Abstract
Summary: We report two cases in which MR imaging was used to diagnose multiple cerebral aneurysms caused by cardiac myxoma. In one case, the diagnosis was confirmed with cerebral angiography. Myxomatous aneurysms characteristically appeared on T1-weighted images as contrast-enhancing focal fusiform dilatations of distal segments of the cerebral arteries. On T2-weighted images, these myxomatous aneurysms appeared as low signal intensity flow voids, sometimes associated with cerebral infarctions.
Cardiac myxoma is a tumor of mesenchymal origin accounting for half of all primary cardiac neoplasms (1–3). When it arises in the left atrium, systemic emboli from a cardiac myxoma can lead to cerebral hemorrhage, infarction, and aneurysm formation. On microscopic examination, myxomatous emboli appear as nests of spindle cells and are associated with variable degrees of penetration by tumor cells into the endothelium, with resultant subintimal growth, destruction of the arterial wall, and fibroblastic proliferation (3–5). This, in turn, can lead to gross pathologic changes within the affected arteries, such as occlusion or narrowing of the lumen, nodular thickening of the vessel wall, and true aneurysmal dilatation. Once detected, cardiac myxomas can have a favorable outcome. In a long-term study of 12 patients with cardiac myxomas, eight were symptom-free after cardiac surgery. Whereas the primary tumor is usually diagnosed with echocardiography, several methods have been used to detect myxomatous cerebral aneurysms, including MR, CT, and angiography. Here we present two cases in which MR imaging demonstrated multiple myxomatous cerebral aneurysms.
Case Reports
Patient 1
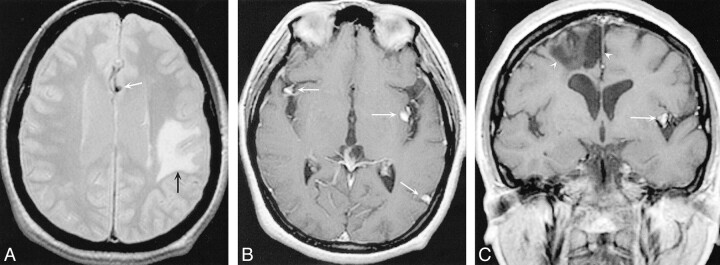
A 75-year-old woman with a history of metastatic melanoma and resected atrial myxoma was referred to our center for gamma knife irradiation for presumed intracranial melanoma metastases. Her neurologic symptoms were limited to a residual aphasia from a previous stroke. As part of an immunotherapy protocol for melanoma, she underwent an outside MR examination that was suggestive of melanoma recurrence with cerebral metastases. Stereotactic MR imaging was performed at our institution in preparation for the gamma knife procedure. Findings on MR imaging included multiple abnormally dilated cortical arteries in the posterior right sylvian fissure, right posterior parietal lobe, and posterior left occipital lobe (Fig 1). The dilated arteries had a fusiform morphology and were surrounded by minimal parenchymal edema. There was also diffuse periventricular white matter T2 prolongation that was consistent with small vessel ischemic changes. No intraparenchymal masses suggestive of melanoma were present. On the basis of the location of the lesions within the subarachnoid space and the fusiform shape of the lesions, the diagnosis of myxomatous aneurysms was suspected and 3D time-of-flight MR angiography was performed followed by cerebral angiography. These revealed multiple fusiform aneurysms in several branches of the right middle cerebral artery, the largest of which measured at least 1 cm in its longest dimension. Other fusiform aneurysms were present in branches of the left middle cerebral artery as well. At the time of writing this report, the patient was being followed with conservative management of these multiple aneurysms.
fig 1.
A 75-year-old woman with a history of melanoma and resected atrial myxoma.
A–C, Axial T1-weighted (600/18/2 [TR/TE/excitations]) postcontrast MR images demonstrate several enhancing foci within the subarachnoid space (arrowheads), consistent with aneurysms of the right middle cerebral artery.
