Abstract
BACKGROUND AND PURPOSE: Interictal hypometabolism has lateralizing value in cases of temporal lobe epilepsy and positive predictive value for seizure-free outcome after surgery to treat epilepsy. Alterations in regional cerebral metabolism can also be inferred from measurements of regional cerebral perfusion. The purpose of this study was to determine the feasibility of detecting cerebral blood flow (CBF) asymmetries in the mesial temporal lobes using continuous arterial spin labeling perfusion MR imaging, which is a noninvasive method for calculating regional CBF.
METHODS: Twelve patients with medically refractory temporal lobe epilepsy who underwent preoperative evaluation for temporal lobectomy and 12 normal control participants were studied retrospectively. Absolute and normalized mesial temporal CBF measurements were compared between the patient and control groups. Lateralization based on a perfusion asymmetry index was compared with metabolic (18[F]-fluorodeoxyglucose positron emission tomography) and hippocampal volumetric asymmetry indices and with clinical lateralization.
RESULTS: Mesial temporal CBF was more asymmetric in patients with temporal lobe epilepsy than in normal control participants, although asymmetric mesial temporal CBF was also found in normal participants, with the left side dominant. Ipsilateral mesial temporal CBF was significantly decreased compared with contralateral mesial temporal CBF in patients with temporal lobe epilepsy. Global CBF measurements were significantly decreased in patients compared with control participants. Asymmetry in mesial temporal blood flow in patients persisted after normalization to global CBF. Lateralization using continuous arterial spin labeling perfusion MR imaging asymmetry index significantly correlated with lateralization based on 18[F]-fluorodeoxyglucose positron emission tomography hypometabolism, hippocampal volumes, and clinical evaluation.
CONCLUSION: Continuous arterial spin labeling perfusion MR imaging can detect interictal asymmetries in mesial temporal lobe perfusion in patients with temporal lobe epilepsy. This technique is readily combined with routine structural assessment and potentially offers an inexpensive and noninvasive means of screening for asymmetries in interictal mesial temporal lobe function.
Epilepsy is among the most common neurologic disorders, affecting approximately 1% of the population. The most common seizure type in adults with medically intractable epilepsy is complex partial seizures, typically arising from the temporal lobe. Temporal lobectomy has been validated as a highly effective treatment for temporal lobe epilepsy. The success of temporal lobectomy in eliminating seizures correlates with the extent of resection and with abnormalities in functional activity in this tissue. Preoperative evaluation typically includes surface and possibly invasive EEG, MR imaging, positron emission tomography (PET), and intracarotid amobarbital testing (Wada test) (1, 2). In some centers, ictal single photon emission CT (SPECT) and MR spectroscopy are also part of the evaluation.
Lateralization of temporal lobe epilepsy is predicted by interictal mesial temporal lobe (mTL) hypometabolism using 18[F]-fluorodeoxyglucose PET (18FDG-PET) (3, 4). The presence of interictal abnormalities on PET or SPECT scans has also been associated with improved outcome from surgery to treat epilepsy (4, 5). In some studies, partial uncoupling of cerebral blood flow (CBF) and metabolism has been shown in the resting state, with 18FDG-PET showing better lateralization than H215O-PET measurements of CBF (6–8). A recent study showed decreased regional cerebral blood volume on the side of the epileptogenic focus using a bolus-contrast MR perfusion technique in patients with temporal lobe epilepsy (9). Although a direct comparison with 18FDG-PET is difficult because such a technique measures perfusion as opposed to metabolism, perfusion MR imaging may provide a practical alternative to PET because it is less expensive, does not involve ionizing radiation, and is more widely available.
Arterial spin labeling (ASL) is a totally noninvasive technique for quantitative perfusion MR imaging. ASL uses electromagnetic fields to label the nuclear spins of hydrogen in the water of the inflowing arterial to make blood itself an endogenous tracer. We have advanced one particular approach to ASL, continuous ASL, to a quantitative multi-section method suitable for clinical applications (10, 11). The purpose of this study was to determine the feasibility of using continuous ASL perfusion MR imaging for detecting CBF asymmetries in the temporal lobes in patients with temporal lobe epilepsy.
