Abstract
BACKGROUND AND PURPOSE: In embolic middle cerebral artery (MCA) trunk occlusion, recanalization with direct percutaneous transluminal angioplasty (PTA) may be preferable to time-consuming thrombolysis. However, distal embolization with small crushed fragments is a complication of direct PTA. We prospectively evaluated combined direct PTA and low-dose native tissue plasminogen activator (t-PA) therapy for acute embolic MCA trunk occlusion.
METHODS: Fifteen patients underwent direct PTA. The embolus was successfully crushed in 12, who received subsequent native t-PA infusion. Direct PTA was performed with a balloon catheter, which was advanced into the occlusion site and inflated several times until recanalization was established. After PTA, 7.2 mg of native t-PA in 100 mL of isotonic sodium chloride solution was infused for 30 minutes. Neurologic status was evaluated at admission and immediately and 1 month after treatment. In all patients, follow-up CT was performed within 24 hours and 3–7 days after onset, and follow-up MR imaging, 1 month after onset.
RESULTS: Direct PTA failed to crush the embolus in three of 15 patients; these three had no clinical improvement. In 11 of 12 patients, combined therapy was successful, with no technical complication. Although no symptomatic intracerebral hemorrhage occurred, one patient had a small hematoma. All patients with successful recanalization had marked clinical improvement. Although angiograms showed distal embolizations in 10, cortical infarctions were confirmed in only three at follow-up.
CONCLUSION: Combined direct PTA and IV low-dose native t-PA therapy may be a safe alternative to thrombolytic therapy in some patients with embolic MCA trunk occlusion.
In patients with embolic middle cerebral artery (MCA) trunk occlusion, the embolus often is so large that it is resistant to thrombolysis and time-consuming thrombolytic therapy. High doses of thrombolytic agents may be required, and these may result in an unfavorable outcome with hemorrhagic complications (1–5). When the lenticulostriate arteries are involved in ischemia or when early CT ischemic changes are present, the therapeutic window may be short. For safety in such cases, mechanical crushing of the embolus and immediate recanalization with direct percutaneous transluminal angioplasty (PTA) may be preferable to time-consuming thrombolytic therapy (4, 5). Although distal embolization with small crush fragments is a noteworthy complication of direct PTA (5–10), thrombolysis of these small fragments is likely to be easier with small amounts of thrombolytic agents (5). Distal embolic occlusion in vessels such as MCA divisions or branches has been reported to be the best condition for the IV administration of tissue plasminogen activator (t-PA) (11). Recently, Nakano et al (12) also demonstrated that IV infusion of low-dose native t-PA might be safe and effective for small distal emboli in the MCA divisions and branches.
On the basis of these considerations, we performed the combined therapy of direct PTA and subsequent IV infusion of low-dose native t-PA in patients with embolic MCA trunk occlusion. The purpose of this study was to prospectively investigate the feasibility of this combination therapy in 12 patients.
Methods
Since 1996, in considering some reperfusion therapies, we performed emergency angiography in 59 patients with acute carotid territory stroke. Among them, 41 patients had embolic MCA occlusion. According to the following protocol, each patient with embolic MCA occlusion was treated with direct PTA or thrombolytic therapy. In patients with occlusion of the MCA divisions or branches, native t-PA was infused. Intraarterial thrombolytic therapy was performed only in those with MCA trunk occlusion with neither involvement of the lenticulostriate arteries nor early CT ischemic changes. Before the initiation of reperfusion therapy, a microcatheter was introduced beyond the thrombus, and superselective local angiography was performed to assess the size of the thrombus and the orifices of the lenticulostriate arteries. When involvement of the lenticulostriate arteries or early CT ischemic changes or both were present, direct PTA was selected as the first choice of treatment. After confirmation that the embolus was crushed, an IV infusion of low-dose native t-PA (7.2 mg of tisokinase) was started to minimize cerebral infarction induced by distal embolization.