D, Coronal postcontrast T1-weighted MR image (600/15/1) demonstrates contrast-enhancing aneurysm of the right middle cerebral artery (arrowhead) and left posterior parietal arteries (arrow).
E, Axial T2-weighted (3000/80/1) MR image. The aneurysm demonstrates a low signal intensity flow void, consistent with a patent aneurysm.
F, Digital subtraction angiography (DSA), anteroposterior view, of the right internal carotid artery demonstrates multiple arterial dilatations, the largest of which is located in the angular branch of the right middle cerebral artery.
G, DSA, lateral view, of the right internal carotid artery demonstrates multiple fusiform aneurysms in the distribution of the right middle cerebral artery. T1-weighted postcontrast image (600/15/1) demonstrates an enhancing left middle cerebral aneurysm (white arrow) and an area of low signal intensity suggestive of old infarction (arrowheads).
Patient 2
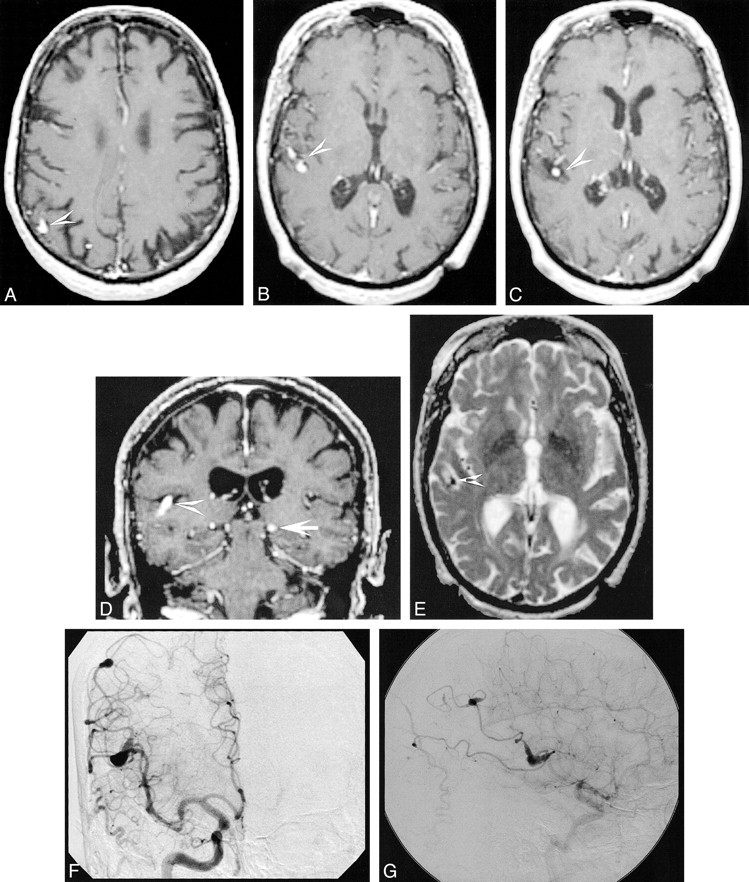
A 73-year-old woman with a history of multiple strokes was examined by echocardiography and was found to have a cardiac myxoma that was subsequently resected. An MR study was obtained to define the extent of her neurologic lesions (Fig 2). Multiple areas of ischemic changes suggestive of old infarcts were noted on T1- and T2-weighted images. T1-weighted images also revealed multiple enhancing foci in the distributions of the right and left middle cerebral arteries. The lesions had a focal fusiform morphology suggestive of myxomatous or mycotic aneurysms. The patient was lost to further follow-up.
fig 2.
A 73-year-old woman with a history of multiple strokes and resected cardiac myxoma.
A, Axial proton density–weighted (2800/40/1) image demonstrates a low signal intensity flow void consistent with a patent aneurysm in the pericallosal artery (white arrow). An area of increased signal intensity is consistent with old infarction (black arrows).