Methods
Participants
Twelve patients with medically refractory temporal lobe epilepsy undergoing preoperative evaluation for temporal lobectomy were studied. Demographic and clinical data were obtained from the patient charts. Clinical information included side of seizure onset, EEG findings, and outcome after surgery. The mean patient age was 31 years (range, 20–41 years), and there were six male and six female patients. Twelve healthy participants also underwent continuous ASL perfusion MR imaging. The mean participant age was 30 years (range, 20–68 years), and there were 11 female and one male participant. All patient and participant studies were conducted within guidelines from the Institutional Review Board, and consent was obtained for all studies.
MR and PET Imaging
MR imaging was performed on a 1.5-T GE Echospeed Horizon clinical scanner (GE Medical Systems, Milwaukee, WI). Sagittal and axial T1-weighted images were obtained (600/14 [TR/TE]). Continuous ASL perfusion images were obtained with 4000/22. CBF was measured in eight contiguous 8-mm axial sections (2-mm spacing between sections); matrix, 64 × 40; field of view, 24 × 15 cm (3.75-mm in-plane resolution). The total imaging time was 6 min. Continuous ASL (or control labeling) was applied at the level of the cervicomedullary junction with a post-labeling delay of 1.2 s (10, 11). Perfusion was calculated from images acquired with arterial or control labeling using echo-planar imaging T1-weighted maps acquired concurrently (10). For measurements of hippocampal volumes, coronal T1-weighted 3D spoiled gradient-echo images were obtained with 35/5; flip angle, 45 degrees; section thickness, 1.5 mm; matrix, 256 × 192; field of view, 24 cm (0.94 ×1.25 mm in-plane resolution).
All PET studies were performed as part of the routine clinical evaluation, using a system custom-designed for brain imaging (HEAD Penn-PET [12]), obtaining 2-mm sections through the brain at an isotropic spatial resolution of 4.5 mm full-width-half-maximum. The field of view was 12.8 cm. 18FDG-PET was IV administered at a dose of 0.037 mCi/kg of body weight, injecting interictally.
Analysis
Right and left mesial temporal lobe (mTL) regions of interest (ROI) were manually drawn on the continuous ASL perfusion images by one investigator (R.L.W.) who was blinded to the laterality of the patients' seizures. Regions were drawn around mesial temporal structures based on a visual inspection of both perfusion maps and T1-weighted spin-echo images obtained at the same section locations, extending the ROI posteriorly to the level of the quadrigeminal plate and including uncus, amygdala, hippocampus, and parahippocampal gyrus. No attempt was made to separate these structures. A perfusion asymmetry index was calculated from the mean flow values in these ROI using the relation asymmetry index = 100[mTL(left) − mTL(right)]/[mTL(left) + mTL(right)]. Because of the potential for a global decrease in CBF due to antiseizure medication (13, 14), normalized mTL CBF ratios were also generated by dividing mean CBF in each mTL ROI by global CBF. The latter was calculated by multiplying perfusion images by a binary mask to exclude background noise and taking the mean CBF over all brain voxels.
Another author (P.M.) drew ROI comparable with those used for calculations of perfusion asymmetry index on axial 18FDG-PET images, again including uncus, amygdala, hippocampus, and parahippocampal gyrus. The metabolic asymmetry index was calculated using the same equation, using mean mTL activity instead of mean flow. As was done for the MR perfusion measurements, normalized mTL activity ratios were generated by dividing mTL activity on each side by global brain activity. Global activity measurements were obtained by averaging counts within ROI covering the supratentorial brain, excluding the background.
For hippocampal volumetric asymmetry index, another author (I.L.R.) traced ROI around each hippocampal formation (amygdala and hippocampus only) using the coronal 3D spoiled gradient-echo images, calculating asymmetry index with the same equation. Each author was unaware of the others' results.
Statistical Analysis
The asymmetry index obtained from continuous ASL perfusion MR imaging was compared with the laterality of temporal lobe epilepsy, as determined clinically, and with surgical outcome. Spearman rank correlations were generated for continuous ASL perfusion MR imaging asymmetry index, PET asymmetry index, and hippocampal volumetric asymmetry index. Finally, a Wilcoxon signed rank test was conducted on mean mTL values and normalized mTL ratios within groups and a Wilcoxon rank sum test performed between groups to search for differences in left versus right mTL perfusion and differences in mTL perfusion between groups.
