Abstract
BACKGROUND AND PURPOSE: Although chronic-stage crossed cerebellar diaschisis (CCD) is reported to be associated with the neurologic state or clinical improvement after infarct, the prognostic value of early-stage CCD remains controversial. Our aim was to determine whether measurements of CCD in the acute and subacute stages obtained at single-photon emission CT (SPECT) facilitate the prediction of stroke outcome.
METHODS: The pattern of cerebral blood flow changes after the occurrence of acute middle cerebral artery ischemia with severe cortical symptoms was examined by using technetium 99m-hexamethylpropyleneamine oxime (99mTc-HMPAO) SPECT. Fifteen patients (mean age, 73 years ± 8 [SD]) with unilateral ischemia were examined in the early subacute stage (10 days ± 5). In 11 patients, SPECT was performed in both the acute (16 hours ± 10) and subacute stages. From the total counts obtained from each cerebellar hemisphere, the asymmetry index (AI) was calculated as follows: [(value in unaffected hemisphere − value in affected hemisphere)/value in unaffected hemisphere] × 100. Clinical outcome (at 60 days) was assessed by means of the Scandinavian Stroke Scale (SSS) and Barthel Index (BI).
RESULTS: AIs in the acute stage and clinical outcome (ie, SSS and BI scores) showed no significant correlation, but the severity of AI in the early subacute stage correlated significantly with both the final SSS (r = −0.69; P < .01) and BI scores (r = −0.82; P < .01).
CONCLUSION: Cerebellar hypoperfusion detected at 99mTc-HMPAO SPECT in the early subacute stage in patients with supratentorial infarct indicates a worse clinical outcome.
Baron et al (1) first demonstrated crossed cerebellar diaschisis (CCD) in patients with supratentorial infarction by using a noninvasive 15O continuous inhalation technique coupled with positron emission tomography (PET). Previous studies demonstrated that CCD matched depression of blood flow and metabolism in the cerebellum contralateral to a supratentorial focal lesion, as detected with PET (1–4). The mechanism underlying CCD reportedly consists of interruption of the cerebropontocerebellar pathway that causes deafferentation and transneural metabolic depression of the contralateral cerebellar hemisphere (1–4).
CCD in the chronic stage is associated with neurologic improvement after infarct in the middle cerebral artery (MCA) territory (5, 6). Some investigators have reported that CCD appears to be prominent in patients with severe hemiparesis in various stages (1, 4). Recent studies showed that CCD in the acute stage is not predictive of neurologic outcome, as quantified with stroke scales for PET (5) or technetium 99m-hexamethylpropyleneamine oxime (99mTc-HMPAO) single-photon emission CT (SPECT) (7, 8). However, to our knowledge, no detailed reports about the predictive value of CCD at stages between the acute and chronic stages have been published.
We performed 99mTc-HMPAO SPECT in patients in the acute and subacute stages of MCA ischemia who presented with severe cortical symptoms. Our aim was to determine whether CCD in the acute and subacute stages, as measured with 99mTc-HMPAO SPECT, facilitates prediction of the outcome and clinical improvement of stroke accompanied by MCA territory infarct.
Methods
Subjects
We examined 15 consecutive patients (11 men and four women; age range, 59–88 years; mean age, 73 years ± 8 [SD]) with acute cortical infarction in the territory of the unilateral MCA (Table). Patients with hemispheric symptoms and persistent hemiparesis were selected. Twenty-six SPECT scans were obtained in 15 patients with stroke; these showed that cerebral hypoperfusion involved the left hemisphere in 10 patients and the right hemisphere in the other five. All patients underwent 99mTc-HMPAO SPECT in the early subacute stage after the stroke episode (10 days ± 5). In 11 patients, SPECT was performed within 48 hours after the onset (16 hours ± 10). None of the patients in this study had clinical symptoms or MR imaging findings suggestive of ischemic episodes in the vertebrobasilar territory; routine MR imaging findings did not suggest gross morphologic alterations in the cerebellum. No thrombolytic agents were administered to any of the patients (9), but routine medications, such as antiplatelet agents, antibiotics, and heparin, were allowed. Patients 5 and 11 received intravenous Argatroban, a thrombin inhibitor, in the acute stage. No recurrences of stroke were noted during the 60-day follow-up period. Patients with complications (eg, chronic heart failure) that affected the activities of daily living and neurologic improvement were excluded. All patients, or their relatives, gave informed consent to participate in this study.