B, Axial T1-weighted (600/20/1) postcontrast image reveals enhancement of multiple fusiform lesions surrounded by regions of low signal intensity, consistent with myxomatous aneurysms (arrows).
C, Coronal T1-weighted postcontrast image (600/15/1) demonstrates an enhancing left middle cerebral aneurysm (white arrow) and an area of low signal intensity suggestive of old infarction (arrowheads).
Discussion
The classic triad of symptoms from cardiac myxomas is heart failure, constitutional symptoms, and embolic manifestations. Not all elements of this triad need to be present to diagnose a cardiac myxoma. Indeed, cardiac myxomas are responsible for a variety of neurologic complications even in the absence of cardiac symptoms (1, 2).
Constitutional symptoms include fever, weight loss, anemia, elevated erythrocyte sedimentation rate, leukocytosis, and hypergammaglobulinemia. It has been hypothesized that these symptoms may be due to interleukin-6 secretion by the tumor cells (2, 6). Cardiac symptoms can be attributed to interference with myocardial function by the primary tumor and may include palpitations, dyspnea on exertion, and syncope. In some cases, however, cardiac myxoma produces no cardiac symptoms (2).
Up to half of cardiac myxomas produce systemic emboli (1, 2). Emboli from cardiac myxomas can lead to cerebral ischemia, infarction, and aneurysm formation. Of these complications, ischemic stroke is the most common, and it has been estimated that 0.5% of all strokes are caused by myxomatous emboli (1). Focal neurologic symptoms can appear suddenly and can include visual disturbances, headache, weakness, and seizures (3). In contrast, chronic embolization from cardiac myxoma can lead to progressive dementia (2). Myxomatous emboli can also lead to hemorrhagic stroke, although this outcome is rare (3). Strokes caused by cardiac myxoma most commonly occur in the middle cerebral artery (3, 7).
Temporary occlusion of cerebral vessels by tumor emboli was once believed to lead to endothelial scarring and subsequent aneurysm formation (8), but a consensus is emerging that the process of aneurysm formation by cardiac myxoma is more elaborate. Histologic evidence is accumulating that supports a model in which emboli from a cardiac myxoma invade the endothelium and proliferate in the vessel wall (3, 9). This, in turn, leads to a weakening of the subintimal tissue and eventual formation of an aneurysm (3, 5). In at least one case, myxomatous emboli penetrated a vessel wall and entered neural parenchyma (4). These findings are compatible with observations that myxomatous aneurysms can grow as well as regress (8, 10). Rupture of myxomatous aneurysms can lead to subarachnoid hemorrhage (3).
MR imaging can be used to image areas of infarct caused by myxoma. MR images of patients with atrial myxoma can demonstrate multiple small infarcts (1). Myxomatous aneurysms on MR imaging can appear as fusiform tubular dilatations of cerebral arteries within the sulci on T1- and T2-weighted images; in some cases a hypointense rim is present around the aneurysm. These lesions are often characterized by vascular dilatation and may be surrounded by edema and hemorrhage (11). Contrast enhancement of fusiform aneurysms was seen in both of our cases and may be secondary to slow flow or possibly enhancing tumor tissue within the wall of the aneurysm. CT of these lesions can demonstrate calcification. MR angiography may confirm the vascular nature of these lesions and is recommended for any tubular enhancing lesions located within the subarachnoid space. Our findings were similar to those previously reported; multiple fusiform arterial dilatations were noted on MR imaging, with associated areas of ischemic changes.
Myxomatous aneurysms typically produce angiographic findings of filling defects, interruption of flow, and local arterial dilatation (8). Morphologically, these aneurysms can appear as fusiform outpouchings or saccular dilatations ranging in size from 3 to 7 mm (3, 5, 6, 8, 9). Aneurysms from cardiac myxomas most commonly occur in the middle cerebral artery, with a mild preponderance of the left side over the right side. In the cases reported here, myxomatous aneurysms were identified with angiography as multiple fusiform arterial dilatations in several branches of the middle cerebral artery. There was no marked preference for left arterial distributions over right arterial distributions.