Results
Demographic and clinical data are shown in Table 1. Seven patients had right temporal lobe epilepsy, and five had left temporal lobe epilepsy. Of the 12 patients in this study, 12 underwent temporal lobectomy and 11 had PET studies. Nine of 12 patients showed abnormal findings on standard preoperative MR images, showing signal and/or parenchymal volume abnormalities in the mTL on the side eventually chosen as the seizure side. Of these, seven had changes consistent with mesial temporal sclerosis (one of which also showed contralateral hippocampal volume loss), two showed hippocampal atrophy, and two were normal. In one case (patient 9), structural MR imaging was initially interpreted as normal and pathologic findings after right temporal lobectomy were also interpreted as normal. However, a symmetric abnormality in migration and cortical organization was identified in the occipital lobes on follow-up imaging.
TABLE 1:
Patient clinical data
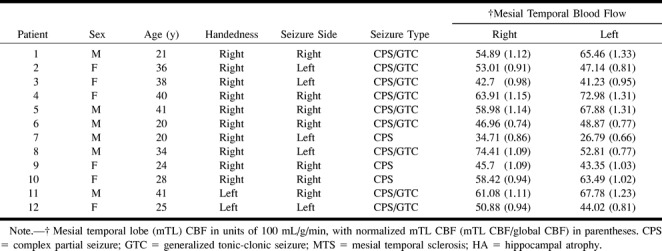
Figure 1 shows a representative example of continuous ASL perfusion MR imaging and 18FDG-PET images of a patient with left mesial temporal hypoperfusion/hypometabolism. Typical ROI placement is shown on T1-weighted images at the same locations. All study images obtained of patients and control participants were of sufficient quality for interpretation. Mean ± SD mTL perfusion in control participants was 74.06 ± 22.38 mL/100 g/min on the right (range, 44.03–125.75) and 78.60 ± 17.75 mL/100 g/min on the left (range, 51.22–109.21). Mean mTL perfusion tended to be higher on the left than on the right and was statistically significant for normalized mTL CBF measurements (P = .03, Wilcoxon signed rank test) but showed a trend only for raw mTL CBF measurements (P = .053).
fig 1.
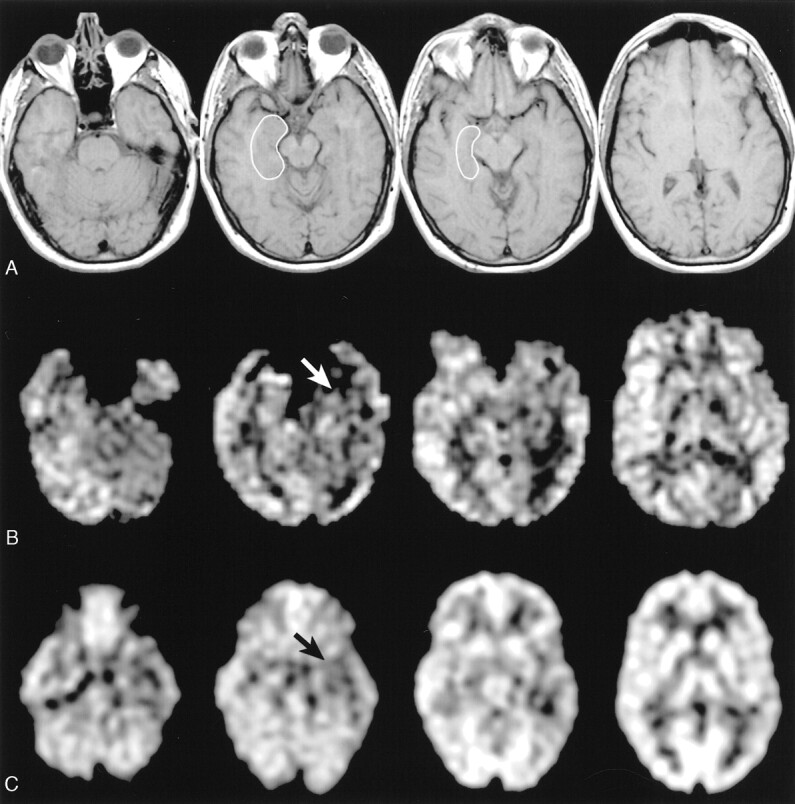
Data acquired from a patient with left temporal lobe epilepsy (patient 8).