According to these criteria, we treated 15 patients with direct PTA. In three patients, however, direct PTA could not provide recanalization, probably because large or hard thrombus could not be crushed into enough small pieces to move distally beyond the MCA bifurcation. In these three patients in whom direct PTA failed to crush the embolus, native t-PA was not infused. The other 12 patients (seven men, five women; age range, 49–80 years; mean age, 68 years) underwent the combination therapy consisting of direct PTA and IV infusion of native t-PA and were selected for this study. Two or three neurosurgeons on duty (T.Y., S.N., H.K.) read the initial pretherapeutic CT scans. Early CT ischemic changes in the deep MCA territory on the initial CT scan were classified according to the classification system proposed by Nakano et al (13). In brief, the grades were as follows: I, normal basal ganglia with subtle hypoattenuation localized to the insula; II, partial obscuration of the posterolateral part of the putamen; and III, hypoattenuation in the entire lentiform nucleus.
In this study, patient selection was performed according to the “early CT ischemic changes < 1/3 of the MCA territory” criterion (14).
The details of the procedure of direct PTA were previously reported (5, 6). In brief, after the demonstration of a large embolus in the MCA trunk at superselective local angiography, we explained the risk of thrombolytic therapy and recommended direct PTA to the patients or their families. After informed consent was obtained, angioplasty was performed. The diameter of the MCA trunk was confirmed by using superselective local angiography, and angioplasty was performed with Stealth angioplasty balloon catheter (Boston Scientific, Natick, MA) with a maximum diameter of 2.0–2.5 mm. The balloon catheter was advanced into the occlusion site and inflated by using a 0.016-inch Radifocus GT guidewire (Terumo, Tokyo, Japan), instead of a ball-valve wire with leakage of inflating pressure. The balloon was inflated to 2 atm initially and subsequently to 3 atm. Several inflations of 30 seconds each were performed until recanalization of the MCA trunk was established. After each inflation, repeat angiography was performed to assess the degree of recanalization and the presence of distal embolic occlusions. Immediately after direct PTA, 7.2 mg of native t-PA dissolved in 100 mL of isotonic sodium chloride solution was infused for 30 minutes at a constant rate.
One of the authors (T.Y.) who had knowledge of the reperfusion therapy evaluated the patient’s neurologic status at admission, just after treatment, and 1 month after treatment by using the National Institute of Health Stroke Scale (NIHSS). Major neurologic improvement was defined as a decrease of four points or more in the stroke score. Follow-up CT scans were obtained within 24 hours and again 3–7 days after onset. Follow-up MR examinations, including T1-weighted imaging (430/20 [TR/TE]), T2-weighted imaging (3000/100), and 3D time-of-flight MR angiography (40/7, flip angle, 25°), also was performed in all patients at 1 month after onset by using a 1.5-T system (Sierra; Yokogawa, Tokyo, Japan). In all patients, complete recanalization was confirmed at follow-up angiography or MR angiography. Two or three neurosurgeons (T.Y., S.N., H.K.) who were in charge of the reperfusion therapy evaluated the follow-up CT and MR images.
Results
The clinical and radiologic characteristics of the 12 patients are shown in the Table. In all patients, neurologic examination revealed complete hemiplegia or incomplete hemiparesis, and six patients with left MCA occlusion also had motor aphasia. The time from symptom onset to the completion of the procedure of direct PTA was 2.5–9.0 hours (average, 4.6 hours). Eight patients had early CT ischemic changes, and in 10 patients, the lenticulostriate arteries were involved. Early CT signs in the deep MCA territory were observed in all eight patients with early CT ischemic changes. Two had a grade I deep MCA sign, four had a grade II sign, and two a had grade III sign. Lenticulostriate artery involvement was not observed in the two patients with a grade I deep MCA sign. In nine patients, the presence of cardiac source for the embolus was confirmed with electrocardiography or echocardiography.