There is still no definitive treatment of aneurysms caused by cardiac myxomas. The recognition that dividing tumor cells are responsible for aneurysm formation suggests the possibility of using chemotherapy to prevent aneurysmal growth, but the results of doxorubicin alone in this regard are equivocal (10, 12). Using similar reasoning, low-dose radiation therapy is used in conjunction with chemotherapy, with more encouraging results (12). Although cardiac surgery to remove the primary cardiac tumor usually eliminates neurologic symptoms, in some cases, aneurysms appear after resection of the primary myxoma, presumably as a result of metastatic seeding prior to surgery (1, 2, 5–7, 11, 12). In light of the potentially preventable nature of these lesions, the diagnosis of myxomatous aneurysm should be considered in any patient with neurologic symptoms and a history of cardiac myxoma.
Footnotes
Address reprint requests to William P. Dillon, MD, Department of Radiology, Box 0628, University of California, San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143.
References
- 1.Knepper LE, Biller J, Adams HP, Bruno A. Neurologic manifestations of atrial myxoma. A 12-year experience and review. Stroke 1988;19:1435-1440 [DOI] [PubMed] [Google Scholar]
- 2.Mattle HP, Maurer D, Sturzenegger M, Ozdoba C, Baumgartner RW, Schroth G. Cardiac myxomas: a long term study. J Neurol 1995;242:689-694 [DOI] [PubMed] [Google Scholar]
- 3.New PF, Price DL, Carter B. Cerebral angiography in cardiac myxoma. Correlation of angiographic and histopathological findings. Radiology 1970;96:335-345 [DOI] [PubMed] [Google Scholar]
- 4.Budzilovich G, Aleksic S, Greco A, Fernandez J, Harris J, Finegold M. Malignant cardiac myxoma with cerebral metastases. Surg Neurol 1979;11:461-469 [PubMed] [Google Scholar]
- 5.Furuya K, Sasaki T, Yoshimoto Y, Okada Y, Fujimaki T, Kirino T. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. Case report. J Neurosurg 1995;83:170-173 [DOI] [PubMed] [Google Scholar]
- 6.Damasio H, Seabra-Gomes R, da Silva JP, Damasio AR, Antunes JL. Multiple cerebral aneurysms and cardiac myxoma. Arch Neurol 1975;32:269-270 [DOI] [PubMed] [Google Scholar]
- 7.Hofmann E, Becker T, Romberg-Hahnloser R, Reichmann H, Warmuth-Metz M, Nadjmi M. Cranial MRI and CT in patients with left atrial myxoma. Neuroradiology 1992;34:57-61 [DOI] [PubMed] [Google Scholar]
- 8.toane L, Allen JH, Collins HA. Radiologic observations in cerebral embolization from left heart myxomas. Radiology 1966;87:262-266 [DOI] [PubMed] [Google Scholar]
- 9.Burton C, Johnston J. Multiple cerebral aneurysms and cardiac myxoma. N Engl J Med 1970;282:35-36 [DOI] [PubMed] [Google Scholar]
- 10.Roeltgen DP, Weimer GR, Patterson LF. Delayed neurologic complications of left atrial myxoma. Neurology 1981;31:8-13 [DOI] [PubMed] [Google Scholar]
- 11.Friedman DP, Rapoport RJ. Giant fusiform oncotic aneurysm: MR and angiographic findings. AJR Am J Roentgenol 1996;167:538-539 [DOI] [PubMed] [Google Scholar]
- 12.Bernet F, Stulz PM, Carrel TP. Long-term remission after resection, chemotherapy, and irradiation of a metastatic myxoma. Ann Thorac Surg 1998;66:1791-1792 [DOI] [PubMed] [Google Scholar]