A, T1-weighted axial images (600/14) show a representative mTL ROI used for calculation of mTL perfusion.
B, Continuous ASL perfusion images obtained at the same anatomic levels show hypoperfusion in the anterior left mTL (white arrow).
C, 18FDG-PET images obtained at approximately the same anatomic levels show hypometabolism in the left mesial temporal structures compared with the right (black arrow).
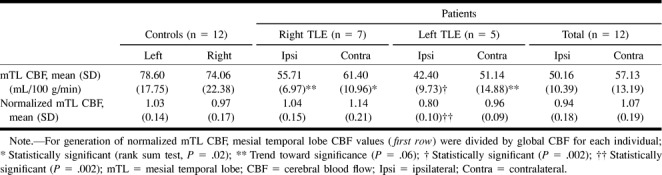
In patients, global CBF was significantly decreased compared with control participants (P = .0001, Wilcoxon rank sum test). Mesial temporal lobe perfusion was 50.16 (±10.39) mL/100 g/min on the ipsilateral (affected) side (range, 26.79–63.91) and 57.13 ± 13.19 mL/100 g/min on the contralateral (unaffected) side (range, 34.71–74.41). The blood flow measurements were significantly different in ipsilateral versus contralateral sides in patients for both normalized (P = .005) and raw measurements (P = .002, Wilcoxon signed rank test). Mesial temporal lobe perfusion was found to be less than in normal participants on the side ipsilateral (P = .06 on right and P = .002 on left, Wilcoxon rank sum test) and contralateral (P = .02 on right and P = .06 on left) to the seizure side. When mean mTL CBF was normalized, only ipsilateral (left) mTL perfusion in patients with left temporal lobe epilepsy were significantly decreased compared with normal participants (P = .002). For right temporal lobe epilepsy, mean normalized CBF ratios were 1.04 (±0.15) ipsilateral (right) to the seizure side and 1.14 (±0.21) contralateral to the seizure side, as opposed to 0.97 (±0.17) on the right and 1.03 (±0.14) on the left in control participants. In this case, normalized CBF values imply increased CBF in bilateral mTL relative to global CBF. These results are summarized in Table 2. Mesial temporal lobe metabolism on PET imaging was significantly decreased on the ipsilateral versus contralateral side for both raw (P = .003, Wilcoxon signed rank test) and normalized measurements (P = .003).
TABLE 2:
Mesial temporal lobe perfusion: patients versus controls
Because patients with both right and left temporal lobe epilepsy were present in the sample, absolute values of asymmetry indices were used for comparisons of control participants and patients. In control participants, the mean absolute asymmetry index ± SD was 4.92 ± 2.76 (range, 0.98–9.65). For the patients, mean absolute asymmetry index ± SD was 6.76 ± 4.28 (range, 1.75–16.98). The difference was not statistically significant. The distribution of asymmetry index values is shown in Table 3, along with results of clinical seizure lateralization by surface and/or intracranial EEG monitoring, 18FDG-PET, and the outcome from temporal lobectomy. 18FDG-PET absolute asymmetry index ± SD was 5.43 ± 2.72 (range, 1.43–9.19) for the patient group.