Clinical and radiologic characteristics in 12 patients
| Patient No./Age (y)/Sex | Early CT Sign |
Occlusion Site† | Lenticulostriate Artery Involvement | Embolic Source‡ | Time to PTA (h) | Complications§ | Initial Symptoms | NIHSS Score |
Cortical Infarct | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deep MCA Grade* | Superficial MCA | Admission | After Treatment | At 1 Mo | ||||||||
| 1/67/M | II | Yes | R M1 | Present | None | 3.0 | None | Hemiplegia | 22 | 12 | 3 | Yes |
| 2/80/M | I | Yes | R M1 | Absent | PAF | 4.0 | None | Hemiplegia | 16 | 2 | None | No |
| 3/72/F | II | No | L M1 | Present | AF | 3.5 | Small hemorrhage | Hemiplegia, aphasia | 13 | 5 | 1 | Yes |
| 4/74/F | None | No | R M1 | Present | AR, MR | 3.5 | None | Hemiplegia | 13 | 6 | 2 | No |
| 5/71/M | II | Yes | L M1 | Present | None | 2.5 | None | Hemiplegia, aphasia | 21 | 6 | 4 | Yes |
| 6/49/M | None | No | R M1 | Present | PAF | 7.0 | None | Hemiparesis | 5 | 0 | 0 | No |
| 7/68/M | None | No | R M1 | Present | None | 5.0 | None | Hemiplegia | 12 | 4 | 2 | No |
| 8/60/F | III | Yes | L M1 | Present | PAF | 4.0 | None | Hemiplegia, aphasia | 21 | 13 | 1 | No |
| 9/65/F | I | Yes | L M1 | Absent | MR | 3.0 | GI bleeding | Hemiplegia, aphasia | 12 | 12 | 4 | Yes |
| 10/70/M | II | No | L M1 | Present | TR | 6.0 | None | Hemiplegia, aphasia | 14 | 4 | 1 | No |
| 11/69/M | III | Yes | L M1 | Present | AF | 4.5 | None | Hemiplegia, aphasia | 24 | 8 | 1 | No |
| 12/75/F | None | No | R M1 | Present | AF | 9.0 | None | Hemiplegia | 15 | 6 | 1 | No |
The grades were as follows: I, normal basal ganglia with subtle hypodensity localized to the insula; II, partial obscuration of the posterolateral part of the putamen; and III, hypodensity in the entire lentiform nucleus.
M1 indicates the M1 portion of the MCA.
AF indicates atrial fibrillation; AR, aortic valve regurgitation; PAF, paroxysmal atrial fibrillation; MR, mitral valve regurgitation; and TR, tricuspid valve regurgitation.
GI indicates gastrointestinal.
In six patients, the emboli were crushed to small pieces with direct PTA, which resulted in the distal embolization of the small cortical arteries. Despite subsequent IV infusion of native t-PA, one (17%) of these patients had a cortical infarction on the follow-up CT scan. In four patients, the emboli were not crushed, but they were flattened with direct PTA, which resulted in the residual stenosis of the MCA. These four patients had no cortical infarction on the follow-up CT scan or MR image. In another two patients, the crushed emboli were relatively large, and direct PTA resulted in M2 occlusion. These two patients had small cortical infarctions. NIHSS scores at admission (mean ± SD, 16.0 ± 5.6) were markedly reduced just after treatment (6.0 ± 3.9) and 1 month later (1.6 ± 1.2). Despite marked clinical improvement, one patient (case 2) died from severe pneumonia after 2 months. Two patients had complications; one (case 3) had a small hemorrhage in the cerebral parenchyma just after treatment, the other (case 9) had gastrointestinal bleeding during native t-PA infusion, and we were obliged to stop the t-PA infusion.
Representative Case
Case 4 involved a 74-year-old woman with aortic and mitral valve regurgitation who presented with sudden-onset consciousness disturbance and left complete hemiplegia. Although the initial CT scan obtained at admission revealed an old infarction in the basal ganglia, no early CT ischemic changes were seen (Fig 1A). Angiography showed complete occlusion of the right MCA trunk and the distal portion of the anterior cerebral artery (ACA) (Fig 1B). Superselective local angiography depicted the short M1 trunk and the lateral lenticulostriate arteries arising from M2 segment. We diagnosed the condition as a cardioembolic stroke and selected rapid recanalization with direct PTA-wand IV infusion of low-dose native t-PA. Because the microcatheter was easily introduced into the ACA after local angiography in the MCA, and because the embolus in the ACA was considered to be small, local thrombolysis with a small amount of native t-PA (3.6 mg) was performed first in the ACA, in passing, and recanalization was successful. Subsequently, direct PTA was performed in the MCA occlusion by using a 2.0 mm × 1.5-cm Stealth balloon catheter with one inflation to 1.2 atm for 30 seconds. Although angiography after angioplasty revealed complete recanalization of the right MCA trunk (Fig 1C), distal embolization of small cortical arteries by the crush fragments was confirmed at several points (Fig 1D). After direct PTA, native t-PA (7.2 mg, 0.18 mg/kg) was infused. The patient recovered well, and she returned to her original condition without consciousness disturbance and hemiplegia after treatment. A follow-up CT scan obtained the next day revealed neither cerebral edema nor a newly developed low-attenuating area (Fig 1E).