TABLE 1:
Clinical characteristics and cerebellar AI
| Patient No./Age (y)/Sex | MCA Side | Infarct | AI | SSS | RI | BI at 60 Days | ||
|---|---|---|---|---|---|---|---|---|
| Mechanism* | Topography† | Acute | At 60 Days | |||||
| 1/78/F | L | E | F, T, P, d | 13.6 | 13 | 38 | 55.5 | 63 |
| 2/72/M | L | E | F, T, P | 13.3 | 16 | 37 | 26.2 | 54 |
| 3/60/M | R | E | F, T, P | 11.9 | 24 | 41 | 50.0 | 48 |
| 4/64/M | L | E | F, T, P, d | 10.6 | 18 | 61 | 32.5 | 42 |
| 5/81/M | L | A | T, P | 10.0 | 20 | 65 | 39.5 | 43 |
| 6/67/F | L | E | F, T, P | 10.0 | 16 | 67 | 26.2 | 71 |
| 7/88/M | R | E | F, T, P | 9.6 | 33 | 43 | 40.0 | 66 |
| 8/75/M | R | E | P | 9.5 | 32 | 34 | 7.7 | 55 |
| 9/77/M | L | E | F, d | 7.3 | 24 | 54 | 88.2 | 87 |
| 10/59/F | R | E | d | 5.9 | 36 | 58 | 100 | 100 |
| 11/81/M | L | A | P, d | 5.7 | 9 | 54 | 91.8 | 86 |
| 12/81/M | L | E | T, d | 5.5 | 12 | 58 | 100 | 90 |
| 13/67/F | R | E | F, d | 4.4 | 22 | 55 | 91.7 | 95 |
| 14/72/M | L | A | P, d | 2.1 | 8 | 44 | 72.0 | 95 |
| 15/82/M | L | E | d | 1.2 | 28 | 54 | 86.7 | 95 |
A indicates atherothrombotic; E, embolic.
d indicates deep MCA territory; F, frontal; P, parietal; T, temporal.
SPECT Imaging Protocol
SPECT imaging was performed 10–20 minutes after the intravenous administration of 740 MBq of 99mTc-HMPAO by using a two-head rotating gamma camera interfaced with a dedicated computer system. Sixty images were acquired within 20 minutes, with a 128 × 128 matrix and a low-energy high-resolution collimator during a 180° rotation. The transaxial sections were reconstructed by means of filtered backprojection with a Butterworth filter. Each reconstructed section was corrected for tissue absorption by using Chang’s method. An average of 20 SPECT image planes, 3.9 mm thick, were required to image the entire brain. All images were resectioned parallel to the orbitomeatal plane before analysis.
Image Analysis
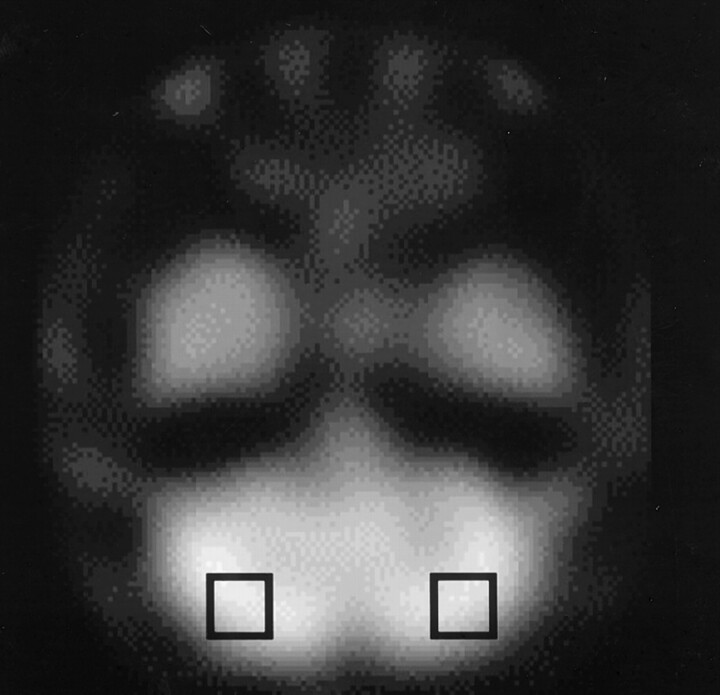
The SPECT images were analyzed semiquantitatively. A 20 × 20-mm region of interest (ROI) was placed on the ipsilateral and contralateral cerebellar hemispheres (Fig 1) (10). From the total counts obtained from each cerebellar hemisphere, the asymmetry index (AI) was calculated by using the following equation: AI = [(value in unaffected hemisphere − value in affected hemisphere)/value in unaffected hemisphere] × 100.