TABLE 3:
CASL perfusion MR imaging, 18FDG-PET, and MR hippocampal volumetric lateralization versus outcome
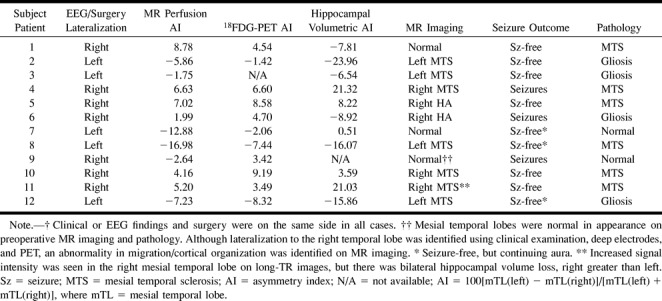
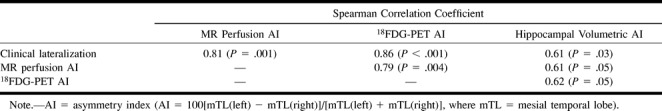
There was good agreement between the continuous ASL perfusion MR asymmetry index and clinical laterality (r = 0.81, P = .001). Correlation coefficients for 18FDG-PET and hippocampal volumetric asymmetry indices were 0.86 (P = .0004) and 0.61 (P = .034), respectively (Table 4). Correlation coefficients for measured asymmetry index were 0.61 (P = .047) for continuous ASL perfusion MR and hippocampal volume, 0.79 (P = .004) for continuous ASL perfusion MR and 18FDG-PET, and 0.62 (P = .054) for 18FDG-PET and hippocampal volumes. Lateralization by continuous ASL and 18FDG-PET showed complete agreement, except in patient 3 (who did not undergo PET imaging) and patient 9 for whom continuous ASL showed left hypoperfusion (although the asymmetry index was <3.0) whereas PET showed hypometabolism on the right. This patient did not achieve a seizure-free outcome from surgery to treat epilepsy. Two other patients (patients 4 and 6) continued to have seizures. In these patients, the asymmetry indices were 6.63 and 1.99, respectively. Pathologic examination revealed mesial temporal sclerosis and/or gliosis in all except two cases, which were interpreted as normal.
TABLE 4:
Continuous ASL perfusion MR imaging, 18FDG-PET, and MR hippocampal volumetric lateralization versus clinical lateralization
Discussion
Measurement of mTL perfusion asymmetry complements a broad range of MR measures, which have shown usefulness in the presurgical management of temporal lobe epilepsy (2). Findings of hippocampal volume loss and increased signal intensity on long-TR images on conventional MR studies are helpful in lateralizing temporal lobe epilepsy (15). Quantitative hippocampal volume measurements have been shown to have additional lateralizing value (16, 17). Mesial temporal lobe function has also been addressed using MR imaging. MR spectroscopy has shown changes in pH and N-acetylaspartate levels, which contribute to localization of the epileptogenic focus (18–20). Functional MR imaging using the BOLD technique has also recently been assessed as a means of noninvasive mapping of eloquent cortex and for assessing memory function in mTL structures (21, 22).
These results show the feasibility of using a continuous ASL perfusion MR imaging technique to provide additional data for seizure lateralization in cases of temporal lobe epilepsy. Mesial temporal blood flow was more asymmetric in patients with temporal lobe epilepsy than in normal control participants, and ipsilateral mesial temporal CBF was significantly decreased compared with contralateral mesial temporal CBF. In addition, decreased mTL perfusion in patients with temporal lobe epilepsy compared with normal participants was seen both ipsilateral and contralateral to the seizure side. The results were statistically significant on the left for ipsilateral seizure foci and on the right for contralateral foci and were trending toward significance (P = .06) on the right for ipsilateral foci and on the left for contralateral foci. Normalization to global CBF indicated that only ipsilateral (left) mTL CBF was significantly decreased in patients with left temporal lobe epilepsy compared with left mTL CBF in control participants. Patients with right temporal lobe epilepsy did not show a statistically significant decrease in normalized ipsilateral (right) mTL perfusion compared with normalized right mTL perfusion in control participants; the normalized values imply that mTL perfusion was actually increased. Variance should increase with normalization, at least partially accounting for this finding, but it may be that normalization to a different perfusion region, such as the cerebellum, may be more appropriate than normalizing to global CBF. Relative mTL perfusion asymmetry was maintained for patients with right temporal lobe epilepsy, however, with ipsilateral mTL CBF lower than contralateral. A true difference will be more difficult to detect for patients with right temporal lobe epilepsy, at least with these populations, because control participants also have baseline asymmetry in this direction.