Fig 1.
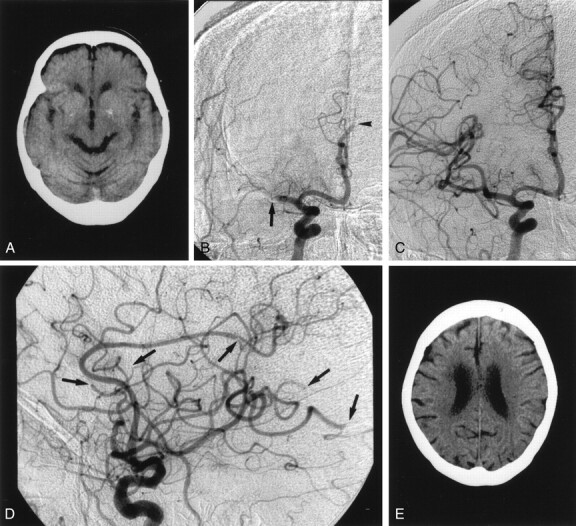
Case 4. A 74-year-old woman with aortic and mitral valve regurgitation who presented with sudden-onset consciousness disturbance and left complete hemiplegia.
A, Pre-PTA CT scan shows an old infarction in the right basal ganglia without any early CT ischemic changes.
B, Pre-PTA angiogram shows complete occlusion of right MCA trunk (arrow) and right ACA (arrowhead).
C, Angiogram obtained after PTA shows complete recanalization of the MCA trunk.
D, Angiogram obtained after PTA shows embolization in the small cortical arteries (arrows).
E, Follow-up CT scan shows no infarction in the cerebral cortex.
Discussion
As previously reported, direct PTA seems to be an effective and safe procedure applicable to not only thrombotic MCA occlusion but also embolic occlusion (5, 6). In embolic occlusion, unlike atherothrombotic MCA occlusion, the balloon catheter only has to crush the embolus; it need not provide dilatation force to the vessel wall. Therefore, when direct PTA is performed in acute embolic stroke, the most important issues are the selection of a balloon catheter with the appropriate diameter, which is less than the average inside diameter of the normal artery, and the inflation of the balloon under leakage of the inflating pressure. The Stealth balloon catheter has an open-ended system, and occlusion of the end hole with a ball-valve wire is required to inflate the balloon with a constant high pressure. A guidewire without a ball valve cannot completely occlude the end hole of the Stealth balloon catheter, resulting in leakage of the inflating pressure. In our direct PTA procedure for embolic occlusion, a common guidewire, instead of the ball-valve wire, was used to avoid excessive dilatation force on the vessel wall. This procedure caused neither arterial rupture nor spasm. Recanalization of the MCA trunk with direct PTA was classified into three patterns as follows: 1) The emboli were crushed small pieces, and the result was the distal embolization to the small cortical arteries. 2) The emboli were not crushed but flattened, and the result was residual stenosis of the MCA trunk. 3) The crushed emboli were relatively large, and the result was M2 occlusion. The emboli were not necessarily crushed into small pieces with direct PTA. In all patients with the type 2 recanalization pattern, the residual stenosis was not apparent on the follow-up angiogram or MR angiogram; no cortical infarctions were present.
The only problem with our procedure is distal embolization of the crushed fragments (5). If distal embolization occurs during the angioplasty procedure, the end hole of the balloon catheter allows intraarterial local injection of thrombolytic agents. Distal embolization in the MCA divisions may be treated with such intraarterial thrombolysis. However, in cases of more distal occlusions, once the balloon catheter is inflated, it cannot be safely introduced into the small vessels, and t-PA infusion may be a better approach that can be applied more quickly and safely. Although angiograms demonstrated distal embolizations in 10 cases (83%), in this study, cortical infarctions were confirmed in only three cases (25%).