Fig 1.
ROIs on a SPECT image of the cerebellum.
Assessment of Patients’ Neurologic Status
Clinical stroke severity was quantified at admission and at day 60 with the Scandinavian Stroke Scale (SSS) (scored from 0 to 58) (11). Functional assessment was performed at 60 days with the Barthel Index (BI) (scored from 0 to 100) (12). The recovery index (RI) was expressed as actual improvement, a percentage reflecting the proportion of potential improvement, as follows: RI = [(final SSS score at 60 days − initial SSS score)/(maximal SSS score − initial SSS score)] × 100 (13).
Statistical Analysis
Changes in AI or SSS were analyzed with the Wilcoxon signed rank sum test. Relationships between the degree of AI and clinical severity were evaluated with the nonparametric Spearman rank test. A P value of less than .05 was considered to indicate a statistically significant difference. Statistical analysis was performed with a statistical software package (SPSS for Macintosh).
Results
The degree of cerebellar hypoperfusion was calculated as the AI in the cerebellar hemispheres. The mean AI was 9.6 ± 1.2 (SE) (n = 11; range, 3.9–16.2 ) in the acute stage and 8.0 ± 1.0 (n = 15; range, 1.2–13.6) in the subacute stage. In 11 cases, both acute- and subacute-stage CCD measurements were available for evaluation of serial changes in cerebellar hypoperfusion. Results indicated that the mean AI of 9.6 ± 0.9 in the subacute stage was not statistically different from the value of 9.6 ± 1.2 in the acute stage (P = 0.86), although the time course of AI differed among patients (Figs 2 and 3).
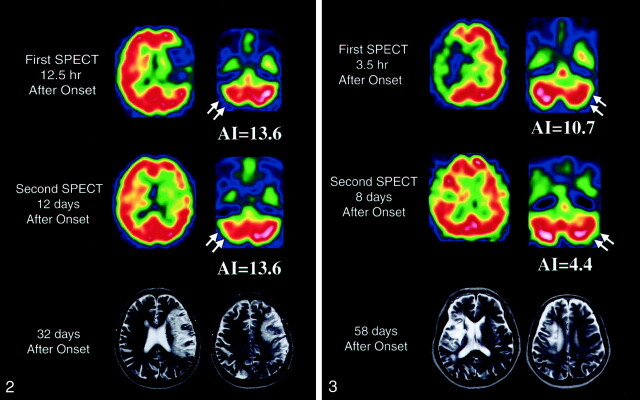
Fig 2.
SPECT images obtained in patient 1 show severe cerebellar hypoperfusion (arrows) in both the acute (top row) and subacute (middle row) stages. This patient had SSS scores of 13 at admission and 38 at 60 days after onset. MR images (bottom row) obtained at 32 days show a large infarct in the left hemisphere.
Fig 3.
SPECT images obtained in patient 13 show cerebellar hypoperfusion (arrows) in both the acute (top row) and subacute (middle row) stages. This patient had SSS scores of 22 at admission and 55 at 60 days after onset. MR images (bottom row) obtained at 58 days show an infarct in the right basal ganglia and frontal lobe.
The clinical data are summarized in the Table. Mean SSS scores improved from 21 ± 2 (SE) in the acute stage to 45 ± 2 at 60 days (P < .01).
First, we investigated the relationship between the AI in the acute stage and clinical outcome at 60 days and found no significant correlation between the first parameter and the final SSS score at 60 days (r = −0.22; P = .49), the final BI score at 60 days (r = −0.04, P = 0.90), or the RI (r = −0.06, P = .85). We also investigated the relation between AI in the subacute stage and clinical outcome at 60 days and found a good correlation with the final SSS score (r = −0.69; P < .01), the final BI score (r = −0.82; P < .01), and the RI (r = −0.64; P < .05) (Fig 4).
Fig 4.
Relationships between the AI in the subacute stage and neurologic state. No significant (N.S.) correlations between the AI in the subacute stage and SSS score at admission were found (top left). AI in the subacute stage was significantly associated with the final SSS score at 60 days (top right), the final BI score at 60 days (bottom left), and the RI (bottom right), as the results of the nonparametric Spearman rank test indicate.
Discussion
The frequency of CCD has been reported to be higher in patients with infarcts involving the frontoparietal lobe, basal ganglia, and internal capsule at various stages (14, 15) than in other patients. Yamauchi et al (16) demonstrated that cerebral hemodynamic and metabolic status can cause CCD, even in small infarcts with unilateral major cerebral artery occlusion. However, little is known about the serial changes in cerebellar hypoperfusion or the prognostic value of CCD observed on 99mTc-HMPAO SPECT scans in infarct patients in the early stage.