Global CBF measurements were significantly decreased in patients compared with normal control participants. Although the populations were not sex-matched (most of the control participants were female), the ages were similar for patients and control participants. One explanation for this finding could be the use of antiseizure medications, which may decrease global CBF (13, 14). Alternatively, several previous reports have shown extratemporal and bilateral temporal lobe abnormalities in cases of temporal lobe epilepsy. For example, previous studies have shown hypometabolic regions ipsilateral to seizure side in 18FDG-PET studies (23), contralateral temporal lobe hyperperfusion in ictal SPECT studies (24), and ipsilateral greater than contralateral extratemporal volume loss (25). Decreased N-acetylaspartate:creatine ratios have been found in bilateral temporal lobes (ipsilateral greater than contralateral) using proton MR spectroscopy (26, 27). Jack et al (28) found worse outcomes associated with atrophy of the contralateral hippocampus. Further studies will be necessary to determine whether contralateral mTL abnormalities or extratemporal abnormalities in continuous ASL perfusion MR imaging similarly contribute useful information for clinical evaluation and management.
Relative mesial temporal hypoperfusion using continuous ASL perfusion MR imaging correlated well with clinical lateralization of seizure side, as well as with 18FDG-PET hypometabolism and hippocampal volume loss. Another recent study showed similar findings using a contrast-bolus MR perfusion technique, measuring interictal relative cerebral blood volume in patients with temporal lobe epilepsy (9). Although both continuous ASL and contrast-bolus perfusion methods are sensitive to hemodynamic changes, continuous ASL provides a diffusable tracer and therefore measures classical tissue perfusion. No injection of contrast material is required, and measurements can be repeated indefinitely (11).
Previous studies have questioned the usefulness of CBF measurements for localization of seizure foci. For example, H215O PET measurements of CBF were not found to be useful in localization of seizures (29). Other studies have shown uncoupling of CBF and metabolism interictally, with 18FDG-PET showing better lateralization than H215O PET measurements of CBF (6–8). However, MR imaging perfusion measurements are typically obtained at higher resolution than are H215O PET measurements of CBF. Although direct comparison with PET (at least 18FDG-PET) is difficult, because this technique measures CBF as opposed to metabolism, perfusion MR imaging may provide a practical alternative to PET because it is less expensive, does not involve ionizing radiation, and is more widely available. Additional studies with larger numbers are necessary to verify that relative mTL hypoperfusion using continuous ASL perfusion MR imaging can be used to lateralize seizures in temporal lobe epilepsy. Although asymmetry index calculated with continuous ASL perfusion MR imaging correlated well with clinical lateralization of seizures, the magnitude of the asymmetry index in patients was not significantly different from that in control participants. With the existing methodology, based on the mean absolute asymmetry index of 4.92 for control participants and 6.76 for patients, a study with approximately 70 participants in each group would have a power of 81% to detect a statistically significant result (α = 0.05), assuming the effect will be in the expected direction (more asymmetric perfusion in patients with temporal lobe epilepsy). Variance in the control population was higher than expected and probably contributed to this finding. Improved segmentation methods for ROI placement and coronal perfusion acquisitions may help address this issue. Interpretation of differences in asymmetry index are also complicated by the presence of bilateral decreased perfusion in the mTL of patients with temporal lobe epilepsy, which would tend to normalize perfusion asymmetry. Absolute perfusion may provide a better objective measure of abnormality, compared with a control population or normalized to global perfusion, although normalization can increase variance in the data.
One patient (patient 9) had discordant findings lateralizing to the left side, whereas EEG and PET findings lateralized to the right (Table 3). Structural MR imaging was initially interpreted as normal in this instance, and pathologic findings after right temporal lobectomy were also interpreted as normal. However, a symmetric abnormality in migration and cortical organization was identified in the occipital lobes on follow-up images. This patient did not attain seizure-free status after surgery. In this case, the magnitude of the asymmetry index was <3.0, indicating a relatively small difference between ipsilateral and contralateral mTL CBF. Two other patients (patients 4 and 6) also continued to have seizures. Perfusion MR imaging lateralized correctly in these cases; however, hippocampal volumetrics were discordant for patient 6. Pathologic abnormality was present in the resected tissue in these cases.
