular), or facial nerve canal, as these features will affect surgical approach, complications, and prognostic implications. We found that dedicated, thin-section, contrast-enhanced MR imaging of the IAC supplemented by high-resolution FSE T2-weighted imaging can help determine extension of a lesion into the labyrinth or facial nerve canal. Temporal bone CT is also useful in the evaluation of dumbbell facial nerve schwannomas for presurgical planning.
Acknowledgments
The authors express their gratitude to Julian Mack from the Medical Illustrations Department of the University of Utah for his work on the drawings. We also gratefully acknowledge the assistance of Tiffany Merrill and Sheila Mulligan-Webb for preparation of the manuscript.
Footnotes
Presented in part at the annual meeting of the American Society of Neuroradiology, Atlanta, Georgia, April 2000.
Address reprint requests to Karen L. Salzman, MD, Section of Neuroradiology, Department of Radiology, 1A71 Medical Center, 50 North Medical Drive, Salt Lake City, UT 84132.
References
- 1.Swartz JD, Harnsberger HR. Imaging of the Temporal Bone. 3rd ed. New York: Thieme;1998:370–435
- 2.Hasso AN, Smith DS. The cerebellopontine angle. Semin Ultrasound CT MR 1989;10:280-301 [PubMed] [Google Scholar]
- 3.Clemis JD, Ballad WJ, Baggot PJ, Lyon ST. Relative frequency of the inferior vestibular schwannoma. Arch Otolaryngol Head Neck Surg 1986;112:190-194 [DOI] [PubMed] [Google Scholar]
- 4.Green JD Jr, McKenzie JD. Diagnosis and management of intralabyrinthine schwannomas. Laryngoscope 1999;109:1626-1631 [DOI] [PubMed] [Google Scholar]
- 5.Schunknecht HF. Pathology of the Ear. 2nd ed. Malvern, PA: Lea and Febiger;1993:460–474
- 6.Mark AS, Seltzer S, Harnsberger HR. Sensorineural hearing loss: more than meets the eye? AJNR Am J Neuroradiol 1993;14:37-45 [PMC free article] [PubMed] [Google Scholar]
- 7.Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 1997;40:248-262 [DOI] [PubMed] [Google Scholar]
- 8.Umezu H, Aiba T, Tsuchida S, Seki Y. Early and late postoperative hearing preservation in patients with acoustic neuromas. Neurosurgery 1996;39:267-272 [DOI] [PubMed] [Google Scholar]
- 9.Glasscock ME, Hays JW, Minor LB, Haynes DS, Carrasco VN. Preservation of hearing in surgery for acoustic neuromas. J Neurosurgery 1993;78:864-870 [DOI] [PubMed] [Google Scholar]
- 10.Haines SJ, Levine SC. Intracanalicular acoustic neuroma: early surgery for preservation of hearing. J Neurosurgery 1993;79:515-520 [DOI] [PubMed] [Google Scholar]
- 11.Shelton C. Hearing preservation in acoustic tumor surgery. Otolaryngol Clin North Am 1992;25:609-621 [PubMed] [Google Scholar]
- 12.Shelton C, Hitselberger WE, House WF, Brackmann DE. Hearing preservation after acoustic tumor removal: long-term results. Laryngoscope 1990;100:115-119 [DOI] [PubMed] [Google Scholar]
- 13.Lhuillier FM, Doyon DL, Halimi PM, Sigal RC, Sterkens JM. Magnetic resonance imaging of acoustic neuromas: pitfalls and differential diagnosis. Neuroradiology 1992;34:144-149 [DOI] [PubMed] [Google Scholar]
- 14.Enzmann DR, O'Donohue J. Optimizing MR imaging for detecting small tumors in the cerebellopontine angle and internal auditory canal. AJNR Am J Neuroradiol 1987;8:99-106 [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels D, Millen S, Meyer G, et al. MR detection of tumor in the internal auditory canal. AJR Am J Roentgenol 1987;148:1219-1222 [DOI] [PubMed] [Google Scholar]
- 16.Allen RW, Harnsberger HR, Shelton C, et al. Low-cost high-resolution fast spin-echo MR of acoustic schwannoma: an alternative to enhanced conventional spin-echo MR? AJNR Am J Neuroradiol 1996;17:1205-1210 [PMC free article] [PubMed] [Google Scholar]