Although the mean AI in the cerebellum did not significantly change from the acute stage to the subacute stage in the series as a whole, the time course of AI differed among patients. Infeld et al (7) found no statistically significant change in cerebellar hypoperfusion between the acute stage (within 36 hours after onset) and chronic stage (6.5 months ± 4), as assessed with 99mTc-HMPAO SPECT. Thus, our findings support their results.
In our study, the degree of AI in the acute stage was not significantly correlated with neurologic severity in the outcome stage. Infeld et al (7) demonstrated that CCD per se in the acute stage, as observed on 99mTc-HMPAO SPECT scans obtained within 72 hours of the onset, was not predictive of clinical outcome according to the Canadian Neurological Scale score or BI scores. Laloux et al (8) also reported that CCD in the acute stage, as determined with 99mTc-HMPAO SPECT (within 36 hours after onset), had no predictive value in terms of functional outcome, as assessed with the Rankin scale. Serrati et al (5) reported no significant correlation between contralateral cerebellar hypometabolism, (ie, oxygen consumption), as evaluated with early PET (within 30 hours after onset), and neurologic state at 60 days. Thus, our results are consistent with those of these three previous studies. At least two possible pathophysiologic explanations may account for our results in the acute stage. First, a reasonable speculation is that the still evolving cerebral perfusion and metabolic disturbances during the acute stage of cerebral infarction might have affected findings. Second, just after a stroke, CCD may still be in a pathophysiologically unsettled status, which can manifest itself as reversible damage and potential tissue recovery (5, 17, 18). Therefore, the degree of cerebellar hypoperfusion in the acute stage does not seem to have prognostic value regarding stroke outcome.
Reperfusion on 99mTc-HMPAO SPECT scans obtained during the subacute stage of ischemic stroke can mask supratentorial ischemic lesions (19–21), and thus, the severity or size of the area of supratentorial hypoperfusion may not be a predictor of stroke outcome. The presence of CCD in the subacute stage may be useful in diagnosing the location and extension of the cerebral ischemic lesion involved in the cerebropontocerebellar pathway (19, 22). However, to our knowledge, no reports about the prognostic clinical value of CCD regarding the outcome of stroke in the subacute stage have been published. These findings have shown that CCD in the early subacute stage after MCA territory infarction with hemispheric symptoms is correlated with neurologic severity and the degree of disability 60 days after the onset of symptoms. The negative direction of these correlations means that, as the degree of cerebellar hypoperfusion in the subacute stage increases, the neurologic outcome worsens.
Serrati et al (5) used the Mathew and Orgogozo scales to study the relationship between crossed cerebellar hypometabolism and clinical outcome on day 60. They reported that crossed cerebellar hypometabolism in the early chronic stage is strongly associated with neurologic outcome, as measured with cerebellar oxygen consumption. In a retrospective study, De Reuck et al (6) reported that PET findings and Orgogozo scores indicated better neurologic improvement in patients without significant CCD in the late chronic stage (4–24 months) of MCA infarcts than that of other patients. We performed SPECT at an earlier stage (ie, early subacute stage); to our knowledge, examination at this stage has not been studied. Cerebellar hypoperfusion in the subacute stage may persist in the chronic stage; therefore, the degree of cerebellar hypoperfusion in the subacute stage may be associated with clinical outcome.
Conclusion
The significant correlation between cerebellar hypoperfusion in the early subacute stage and clinical outcome at 60 days was revealed in this study. This finding suggests that CCD in the early subacute stage may be a valuable prognostic factor in patients with stroke.
Acknowledgments
We thank Shigeru Yoneda and Hiroshi Nakao of the Department of Radiology for technical support in performing brain SPECT imaging.