One potential confounding factor for both perfusion MR imaging and 18FDG-PET is that observed decreases in mean blood flow or metabolism could be due to mesial temporal atrophy. However, in one case (patient 7), a clear asymmetry in perfusion (asymmetry index = −12.88) was identified despite normal structural MR imaging (hippocampal volume asymmetry index = 0.51). This finding suggests dissociation between structure and function. Additional evidence supporting structure-function dissociation can be found in the data regarding patients 1 and 6 in this series, which indicate that the functional parameters, MR perfusion asymmetry index and PET asymmetry index, lateralized to the right and the structural parameter, MR volumetric asymmetry index, lateralized to the left. Patient 6 continued to have seizures, but patient 1 was seizure-free after surgery. We also note that lateralization using functional tests (perfusion MR imaging and 18FDG-PET) correlated more strongly with clinical lateralization than did the structural test (hippocampal volumetrics). Because MR hippocampal volume measurements indicate a later stage in mesial temporal sclerosis, a functional technique such as perfusion MR imaging, proton MR spectroscopy, and 18FDG-PET may be useful in identifying the process in its early stages (ie, before volume loss) or in helping to resolve difficulties in lateralization.
Conclusion
In conclusion, our preliminary findings suggest that continuous ASL perfusion MR imaging can successfully detect interictal asymmetry in mTL perfusion in patients with temporal lobe epilepsy. Lateralization using functional measurements provides complementary information to tests that assess structure. Continuous ASL perfusion MR imaging is readily combined with routine structural assessment (and also functional assessment using MR spectroscopy) and offers an inexpensive and completely noninvasive means of screening for asymmetry in interictal mTL function, providing a practical alternative to PET imaging.
Acknowledgments
We thank Jesse Chittams, MS, Senior Biostatistician, Division of Biostatistics, Department of Biostatistics and Epidemiology, for advice and assistance with the statistical analyses.
Footnotes
This work was supported by NS02079 and NS37488 (J.A.D.), National Institutes of Health Training Grant T32 CA 7478 (R.L.W.), and the Whitaker Foundation (D.C.A.).
This work was presented in part at the Annual Meeting of the International Society for Magnetic Resonance in Medicine, Philadelphia, PA, May 1999, and at the Annual Meeting of the American Epilepsy Society, Orlando, FL, December 1999 (Young Investigator Award Recipient).
Address reprint requests to Ronald L. Wolf, MD, PhD, University of Pennsylvania Medical Center, Department of Radiology, 3400 Spruce Street, Philadelphia, PA 19104.
References
- 1.Fisher R, Stein A, Karis J. Epilepsy for the neuroradiologist. AJNR Am J Neuroradiol 1997;18:851-863 [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan J. Imaging and epilepsy. Brain 1997;120:339-377 [DOI] [PubMed] [Google Scholar]
- 3.Henry T, Engel J Jr, Mazziotta J. Clinical evaluation of interictal fluorine-18-fluorodeoxyglucose PET in partial epilepsy. J Nucl Med 1993;34:1892-1898 [PubMed] [Google Scholar]
- 4.Weinand M, Carter L. Surface cortical cerebral blood flow monitoring and single photon emission computed tomography: prognostic factors for selecting temporal lobectomy candidates. Seizure 1994;3:55-59 [DOI] [PubMed] [Google Scholar]
- 5.Manno E, Sperling M, Ding X, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology 1994;44:2331-2336 [DOI] [PubMed] [Google Scholar]
- 6.Gaillard W, Fazilat S, White S, et al. Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology 1995;45:1841-1847 [DOI] [PubMed] [Google Scholar]