- 17.Fukui MB, Weissman JL, Curtin HD, Kanal E. T2-weighted MR characteristics of internal auditory canal masses. AJNR Am J Neuroradiol 1996;17:1211-1218 [PMC free article] [PubMed] [Google Scholar]
- 18.Kumon Y, Sakaki S, Ohue S, Ohta S, Kikuchi K, Miki H. Usefulness of heavily T2-weighted magnetic resonance imaging in patients with cerebellopontine angle tumors. Neurosurgery 1998;43:1338-1343 [DOI] [PubMed] [Google Scholar]
- 19.Doyle KJ, Brackmann DE. Intralabyrinthine schwannomas. Otolaryngol Head Neck Surg 1994;110:517-523 [DOI] [PubMed] [Google Scholar]
- 20.Susilawati S, Adler J, Fagan P. Schwannoma in the vestibule and cochlea. Australas Radiol 1997;41:109-111 [PubMed] [Google Scholar]
- 21.Ginsberg LE, DeMonte F. Diagnosis please. Case 16: facial nerve schwannoma with middle cranial fossa involvement. Radiology 1999;213:364-368 [DOI] [PubMed] [Google Scholar]
- 22.Saleh E, Achilli V, Naguib M, et al. Facial nerve neuromas: diagnosis and management. Am J Otol 1995;16:521-526 [PubMed] [Google Scholar]
- 23.Parnes LS, Lee DH, Peerless SJ. Magnetic resonance imaging of facial nerve neuromas. Laryngoscope 1991;101:31-35 [DOI] [PubMed] [Google Scholar]
- 24.Kumon Y, Sakiki S, Ohta S, Ohue S, Nakagawa K, Tanaka K. Greater superficial petrosal nerve neuroma. Case report. J Neurosurg 1999;91:691-696 [DOI] [PubMed] [Google Scholar]
- 25.Martin N, Sterkers O, Mompoint D, Nahum H. Facial nerve neuromas: MR imaging. Report of four cases. Neuroradiology 1992;34:62-67 [DOI] [PubMed] [Google Scholar]
- 26.McMenomey SO, Glasscock ME 3rd, Minor LB, Jackson CG, Strasnick B. Facial nerve neuromas presenting as acoustic tumors. Am J Otol 1994;15:307-312 [PubMed] [Google Scholar]
- 27.Pulec JL. Symposium on ear surgery: II. Facial nerve neuroma. Laryngoscope 1972;82:1160-1176 [DOI] [PubMed] [Google Scholar]
- 28.Chung SY, Kim DI, Lee BH, Yoon PH, Jeon P, Chung TS. Facial nerve schwannomas: CT and MR findings. Yonsi Med J 1998;39:148-153 [DOI] [PubMed] [Google Scholar]
- 29.Shelton C. Facial nerve tumors. In: Brackmann DE, Shelton C, Arriaga MA, eds. Otologic Surgery. Philadelphia, PA: W.B. Saunders Company;1994:414–423
- 30.O'Donoghue GM, Brackmann DE, House JW, Jackler RK. Neuromas of the facial nerve. Am J Otol 1989;10:49-54 [PubMed] [Google Scholar]
- 31.Lipkin AF, Coker NJ, Jenkins HA, Alford BR. Intracranial and intratemporal facial neuroma. Otolaryngol Head Neck Surg 1987;96:71-79 [DOI] [PubMed] [Google Scholar]
- 32.Dubrelle F, Ernst O, Vincent C, Vaneecloo FM, Lejeune JP, Lemaitre L. Cochlear fossa enhancement at MR evaluation of vestibular schwannoma: correlation with success at hearing preservation surgery. Radiology 2000;215:458-462 [DOI] [PubMed] [Google Scholar]
- 33.Deen HG, Ebersold MJ, Harner SG, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery 1996;39:260-266 [DOI] [PubMed] [Google Scholar]
- 34.Wiet RJ, Zappia JJ, Hecht CS, O'Connor CA. Conservative management of patients with small acoustic tumors. Laryngoscope 1995;105:795-800 [DOI] [PubMed] [Google Scholar]
- 35.Mirz F, Jorgensen B, Fiirgaard B, Lundorf E, Pedersen CB. Investigations into the natural history of vestibular schwannomas. Clin Otolaryngol 1999;24:13-18 [DOI] [PubMed] [Google Scholar]
- 36.Fucci MJ, Buchman CA, Brackmann DE, Berliner KI. Acoustic tumor growth: implications for treatment choices. Am J Otol 1999;20:495-499 [PubMed] [Google Scholar]
- 37.Harnsberger HR. Head and Neck Digital Teaching File. Salt Lake City, UT: Electronic Medical Education Resource Group;2000