References
- 1.Baron JC, Bousser MG, Comar D, Castaigne P. Crossed cerebellar diaschisis in human supratentorial brain infarction. Trans Am Neurol Assoc 1980;105:459–461 [PubMed] [Google Scholar]
- 2.Lenzi GL, Frackowiak RS, Jones T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. J Cereb Blood Flow Metab 1982;2 ;321–335 [DOI] [PubMed] [Google Scholar]
- 3.Kushner M, Alavi A, Reivich M, Dann R, Burke A, Robinson G. Contralateral cerebellar hypometabolism following cerebral insult: a positron emission tomographic study. Ann Neurol 1984;15:425–434 [DOI] [PubMed] [Google Scholar]
- 4.Pantano P, Baron JC, Samson Y, Bousser MG, Derouesne C, Comar D. Crossed cerebellar diaschisis: further studies. Brain 1986;109:677–694 [DOI] [PubMed] [Google Scholar]
- 5.Serrati C, Marchal G, Rioux P, et al. Contralateral cerebellar hypometabolism: a predictor for stroke outcome. J Neurol Neurosurg Psychiatry 1994;57:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Reuck J, Decoo D, Lemahieu I, Strijckmans K, Goethals P, Maele GV. Crossed cerebellar diaschisis after cerebral artery infarction. Clin Neurology Neurosurg 1997;99:11–16 [DOI] [PubMed] [Google Scholar]
- 7.Infeld B, Davis SM, Lichtenstein M, Mitchell PJ, Hopper JL. Crossed cerebellar diaschisis and brain recovery after stroke. Stroke 1995;26:90–95 [DOI] [PubMed] [Google Scholar]
- 8.Laloux P, Richelle F, Jamart J, Coster PD, Laterre C. Comparative correlations of HMPAO SPECT indices, neurological score, and stroke subtypes with clinical outcome in acute carotid infarcts. Stroke 1995;26:816–821 [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1996;27:1711–1718 [PubMed] [Google Scholar]
- 10.Kuwabara Y, Ichiyama Y, Sasaki M, et al. Cerebellar vascular response to acetazolamide in crossed cerebellar diaschisis: a comparison of 99mTc-HMPAO single-photon emission tomography with 15O-H2O positron emission tomography. Eur J Nucl Med 1996;23:683–689 [DOI] [PubMed] [Google Scholar]
- 11.Scandinavian Stroke Study Group. Multicenter trial of hemodilution in ischemic stroke. Stroke 1985;16:885–890 [DOI] [PubMed] [Google Scholar]
- 12.Granger CV, Hamilton BB, Gresham GE, Kramer AA. The stroke rehabilitation outcome study, II: relative merits of the total Barthel Index score and a four-item subscore in predicting patient outcome. Arch Phys Med Rehabil 1989;70:100–103 [PubMed] [Google Scholar]
- 13.Shah S, Vanclay F, Cooper B. Efficiency, effectiveness, and duration of stroke rehabilitation. Stroke 1990;21:241–246 [DOI] [PubMed] [Google Scholar]
- 14.Kim SE, Choi CW, Yoon BW, et al. Crossed-cerebellar diaschisis in cerebral infarction: Technetium-99m-HMPAO SPECT and MRI. J Nucl Med 1997;38:14–19 [PubMed] [Google Scholar]
- 15.Kushner M, Kaasik AE, Nencini P, et al. Contralateral cerebellar hypometabolism following cerebral infarction: an acute and follow-up study. Neurology 1988;38:147 [Google Scholar]
- 16.Yamauchi H, Fukuyama H, Yamaguchi S, et al. Crossed cerebellar hypoperfusion in unilateral major cerebral artery occlusive disorders. J Nucl Med 1992;33:1632–1636 [PubMed] [Google Scholar]
- 17.Reivich M. Crossed cerebellar diaschisis. AJNR Am J Neuroradiol 1992;13:62–64 [PMC free article] [PubMed] [Google Scholar]
- 18.Heiss WD. Experimental evidence of ischemic thresholds and functional recovery. Stroke 1992;23:1668–1672 [DOI] [PubMed] [Google Scholar]
- 19.Moretti JL, Defer G, Cinotti L, et al. “Luxury perfusion” with Tc-99m HMPAO and I-123 IMP SPECT imaging during the subacute phase of stroke. Eur J Nucl Med 1990;16:17–22 [DOI] [PubMed] [Google Scholar]
- 20.Sperling B, Lassen NA. Hyperfixation of HMPAO in subacute ischemic stroke leading to spuriously high estimates of cerebral blood flow by SPECT. Stroke 1993;24:193–194 [DOI] [PubMed] [Google Scholar]
- 21.Bowler JV, Wade JPH, Jones BE, Nijran KS, Steiner TJ. Natural history of the spontaneous reperfusion of human cerebral infarcts as assessed by 99mTc HMPAO SPECT. J Neurol Neurosurg Psychiatry 1998;64:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin WY, Kao CH, Wang PY, Changlai SP, Wang SJ. Serial changes in regional blood flow in the cerebrum and cerebellum of stroke patients imaged by 99mTc-HMPAO SPECT. Nucl Med Commun 1996;17:208–211 [DOI] [PubMed] [Google Scholar]