- 7.Leiderman D, Balish M, Sato S, et al. Comparison of PET measurements of cerebral blood flow and glucose metabolism for the localization of human epileptic foci. Epilepsy Res 1992;13:153-157 [DOI] [PubMed] [Google Scholar]
- 8.Fink GR, Pawlik G, Stefan H, Pietrzyk U, Wienhard K, Heiss W-D. Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesial structures. J Neurol Sci 1996;137:28-34 [DOI] [PubMed] [Google Scholar]
- 9.Wu R, Bruening R, Noachtar S, et al. MR measurement of regional relative cerebral blood volume in epilepsy. J Magn Reson Imaging 1999;9:435-440 [DOI] [PubMed] [Google Scholar]
- 10.Alsop D, Detre J. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236-1249 [DOI] [PubMed] [Google Scholar]
- 11.Alsop D, Detre J. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 1998;208:410-416 [DOI] [PubMed] [Google Scholar]
- 12.Karp JS, Freifelder R, Geagan MJ, et al. Three-dimensional imaging characteristics of the HEAD PENN-PET scanner. J Nucl Med 1997;38:636-643 [PubMed] [Google Scholar]
- 13.Theodore WH, Bromfield E, Onorati L. The effect of carbamazepine on cerebral glucose metabolism. Ann Neurol 1989;25:516-520 [DOI] [PubMed] [Google Scholar]
- 14.Gaillard WD, Zeffiro T, Fazilat S, DeCarli C, Theodore WH. Effect of valpoate on cerebral metabolism and blood flow: an 18F–2-deoxyglucose and 15O water positron emission tomography study. Epilepsia 1996;37:512-521 [DOI] [PubMed] [Google Scholar]
- 15.Jackson G, Berkovic S, Tress B, Kalnins R, Fabinyi G, Bladin P. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990;40:1869-1875 [DOI] [PubMed] [Google Scholar]
- 16.Jack CJ, Sharbrough F, Marsh W. Use of MR imaging for quantitative evaluation of resection for temporal lobe epilepsy. Radiology 1988;169:463-468 [DOI] [PubMed] [Google Scholar]
- 17.Jack CR Jr, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 1990;175:423-429 [DOI] [PubMed] [Google Scholar]
- 18.Laxer K, Hubesch B, Sappey-Marinier D, Weiner M. Increased pH and inorganic phosphate in temporal seizure foci demonstrated by [31P]MRS. Epilepsia 1992;33:618-623 [DOI] [PubMed] [Google Scholar]
- 19.Hugg J, Laxer K, Matson G, Maudsley A, Weiner M. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol 1993;34:788-794 [DOI] [PubMed] [Google Scholar]
- 20.Ng T, Comair Y, Xue M, et al. Temporal lobe epilepsy: presurgical localization with proton chemical shift imaging. Radiology 1994;193:465-472 [DOI] [PubMed] [Google Scholar]
- 21.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996;46:978-984 [DOI] [PubMed] [Google Scholar]
- 22.Detre JA, Maccotta L, King D, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology 1998;50:926-932 [DOI] [PubMed] [Google Scholar]
- 23.Henry TR, Mazziotta JC, Engel J Jr. Interictal metabolic anatomy of mesial temporal lobe epilepsy. Arch Neurol 1993;50:582-589 [DOI] [PubMed] [Google Scholar]
- 24.Ho SS, Berkovic SF, McKay WJ, Kalnins RM, Bladin PF. Temporal lobe epilepsy subtypes: differential patterns of cerebral perfusion on ictal SPECT. Epilepsia 1996;37:788-795 [DOI] [PubMed] [Google Scholar]
- 25.DeCarli C, Hatta J, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998;43:41-45 [DOI] [PubMed] [Google Scholar]
- 26.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology 1994;44:1411-1417 [DOI] [PubMed] [Google Scholar]
- 27.Cross JH, Connelly A, Jackson GD, Johnson CL, Neville BG, Gadian DG. Proton magnetic resonance spectroscopy in children with temporal lobe epilepsy. Ann Neurol 1996;39:107-113 [DOI] [PubMed] [Google Scholar]
- 28.Jack CR Jr, Sharbrough FW, Cascino GD, Hirschorn KA, O'Brien PC, Marsh WR. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 1992;31:138-146 [DOI] [PubMed] [Google Scholar]
- 29.Theodore W, Gaillard W, Sato S, Kufta C, Leiderman D. Positron emission tomographic measurement of cerebral blood flow and temporal lobectomy. Ann Neurol 1994;36:241-244 [DOI] [PubMed] [Google Scholar]