The t-PA infusion may be useful to prevent or reduce cortical infarction due to distal embolization. As shown in the t-PA Acute Stroke Study Group trial, distal occlusions such as MCA divisions and branches recanalization is likely to be easy with t-PA infusion. Recently, investigators have reported that even low-dose native t-PA (7.2 mg tisokinase) may be effective for small distal emboli in the MCA divisions or branches when early CT ischemic changes and involvement of the lenticulostriate arteries are absent (12). Our present findings also demonstrated that IV infusion of low-dose native t-PA may be feasible for distal embolization after direct PTA for embolic MCA trunk occlusion, even in patients with early CT ischemic changes or involvement of the lenticulostriate arteries. As previous reports (4–6, 15–17) have shown, when ischemia involves the lenticulostriate arteries, thrombolytic therapy may be difficult to use in rescuing the lentiform nucleus, and it may be associated with a high risk of hemorrhagic transformation. To be sure, any treatment may be ineffective in rescuing the lentiform nucleus from the ischemic insults, because the lenticulostriate arteries are terminal vessels with poor collaterals. Also, in most patients in our study, the lenticulostriate arteries were involved, and the lentiform nucleus could not escape from the cerebral infarction. However, none of these patients had neurologic deterioration due to hemorrhagic complications. Some patients had almost complete neurologic recovery despite the striatocapsular infarction.
Conclusion
The preliminary results from this study suggest that direct PTA and subsequent IV infusion of low-dose native t-PA may be a feasible therapy for acute embolic MCA trunk occlusion, particularly in patients with early CT ischemic changes or involvement of lenticulostriate arteries. Although the safety and effectiveness of our procedure should be evaluated with randomized controlled clinical trials in a large number of patients, our present results encourage us to perform further trials of this combination therapy for acute embolic MCA trunk occlusion.
References
- 1.del Zoppo GJ. Thrombolytic therapy in cerebrovascular disease. Stroke 1988;19:1174–1179 [DOI] [PubMed] [Google Scholar]
- 2.Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989;20:598–603 [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Waldlow CP. Thrombolysis in acute ischemic stroke: does it work? Stroke 1992;23:1826–1839 [DOI] [PubMed] [Google Scholar]
- 4.Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 1996;39:1102–1107 [DOI] [PubMed] [Google Scholar]
- 5.Nakano S, Yokogami K, Ohta H, Yano T, Ohnishi T. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 1998;19:767–772 [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano S, Yokogami K, Ohta H, Goya T, Wakisaka S. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion: report of two cases. Angiology 1997;6:254–256 [Google Scholar]
- 7.Purdy PD, Devous MD, Unwin DH, Giller CA, Batjer HH. Angioplasty of an atherosclerotic middle cerebral artery associated with improvement in regional cerebral blood flow. AJNR Am J Neuroradiol 1990;11:878–880 [PMC free article] [PubMed] [Google Scholar]
- 8.Ahuja A, Guterman LR, Hopkins LN. Angioplasty for basilar artery atherosclerosis: case report. J Neurosurg 1991;77:941–944 [DOI] [PubMed] [Google Scholar]
- 9.Tsai FY, Higashida RT, Matovich V, Alfieri K, Kobayashi S. Seven years’ experience with percutaneous transluminal angioplasty for carotid stenosis. Neuroradiology 1991;33:397–398 [Google Scholar]
- 10.Higashida RT, Tsai FY, Halbach VV, et al. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg 1993;78:192–198 [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]
- 12.Nakano S, Iseda T, Yoneyama T, Ikeda T, Wakisaka S. Intravenous low-dose native tissue plasminogen activator for distal embolism in the middle cerebral artery divisions or branches: a pilot study. Neurosurgery 2000;46:853–859 [DOI] [PubMed] [Google Scholar]
- 13.Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories and angiographic site of arterial occlusion. AJNR Am J Neuroradiol 2001;22:654–659 [PMC free article] [PubMed] [Google Scholar]
- 14.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke: potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:2109–2118 [DOI] [PubMed] [Google Scholar]
- 15.Bozzao L, Angeloni U, Bastianello S, Fantozzi LM, Pierallini A, Fieschi C. Early angiographic and CT findings in patients with hemorrhagic infarction in the distribution of the middle cerebral artery. AJNR Am J Neuroradiol 1991;12:1115–1121 [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzao L, Bastianello S, Fantozzi LM, Angeloni U, Argentino C, Fieschi C. Correlation of angiographic and sequential CT findings in patients with evolving cerebral infarction. AJNR Am J Neuroradiol 1989;10:1215–1222 [PMC free article] [PubMed] [Google Scholar]
- 17.Theron J, Courtheoux P, Casasco A, et al. Local intraarterial fibrinolysis in the carotid territory. AJNR Am J Neuroradiol 1989;10:753–765 [PMC free article] [PubMed] [Google Scholar]


